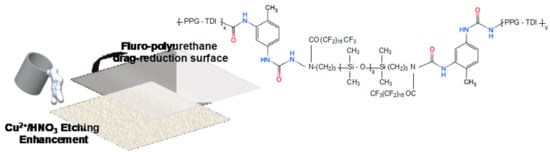
Fabrication of Zinc Substrate Encapsulated by Fluoropolyurethane and Its Drag-Reduction Enhancement by Chemical Etching
Abstract
:1. Introduction
2. Experiment and Methods
2.1. Chemicals
2.2. Fabrication of Drag-Reduction Substrate
2.2.1. Etching Treatment of Zinc Substrate
2.2.2. Fluoro-Modified Polyurethane Encapsulated Treatment
2.3. Surface Characterization
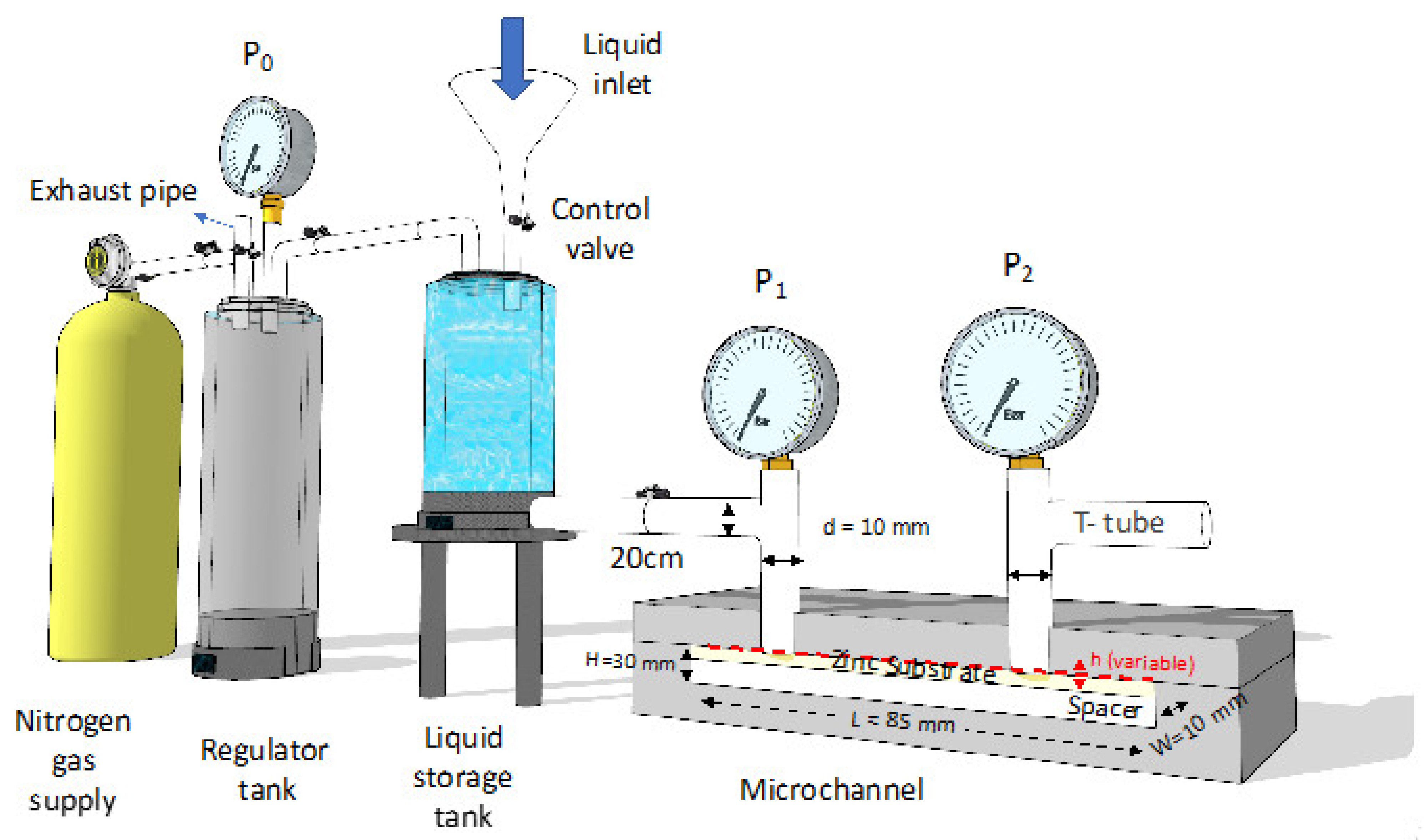
2.4. Microchannel Drag Reduction Test
3. Results and Discussion
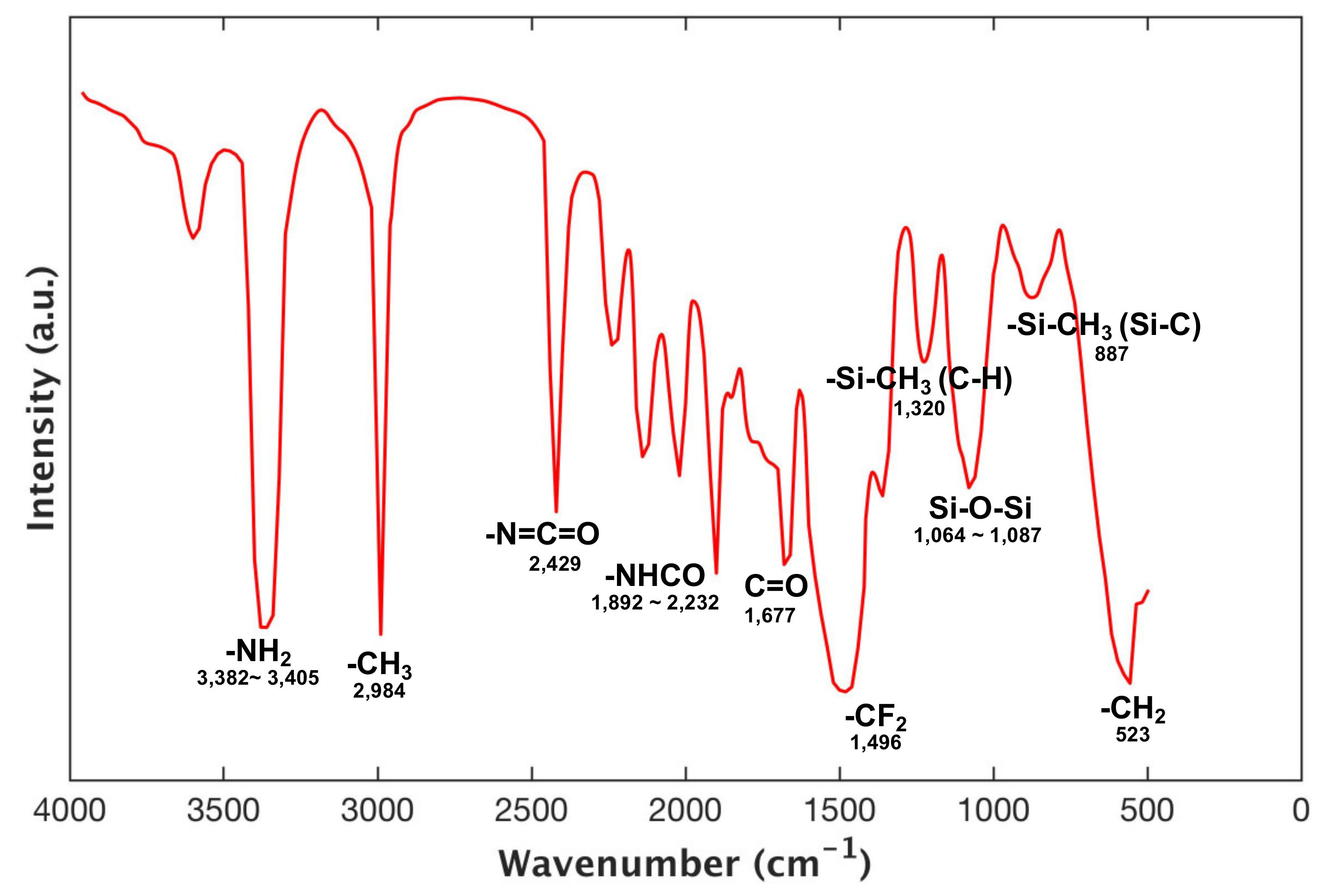
3.1. FTIR Characterization of Fluoropolyurethane Prepolymer
3.2. Surface Morphology
3.3. Surface Wettability Analysis
3.4. Microchannel Drag-Reduction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, M.; Randal, M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Ong, C.; Murthe, S.S.; Mohamed, N.; Perumal, V.; Mohamed Saheed, M. Nanoscaled Surface Modification of Poly(dimethylsiloxane) Using Carbon Nanotubes for Enhanced Oil and Organic Solvent Absorption. ACS Omega 2018, 3, 15907–15915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.; Luo, Y. Synthesis and characterization of siloxane-modified two-component waterborne polyurethane. Prog. Org. Coat. 2013, 76, 1522–1526. [Google Scholar] [CrossRef]
- Philipp, C. Waterborne polyurethane wood coatings based on rapeseed fatty acid methyl esters. Prog. Org. Coat. 2012, 74, 705–711. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Mathew, A.; Kurmvanshi, S.; Mohanty, S.; Nayak, S. Preparation and characterization of siloxane modified: Epoxy terminated polyurethane-silver nanocomposites. Polym. Compos. 2018, 39, E2390–E2396. [Google Scholar] [CrossRef]
- Galhenage, T.; Webster, D.; Moreira, A.; Burgett, R.; Stafslien, S.; Vanderwal, L.; Clare, A. Poly(ethylene) glycol-modified, amphiphilic, siloxane–polyurethane coatings and their performance as fouling-release surfaces. J. Coat. Technol. Res. 2017, 14, 307–322. [Google Scholar] [CrossRef]
- Zhou, X.X.; Sun, B.; Wu, S.; Zhang, X.; Liu, Q.; Xiao, Y. Reports on Materials Science Findings from Wuhan University of Technology Provide New Insights (Evaluation On Self-healing Mechanism and Hydrophobic Performance of Asphalt Modified By Siloxane and Polyurethane).Report. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.; Tabasum, S.; Aftab, W.; Shahid, M.; Zuber, M. Structural elucidation and biological aptitude of modified hydroxyethylcellulose-polydimethyl siloxane based polyurethanes. Int. J. Biol. Macromol. 2020, 150, 426–440. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y. Super-hydrophobic surfaces from natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Cottin, B.; Barrat, J.; Bocquet, L. Low-friction flows of liquid at nanopatterned interfaces. Nat. Mater. 2003, 2, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, S.; Charlaix, E.; Benyagoub, A. Contact angle hysteresis on nano- structured surfaces. Surf. Sci. 2003, 540, 355–362. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, W.; Park, H.C.; Lee, K.H. Super-hydrophobic nano-wire entanglement structures. J. Micromech. Microeng. 2006, 16, 2593. [Google Scholar] [CrossRef]
- Cai, X.; Xiao, X. Research Progress of Superhydrophobic Surface Coatings. Mod. Chem. 2013, 23, 22–25. [Google Scholar]
- Feng, L.; Song, Y.; Zhai, J.; Liu, B.; Xu, J.; Jiang, L.; Zhu, D. Creation of a super- hydrophobic surface from an amphiphilic polymer. Angew. Chem. Int. Ed. 2003, 42, 800–802. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Superhydrophobic, passive microvalves with controllable opening threshold: Exploiting plasma nanotextured microfluidics for a programmable flow switchboard. Microfluid. Nanofluidics 2014, 17, 489–498. [Google Scholar] [CrossRef]
- Tadanaga, K.; Morinaga, J.; Matsuda, A.; Minami, T. Super-hydrophobic–super- hydrophilic micropatterning on flowerlike alumina coating film by the sol–gel method. Chem. Mater. 2000, 12, 590–592. [Google Scholar] [CrossRef]
- Youngblood, J.; McCarthy, T. Ultrahydrophobic polymer surfaces prepared by simultaneous ablation of polypropylene and sputtering of poly(tetrafluoroethylene) using radio frequency plasma. Macromolecules 1999, 32, 6800–6806. [Google Scholar] [CrossRef]
- Liu, H.; Feng, L.; Zhai, J.; Jiang, L.; Zhu, D. Reversible wettability of a chemical vapor deposition prepared ZnO film between super-hydrophobicity and super- hydrophilicity. Langmuir 2004, 20, 5659–5661. [Google Scholar] [CrossRef]
- Hirano, T.; Nakade, K.; Li, S.; Kawai, K.; Arima, K. Chemical etching of a semiconductor surface assisted by single sheets of reduced graphene oxide. Carbon 2018, 127, 681–687. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Yu, X.; Shi, F.; Xu, H.; Zhang, X.; Smet, M.; Dehaen, W. Self-assembled monolayers of dendron thiols for electrodeposition of gold nanostructures: Toward fabrication of super-hydrophobic/super-hydrophilic surfaces and pH-responsive surfaces. Langmuir 2005, 21, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Geyer, F.; Pilat, D.W.; Wooh, S.; Vollmer, D.; Butt, H.J.; Berger, R. How drops start sliding over solid surfaces. Nat. Phys. 2018, 14, 191–196. [Google Scholar] [CrossRef]
- Sarkiris, P.; Ellinas, K.; Gkiolas, D.; Mathioulakis, D.; Gogolides, E. Motion of Drops with Different Viscosities on Micro-Nanotextured Surfaces of Varying Topography and Wetting Properties. Adv. Funct. Mater. 2019, 29, 1902905. [Google Scholar] [CrossRef]
- Lagubeau, G.; Le Merrer, M.; Clanet, C.; Quere, D. Leidenfrost on a ratchet. Nat. Phys. 2011, 7, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable superhydrophobic and superamphiphobic polymeric surfaces and their applications: A review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef]
- Wu, C.; Ma, G.; Zhou, P. Research progress on boundary slip of fluid flow. Adv. Mech. 2008, 38, 265–282. [Google Scholar]
- Hao, X.; Wang, L.; Ding, Y. Study on drag reduction of superhydrophobic surface. Lubr. Eng. 2009, 34, 25–28. [Google Scholar]
- Poetes, R.; Holtzmann, K.; Franze, K.U. Steiner, Metastable underwater superhydrophobicity. Phys. Rev. Lett. 2010, 105, 166104. [Google Scholar] [CrossRef]
- Pan, S.; Kota, A.; Mabry, J.; Tuteja, A. Superomniphobic surfaces for effective chemical shielding. J Am Chem. Soc. 2013, 135, 578–581. [Google Scholar] [CrossRef]
- Ellinas, K.; Pujari, S.; Dragatogiannis, D.; Charitidis, C.; Tserepi, A. Zuilhof, Plasma micro-nanotextured, scratch, water and hexadecane resistant, superhydrophobic, and superamphiphobic polymeric surfaces with perfluorinated monolayers. ACS Appl. Mater. Interfaces 2014, 6, 6510–6524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Chen, H.; Zhu, W.; Zhang, P. A novel damage-tolerant superhydrophobic and superoleophilic material. J. Mater. Chem. A 2014, 2, 9002. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, E.; Zhang, X.; Yuan, R.; Wu, S.; Zhu, Y. Facile preparation of superamphiphobic epoxy resin/modified poly(vinylidene fluoride)/fluorinated ethylene propylene composite coating with corrosion/wear-resistance. Appl. Surf. Sci. 2015, 357, 229–235. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Men, X.; Yang, J.; Wang, K.; Xu, X.; Zhou, X.; Xue, Q. Robust superhydrophobic surfaces with mechanical durability and easy repairability. J. Mater. Chem. 2011, 21, 15793–15797. [Google Scholar] [CrossRef]
- Choi, C.; Kim, C. Large slip of aqueous liquid flow over a nanoengineered super-hydrophobic surface. Phys. Rev. Lett. 2006, 96, 066001. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Luo, B.; Guet, C.; Narasimalu, S.; Dong, L. Preparation and formula analysis of anti-biofouling titania–polyurea spray coating with nano/micro-structure. Coatings 2019, 9, 560. [Google Scholar] [CrossRef] [Green Version]
- Huo, S.; Yu, Z.; Li, Y. Flow characteristics of water in superhydrophobic microchannels. Chem. J. China 2007, 58, 2721–2726. [Google Scholar]
- Tsai, P.; Peters, A.M.; Pirat, C.; Wessling, M.; Lammertink, R.G.H.; Lohse, D. Quan-tifying effective slip length over micropatterned hydrophobic surfaces. Phys. Fluids 2009, 21, 112002–112008. [Google Scholar] [CrossRef] [Green Version]
- Natrajan, V.; Christensen, K. The impact of surface roughness on flow through a rectangular microchannel from the laminar to turbulent regimes. Microfluid. Nanofluidics 2010, 9, 95–121. [Google Scholar] [CrossRef]
- Hong, C.; Yamada, T.; Asako, Y.; Faghri, M. Experimental investigations of laminar, transitional and turbulent Gas flow in microchannels. Int. J. Heat Mass Transf. 2012, 55, 4397–4403. [Google Scholar] [CrossRef]
- Arjun, K.; Rakesh, K. CFD analysis of thermal performance of microchannel nanofluid flow at different Reynolds numbers. Songklanakarin J. Sci. Technol. 2019, 41, 109–116. [Google Scholar]
- Gatapova, E.; Dmitry, S.G. The Drag Reduction of Microchannel Flow by Contrast Wettability. MATEC Web Conf. 2016, 72, 4. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Deng, Y.; Yi, P.; Peng, L. Experimental and numerical investigation on thin sheet metal roll forming process of micro channels with high aspect ratio. Int. J. Adv. Manuf. Technol. 2019, 100, 117–129. [Google Scholar] [CrossRef]
- Madavan, N.; Deutsch, S.; Merkle, C. Measurments of local skin friction in a microbubblemodified turbulent boundary layer. J. Fluid Mech. 1985, 156, 237–256. [Google Scholar] [CrossRef]
- Byun, D.; Kim, J.; Han, S.; Hoon, C. Direct measurement of slip flows in superhydrophobic microchannels with transverse grooves. Phys. Fluids 2008, 20, 113601. [Google Scholar] [CrossRef]
- Deutsch, S.; Castano, J. Microbubble skin friction reduction on an axisymmetric body. Phys. Fluids 1986, 29, 3590–3596. [Google Scholar] [CrossRef]
- Zhou, Y.; Chang, H. Numerical simulation of hydrodynamic and heat transfer characteristics of slug flow in serpentine microchannel with various curvature ratio. Int. J. Heat Mass Transf. 2019, 55, 3343–3358. [Google Scholar] [CrossRef]
- Papageorgiou, D.P.; Tsougeni, K.; Tserepi, A.; Gogolides, E. Superhydrophobic, hierarchical, plasma-nanotextured polymeric microchannels sustaining high-pressure flows. Microfluid. Nanofluid. 2013, 14, 247–255. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cui, Z.; Zhu, Q.; Narasimalu, S.; Dong, Z. Fabrication of Zinc Substrate Encapsulated by Fluoropolyurethane and Its Drag-Reduction Enhancement by Chemical Etching. Coatings 2020, 10, 377. https://doi.org/10.3390/coatings10040377
Li Y, Cui Z, Zhu Q, Narasimalu S, Dong Z. Fabrication of Zinc Substrate Encapsulated by Fluoropolyurethane and Its Drag-Reduction Enhancement by Chemical Etching. Coatings. 2020; 10(4):377. https://doi.org/10.3390/coatings10040377
Chicago/Turabian StyleLi, Yuanzhe, Zhe Cui, Qiucheng Zhu, Srikanth Narasimalu, and Zhili Dong. 2020. "Fabrication of Zinc Substrate Encapsulated by Fluoropolyurethane and Its Drag-Reduction Enhancement by Chemical Etching" Coatings 10, no. 4: 377. https://doi.org/10.3390/coatings10040377