Investigation on the Stability of Derivative Melam from Melamine Pyrolysis under High Pressure
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Characterizations
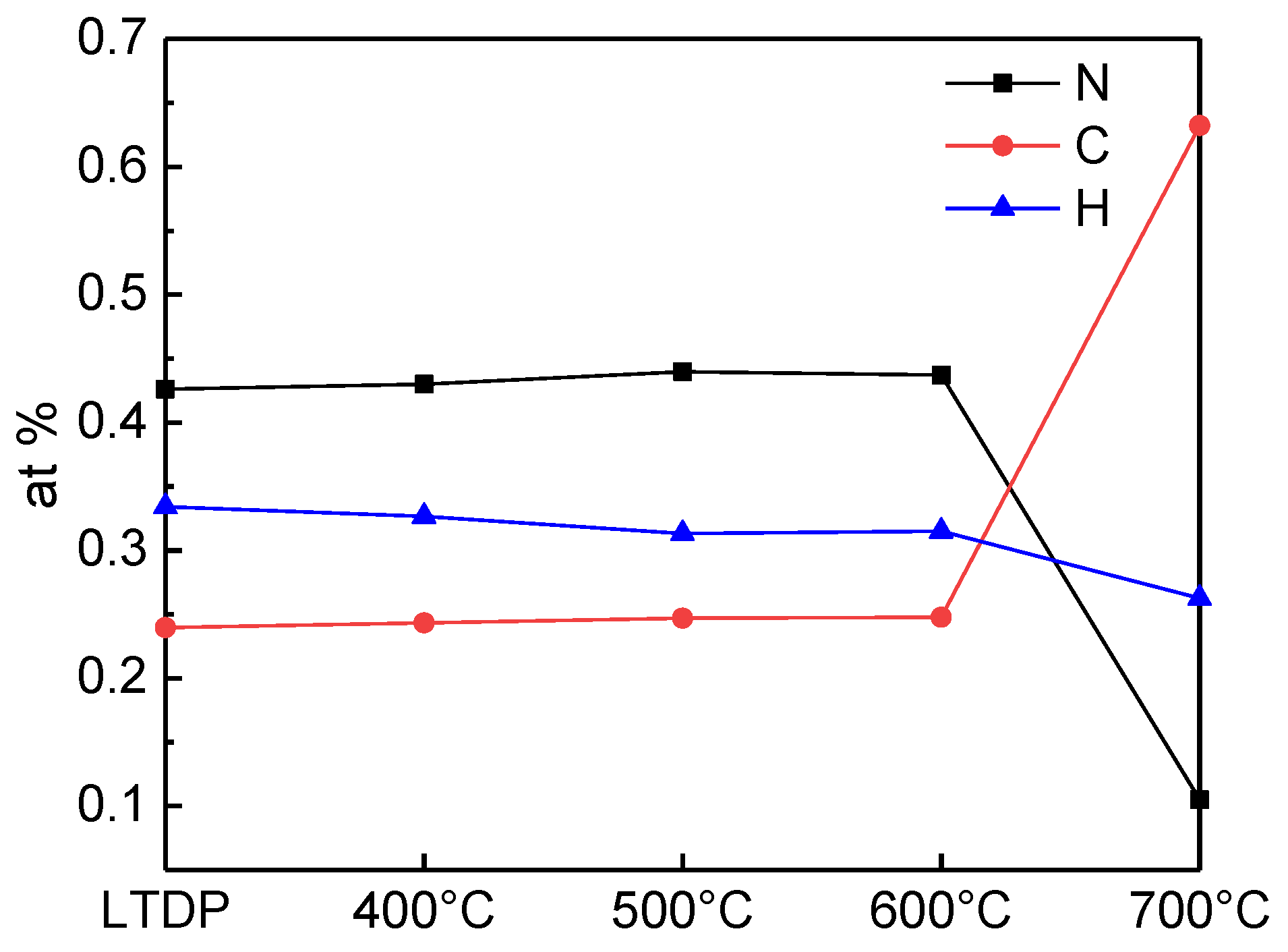
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohen, M.L. Calculation of bulk moduli of diamond and zinc-blende solids. Phys. Rev. B 1985, 32, 7988–7991. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of new low compressibility solids. Science 1989, 245, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Teter, D.M.; Hemley, R.J. Low-Compressibility Carbon Nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Sung, C.-M.; Sung, M. Carbon nitride and other speculative superhard materials. Mater. Chem. Phys. 1996, 43, 1–18. [Google Scholar] [CrossRef]
- Dong, H.; Oganov, A.R.; Zhu, Q.; Qian, G.R. The phase diagram and hardness of carbon nitrides. Sci. Rep. 2015, 5, 9870. [Google Scholar] [CrossRef] [PubMed]
- Pickard, C.J.; Salamat, A.; Bojdys, M.J.; Needs, R.J.; McMillan, P.F. Carbon nitride frameworks and dense crystalline polymorphs. Phys. Rev. B 2016, 94, 1–11. [Google Scholar] [CrossRef]
- Martin-Gil, J.; Martin-Gil, F.J.; Sarikaya, M.; Qian, M.; José-Yacamán, M.; Rubio, A. Evidence of a low compressibility carbon nitride with defect-zincblende structure. J. Appl. Phys. 1997, 81, 2555–2559. [Google Scholar] [CrossRef]
- Kroke, E.; Schwarz, M. Novel group 14 nitrides. Coord. Chem. Rev. 2004, 248, 493–532. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Correa-Guimaraes, A.; Martín-Gil, J. Synthesis of graphitic carbon nitride by reaction of melamine and uric acid. Mater. Chem. Phys. 2011, 130, 1094–1102. [Google Scholar] [CrossRef]
- Solozhenko, V. Equation of state and phase stability of turbostratic carbon nitride. J. Phys. Chem. Solids 2003, 64, 1265–1270. [Google Scholar] [CrossRef]
- Fang, L.; Ohfuji, H.; Shinmei, T.; Irifune, T. Experimental study on the stability of graphitic C3N4 under high pressure and high temperature. Diam. Relat. Mater. 2011, 20, 819–825. [Google Scholar] [CrossRef]
- Kojima, Y.; Ohfuji, H. Structure and stability of carbon nitride under high pressure and high temperature up to 125 GPa and 3000 K. Diam. Relat. Mater. 2013, 39, 1–7. [Google Scholar] [CrossRef]
- Badding, J.V.; Nesting, D.C. Thermodynamic analysis of the formation of carbon nitrides under pressure. Chem. Mater. 1996, 8, 535–540. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Yin, G.; Li, J.; Zheng, Y.; Chen, L.; Jia, Y. Optical properties of cubic Ti3N4, Zr3N4, and Hf3N4. Appl. Phys. Lett. 2006, 89, 151908. [Google Scholar] [CrossRef]
- Zinin, P.V.; Ming, L.C.; Sharma, S.K.; Hong, S.M.; Xie, Y.; Irifune, T.; Shinmei, T. Synthesis of new cubic C3N4 and diamond-like BC3 phases under high pressure and high temperature. J. Phys. Conf. Ser. 2008, 121, 62002. [Google Scholar] [CrossRef]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Posada, P.; Martín-Ramos, P.; Sánchez-Arévalo, F.M.; Dante, R.C. Molecular dynamics simulations of nanosheets of polymeric carbon nitride and comparison with experimental observations. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 137–144. [Google Scholar] [CrossRef]
- May, H. Pyrolysis of melamine. J. Appl. Chem. 1959, 9, 340–344. [Google Scholar] [CrossRef]
- Costa, L.; Camino, G. Thermal behaviour of melamine. J. Therm. Anal. 1988, 34, 423–429. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Schnick, W. New Light on an Old Story: Formation of Melam during Thermal Condensation of Melamine. Chem. A Eur. J. 2007, 13, 4956–4968. [Google Scholar] [CrossRef] [PubMed]
- Sattler, A.; Pagano, S.; Zeuner, M.; Zurawski, A.; Gunzelmann, D.; Senker, J.; Müller-Buschbaum, K.; Schnick, W. Melamine-Melem Adduct Phases: Investigating the Thermal Condensation of Melamine. Chem. A Eur. J. 2009, 15, 13161–13170. [Google Scholar] [CrossRef] [PubMed]
- Wirnhier, E.; Mesch, M.B.; Senker, J.; Schnick, W. Formation and characterization of melam, melam hydrate, and a melam-melem adduct. Chem. A Eur. J. 2013, 19, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, B.; Irran, E.; Senker, J.; Kroll, P.; Müller, H.; Schnick, W. Melem (2,5,8-Triamino-tris-triazine), an Important Intermediate during Condensation of Melamine Rings to Graphitic Carbon Nitride: Synthesis, Structure Determination by X-ray Powder Diffractometry, Solid-State NMR, and Theoretical Studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural Investigation of Graphitic Carbon Nitride via XRD and Neutron Diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Gillan, E.G. Synthesis of Nitrogen-Rich Carbon Nitride Networks from an Energetic Molecular Azide Precursor. Chem. Mater. 2000, 12, 3906–3912. [Google Scholar] [CrossRef]
- Horibe, T.; Kusaba, K.; Niwa, K.; Hasegawa, M.; Yasuda, K.; Ishigami, R. Molecular routes syntheses of graphite-like C–N compounds with various N/C ratios in high pressure and temperature. J. Ceram. Soc. Jpn. 2016, 124, 1013–1016. [Google Scholar] [CrossRef]
- Goglio, G.; Foy, D.; Demazeau, G. State of Art and recent trends in bulk carbon nitrides synthesis. Mater. Sci. Eng. R Rep. 2008, 58, 195–227. [Google Scholar] [CrossRef]
- Zhang, Z.; Leinenweber, K.; Bauer, M.; Garvie, L.A.J.; McMillan, P.F.; Wolf, G.H. High-Pressure Bulk Synthesis of Crystalline C6N9H3·HCl: A Novel C3N4 Graphitic Derivative. J. Am. Chem. Soc. 2001, 123, 7788–7796. [Google Scholar] [CrossRef] [PubMed]
- Horvath-Bordon, E.; Riedel, R.; McMillan, P.F.; Kroll, P.; Miehe, G.; Van Aken, P.A.; Zerr, A.; Hoppe, P.; Shebanova, O.; McLaren, I.; et al. High-pressure synthesis of crystalline carbon nitride imide, C2N2(NH). Angew. Chem. Int. Ed. 2007, 46, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.A.; Jia, X.; Cui, Q.L.; Pan, Y.W.; Zhu, P.W.; Liu, B.B.; Liu, H.J.; Wang, X.C.; Liu, J.; Zou, G.T. Crystal structures of C3N6H6 under high pressure. Chem. Phys. Lett. 2003, 368, 668–672. [Google Scholar] [CrossRef]
- Hong, J.; Xia, X.; Wang, Y.; Xu, R. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. 2012, 22, 15006. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Maeda, K.; Domen, K.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Sulfur-mediated synthesis of carbon nitride: Band-gap engineering and improved functions for photocatalysis. Energy Environ. Sci. 2011, 4, 675–678. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Metal-free activation of H2O2 by g-C3N4 under visible light irradiation for the degradation of organic pollutants. Phys. Chem. Chem. Phys. 2012, 14, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, B.; Yang, S.; Wu, H.; Wu, Y.; Wu, L.; Pan, J.; Xiong, X. In situ construction of an SnO2/g-C3N4 heterojunction for enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- Martín-Ramos, P.; Martín-Gil, J.; Dante, R.C.; Vaquero, F.; Navarro, R.M.; Fierro, J.L.G. A simple approach to synthesize g-C3N4 with high visible light photoactivity for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 7273–7281. [Google Scholar] [CrossRef]
- De Lourdes Mendes Finete, V.; Martins Gouvêa, M.; De Carvalho Marques, F.F.; Duarte Pereira Netto, A. Characterization of newfound natural luminescent properties of melamine, and development and validation of a method of high performance liquid chromatography with fluorescence detection for its determination in kitchen plastic ware. Talanta 2014, 123, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Li, J.; Kalytchuk, S.; Wang, Y.; Li, Q.; Lau, T.C.; Niehaus, T.A.; Rogach, A.L.; Zhang, R.-Q. Efficient Emission Facilitated by Multiple Energy Level Transitions in Uniform Graphitic Carbon Nitride Films Deposited by Thermal Vapor Condensation. Chem. Phys. Chem. 2015, 16, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Luo, K.; Zhang, K.; He, J.; Zhao, Y.; Yu, D. Combinatorial Vibration-Mode Assignment for the FTIR Spectrum of Crystalline Melamine: A Strategic Approach toward Theoretical IR Vibrational Calculations of Triazine-Based Compounds. J. Phys. Chem. A 2016, 120, 7427–7433. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J.; Rodil, S.E.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 67, 1–20. [Google Scholar] [CrossRef]
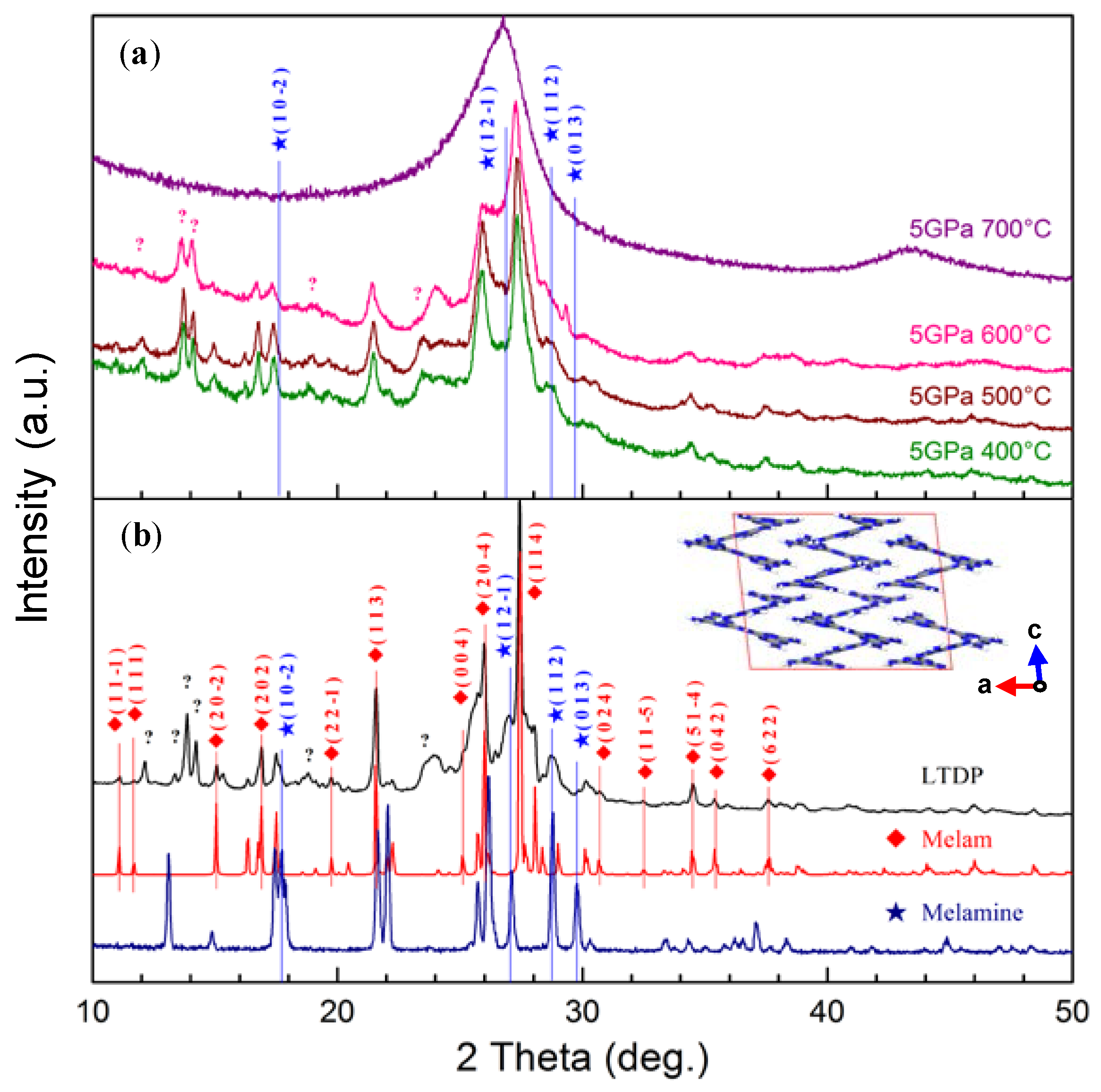

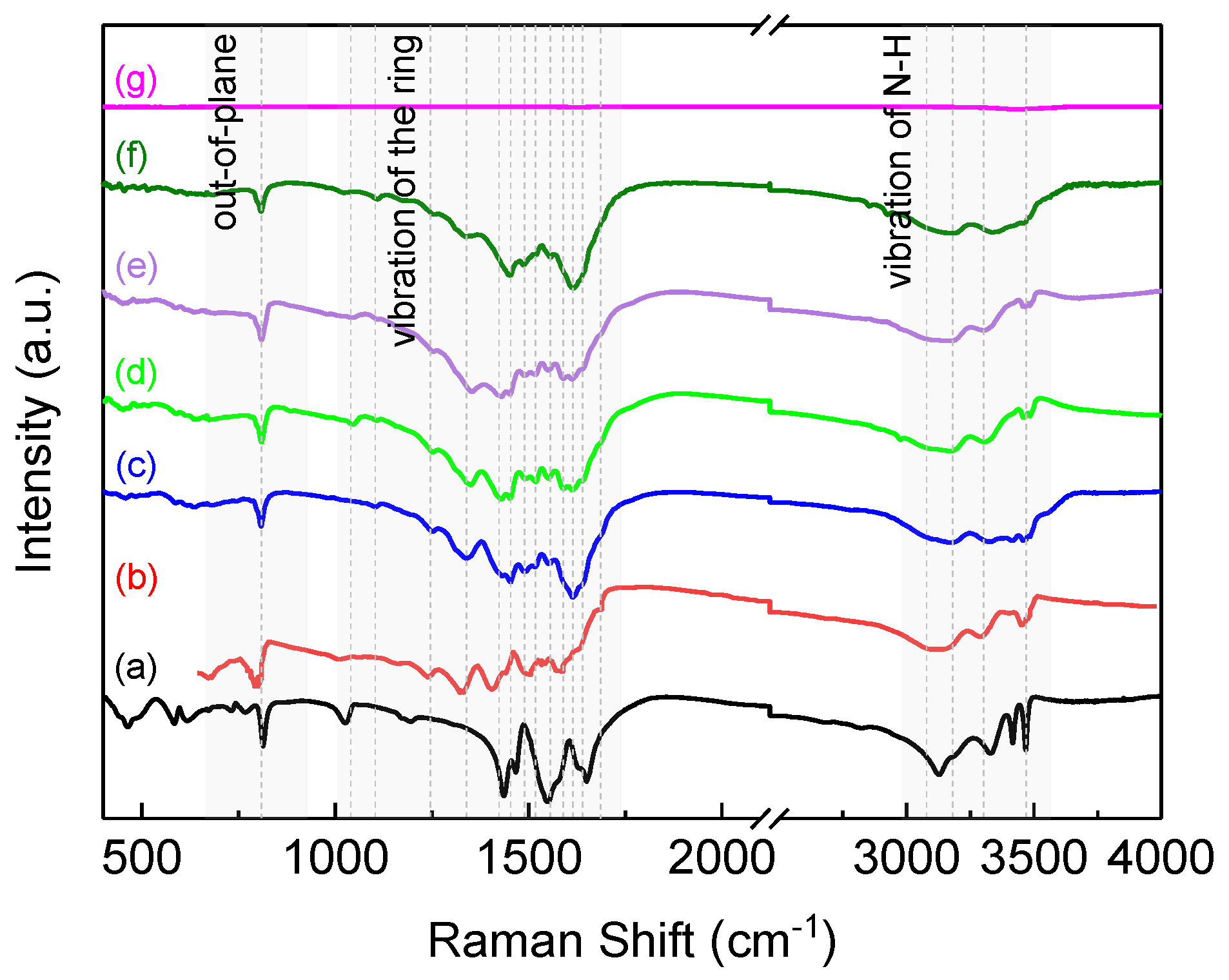
| Vibrations | Melam (cm−1) | Melamine (cm−1) | LTDP (cm−1) |
|---|---|---|---|
| N–H | 3483.8 (vw) | 3468 (m) | 3485 (vw) |
| 3456.5 (vw) | 3420 (m) | 3460 (w) | |
| 3300.2 (w) | 3331 (m) | 3320 (w) | |
| 3165.4(w) | 3129 (m) | 3182 (w) | |
| Ring | 1687 (vw) | - | - |
| 1639.5 (m) | - | - | |
| 1610.0 (m) | - | - | |
| 1583.8 (s) | 1650 (vs) | 1614 (vs) | |
| 1545.8 (s) | 1548 (vs) | 1552 (m) | |
| 1513.1 (s) | - | 1515 (m) | |
| 1450.8 (s) | 1469 (vs) | 1452 (vs) | |
| 1414.7 (vs) | 1438 (vs) | 1429 (vs) | |
| 1338.4 (vs) | - | 1337 (vs) | |
| 1249.8 (s) | - | 1250 (m) | |
| 1174.9 (w) | - | - | |
| 1069.9 (vw) | - | - | |
| 1021.1 (vw) | - | - | |
| 972.2 (vw) | - | - | |
| Out-of-Plane | 806.2 (vs) | 814 (vs) | 808 (vs) |
| 782.1 (w) | - | - | |
| 748.0 (vw) | - | - | |
| 682.6 (m) | - | 681 (w) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Luo, K.; Wu, Y.; He, J.; Zhao, Z.; Yu, D. Investigation on the Stability of Derivative Melam from Melamine Pyrolysis under High Pressure. Nanomaterials 2018, 8, 172. https://doi.org/10.3390/nano8030172
Yuan X, Luo K, Wu Y, He J, Zhao Z, Yu D. Investigation on the Stability of Derivative Melam from Melamine Pyrolysis under High Pressure. Nanomaterials. 2018; 8(3):172. https://doi.org/10.3390/nano8030172
Chicago/Turabian StyleYuan, Xiaohong, Kun Luo, Yingju Wu, Julong He, Zhisheng Zhao, and Dongli Yu. 2018. "Investigation on the Stability of Derivative Melam from Melamine Pyrolysis under High Pressure" Nanomaterials 8, no. 3: 172. https://doi.org/10.3390/nano8030172
APA StyleYuan, X., Luo, K., Wu, Y., He, J., Zhao, Z., & Yu, D. (2018). Investigation on the Stability of Derivative Melam from Melamine Pyrolysis under High Pressure. Nanomaterials, 8(3), 172. https://doi.org/10.3390/nano8030172





