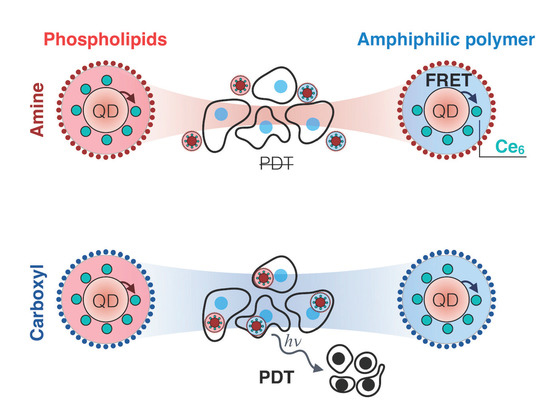
Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spectral Studies
2.3. Cell Culturing and Labeling
2.4. PDT Studies
2.5. Cellular Microscopy
3. Results and Discussion
3.1. QD-Ce6 Complex Formation
3.2. FRET from QDs to Ce6
3.3. Cellular Accumulation of QD-Ce6 in Serum-Free Environment and PDT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samia, A.C.; Chen, X.; Burda, C. Semiconductor Quantum Dots for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, N.; Spillmann, C.M.; Algar, W.R.; Pons, T.; Stewart, M.H.; Oh, E.; Susumu, K.; Diaz, S.A.; Delehanty, J.B.; Medintz, I.L. Energy Transfer with Semiconductor Quantum Dot Bioconjugates: A Versatile Platform for Biosensing, Energy Harvesting, and Other Developing Applications. Chem. Rev. 2016, 117, 536–711. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hernandez, B.; Selke, M. Singlet Oxygen Generation from Water-Soluble Quantum Dot-Organic Dye Nanocomposites. J. Am. Chem. Soc. 2006, 128, 6278–6279. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-M.; Sun, X.-J.; Wang, L.-L.; Fei, M.-Y.; Yan, Z.-Y. Singlet Oxygen-Generating from Fluorescence Probes Based on Denatured Bovine Serum Albumin-Conjugated Cdte Quantum Dots and Photosensitizer Chlorin E6. J. Nanopart. Res. 2014, 16, 2701. [Google Scholar] [CrossRef]
- Charron, G.; Stuchinskaya, T.; Edwards, D.R.; Russell, D.A.; Nann, T. Insights into the Mechanism of Quantum Dot-Sensitized Singlet Oxygen Production for Photodynamic Therapy. J. Phys. Chem. C 2012, 116, 9334–9342. [Google Scholar] [CrossRef]
- Tsay, J.M.; Trzoss, M.; Shi, L.; Kong, X.; Selke, M.; Jung, M.E.; Weiss, S. Singlet Oxygen Production by Peptide-Coated Quantum Dot-Photosensitizer Conjugates. J. Am. Chem. Soc. 2007, 129, 6865–6871. [Google Scholar] [CrossRef]
- Yaghini, E.; Pirker, K.F.; Kay, C.W.; Seifalian, A.M.; MacRobert, A.J. Quantification of Reactive Oxygen Species Generation by Photoexcitation of Pegylated Quantum Dots. Small 2014, 10, 5106–5115. [Google Scholar] [CrossRef]
- Dayal, S.; Burda, C. Semiconductor Quantum Dots as Two-Photon Sensitizers. J. Am. Chem. Soc. 2008, 130, 2890–2891. [Google Scholar] [CrossRef]
- Wen, Y.-N.; Song, W.-S.; An, L.-M.; Liu, Y.-Q.; Wang, Y.-H.; Yang, Y.-Q. Activation of Porphyrin Photosensitizers by Semiconductor Quantum Dots Via Two-Photon Excitation. Appl. Phys. Lett. 2009, 95, 143702. [Google Scholar] [CrossRef]
- Skripka, A.; Valanciunaite, J.; Dauderis, G.; Poderys, V.; Kubiliute, R.; Rotomskis, R. Two-Photon Excited Quantum Dots as Energy Donors for Photosensitizer Chlorin E6. J. Biomed. Opt. 2013, 18, 078002. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.-C.; Yang, M.-J.; Hsu, C.-C.; Lai, C.-W.; Hsieh, C.-C.; Lin, S.H.; Cheng, Y.-M.; Chou, P.-T. The Empirical Correlation between Size and Two-Photon Absorption Cross Section of Cdse and Cdte Quantum Dots. Small 2006, 2, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.O.; Martynenko, I.V.; Maslov, V.G.; Fedorov, A.V.; Gun’ko, Y.K.; Baranov, A.V. Investigation of Complexes of Cdte Quantum Dots with the Aloh-Sulphophthalocyanine Molecules in Aqueous Media. J. Phys. Chem. C 2013, 117, 23425–23431. [Google Scholar] [CrossRef]
- Zenkevich, E.; Cichos, F.; Shulga, A.; Petrov, E.P.; Blaudeck, T.; von Borczyskowski, C. Nanoassemblies Designed from Semiconductor Quantum Dots and Molecular Arrays. J. Phys. Chem. B 2005, 109, 8679–8692. [Google Scholar] [CrossRef] [PubMed]
- Valanciunaite, J.; Klymchenko, A.S.; Skripka, A.; Richert, L.; Steponkiene, S.; Streckyte, G.; Mely, Y.; Rotomskis, R. A Non-Covalent Complex of Quantum Dots and Chlorin E6: Efficient Energy Transfer and Remarkable Stability in Living Cells Revealed by Flim. RSC Adv. 2014, 4, 52270–52278. [Google Scholar] [CrossRef]
- Valanciunaite, J.; Skripka, A.; Streckyte, G.; Rotomskis, R. Complex of Water-Soluble Cdse/Zns Quantum Dots and Chlorin E6: Interaction and Fret. Laser Appl. Life Sci. 2010, 7376, 737607. [Google Scholar] [CrossRef]
- Steponkiene, S.; Valanciunaite, J.; Skripka, A.; Rotomskis, R. Cellular Uptake and Photosensitizing Properties of Quantum Dot-Chlorin E6 Complex: In Vitro Study. J. Biomed. Nanotechnol. 2014, 10, 679–686. [Google Scholar] [CrossRef]
- Karabanovas, V.; Skripka, A.; Valanciunaite, J.; Kubiliute, R.; Poderys, V.; Rotomskis, R. Formation of Self-Assembled Quantum Dot–Chlorin E6 Complex: Influence of Nanoparticles Phospholipid Coating. J. Nanopart. Res. 2014, 16, 2508. [Google Scholar] [CrossRef]
- Valančiūnaitė, J.; Skripka, A.; Araminaitė, R.; Kalantojus, K.; Streckytė, G.; Rotomskis, R. Spectroscopic Study of Non-Covalent Complex Formation between Different Porphyrin Analogues and Quantum Dots with Lipidbased Coating. Chemija 2011, 22, 181–187. [Google Scholar]
- Skripka, A.; Marin, R.; Benayas, A.; Canton, P.; Hemmer, E.; Vetrone, F. Covering the Optical Spectrum through Collective Rare-Earth Doping of NaGdF4 Nanoparticles: 806 and 980 nm Excitation Routes. Phys. Chem. Chem. Phys. 2017, 19, 11825–11834. [Google Scholar] [CrossRef] [PubMed]
- Dapkute, D.; Steponkiene, S.; Bulotiene, D.; Saulite, L.; Riekstina, U.; Rotomskis, R. Skin-Derived Mesenchymal Stem Cells as Quantum Dot Vehicles to Tumors. Int. J. Nanomed. 2017, 12, 8129–8142. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.A.; Zenkevich, E.I.; Gurinovich, G.P.; Kochubeyev, G.A. Chlorin E6-Liposome Interaction. Investigation by the Methods of Fluorescence Spectroscopy and Inductive Resonance Energy Transfer. J. Photochem. Photobiol. B 1990, 7, 43–56. [Google Scholar] [CrossRef]
- Mojzisova, H.; Bonneau, S.; Vever-Bizet, C.; Brault, D. The Ph-Dependent Distribution of the Photosensitizer Chlorin E6 among Plasma Proteins and Membranes: A Physico-Chemical Approach. Biochim. Biophys. Acta 2007, 1768, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Sotgiu, G.; Ferroni, C.; Duchi, S.; Lucarelli, E.; Martini, C.; Posati, T.; Guerrini, A.; Ballestri, M.; Corticelli, F.; et al. Chlorin E6 Keratin Nanoparticles for Photodynamic Anticancer Therapy. RSC Adv. 2016, 6, 33910–33918. [Google Scholar] [CrossRef]
- Parak, W.J.; Pellegrino, T.; Plank, C. Labelling of Cells with Quantum Dots. Nanotechnology 2005, 16, R9–R25. [Google Scholar] [CrossRef]
- Pellegrino, T.; Manna, L.; Kudera, S.; Liedl, T.; Koktysh, D.; Rogach, A.L.; Keller, S.; Rädler, J.; Natile, G.; Parak, W.J. Hydrophobic Nanocrystals Coated with an Amphiphilic Polymer Shell: A General Route to Water Soluble Nanocrystals. Nano Lett. 2004, 4, 703–707. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Orlova, A.O.; Maslov, V.G.; Baranov, A.V.; Fedorov, A.V.; Artemyev, M. Energy Transfer in Complexes of Water-Soluble Quantum Dots and Chlorin E6 Molecules in Different Environments. Beilstein J. Nanotechnol. 2013, 4, 895–902. [Google Scholar] [CrossRef]
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent Dependence of the Fluorescence Lifetimes of Xanthene Dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (Cdse)Zns Core−Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and Properties of Biocompatible Water-Soluble Silica-Coated Cdse/Zns Semiconductor Quantum Dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; Volume 1. [Google Scholar]
- Howland, M.C.; Szmodis, A.W.; Sanii, B.; Parikh, A.N. Characterization of Physical Properties of Supported Phospholipid Membranes Using Imaging Ellipsometry at Optical Wavelengths. Biophys. J. 2007, 92, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Lü, C.; Yang, B. High Refractive Index Organic–Inorganic Nanocomposites: Design, Synthesis and Application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Jennings, T.L.; Becker-Catania, S.G.; Triulzi, R.C.; Tao, G.; Scott, B.; Sapsford, K.E.; Spindel, S.; Oh, E.; Jain, V.; Delehanty, J.B.; et al. Reactive Semiconductor Nanocrystals for Chemoselective Biolabeling and Multiplexed Analysis. ACS Nano 2011, 5, 5579–5593. [Google Scholar] [CrossRef] [PubMed]
- Mercatali, L.; La Manna, F.; Groenewoud, A.; Casadei, R.; Recine, F.; Miserocchi, G.; Pieri, F.; Liverani, C.; Bongiovanni, A.; Spadazzi, C.; et al. Development of a Patient-Derived Xenograft (PDX) of Breast Cancer Bone Metastasis in a Zebrafish Model. Int. J. Mol. Sci. 2016, 17, 1375. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Stewart, R.L.; Chen, J.; Gao, T.; Scott, T.L.; Samayoa, L.M.; O’Connor, K.; Lane, A.N.; Xu, R. Collagen Prolyl 4-Hydroxylase 1 Is Essential for Hif-1α Stabilization and Tnbc Chemoresistance. Nat. Commun. 2018, 9, 4456. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, C.C.; Payne, C.K. Nanoparticle Surface Charge Mediates the Cellular Receptors Used by Protein-Nanoparticle Complexes. J. Phys. Chem. B 2012, 116, 8901–8907. [Google Scholar] [CrossRef]
- Frohlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Karabanovas, V.; Zitkus, Z.; Kuciauskas, D.; Rotomskis, R.; Valius, M. Surface Properties of Quantum Dots Define Their Cellular Endocytic Routes, Mitogenic Stimulation and Suppression of Cell Migration. J. Biomed. Nanotechnol. 2014, 10, 775–786. [Google Scholar] [CrossRef]
- Damalakiene, L.; Karabanovas, V.; Bagdonas, S.; Rotomskis, R. Fluorescence-Lifetime Imaging Microscopy for Visualization of Quantum Dots’ Endocytic Pathway. Int. J. Mol. Sci. 2016, 17, 473. [Google Scholar] [CrossRef]
- Damalakiene, L.; Karabanovas, V.; Bagdonas, S.; Valius, M.; Rotomskis, R. Intracellular Distribution of Nontargeted Quantum Dots after Natural Uptake and Microinjection. Int. J. Nanomed. 2013, 8, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Variables Influencing Interactions of Untargeted Quantum Dot Nanoparticles with Skin Cells and Identification of Biochemical Modulators. Nano Lett. 2007, 7, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailander, V.; Landfester, K.; et al. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernandez, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Quantity | L-QD(amine) | L-QD(carboxyl) | P-QD(amine) | P-QD(carboxyl) | ||||
|---|---|---|---|---|---|---|---|---|
| QY | 0.14 | 0.18 | 0.34 | 0.37 | ||||
| J, 10−13 M−1cm3 | 1.16 | 1.19 | 1.26 | 1.22 | ||||
| R0, Å | 38.0 | 39.8 | 44.7 | 45.0 | ||||
| m | E, % | r, Å | E, % | r, Å | E, % | r, Å | E, % | r, Å |
| 0.5 | 23.9 | 41.1 | 20.8 | 44.3 | 10.9 | 56.5 | 5.4 | 64.7 |
| 5 | 74.9 | 41.5 | 70.6 | 45.0 | 47.3 | 59.5 | 24.4 | 71.1 |
| 10 | 82.8 | 43.0 | 83.7 | 44.5 | 66.9 | 58.3 | 40.1 | 70.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skripka, A.; Dapkute, D.; Valanciunaite, J.; Karabanovas, V.; Rotomskis, R. Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy. Nanomaterials 2019, 9, 9. https://doi.org/10.3390/nano9010009
Skripka A, Dapkute D, Valanciunaite J, Karabanovas V, Rotomskis R. Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy. Nanomaterials. 2019; 9(1):9. https://doi.org/10.3390/nano9010009
Chicago/Turabian StyleSkripka, Artiom, Dominyka Dapkute, Jurga Valanciunaite, Vitalijus Karabanovas, and Ricardas Rotomskis. 2019. "Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy" Nanomaterials 9, no. 1: 9. https://doi.org/10.3390/nano9010009