Enhanced Biocompatibility and Osteogenic Property of Biodegradable Zn-0.5Li Alloy through Calcium–Phosphorus Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Surface Pretreatment
2.2. Coating Preparation
2.3. Coating Characterization
2.4. Electrochemical Measurements
2.5. Immersion Test in SBF
2.6. In Vitro Cell Culture
2.6.1. Cell Proliferation
2.6.2. Live/Dead Cell Staining
2.6.3. Cell Morphology
2.6.4. Alkaline Phosphatase Measurement
2.7. Statistical Analysis
3. Results
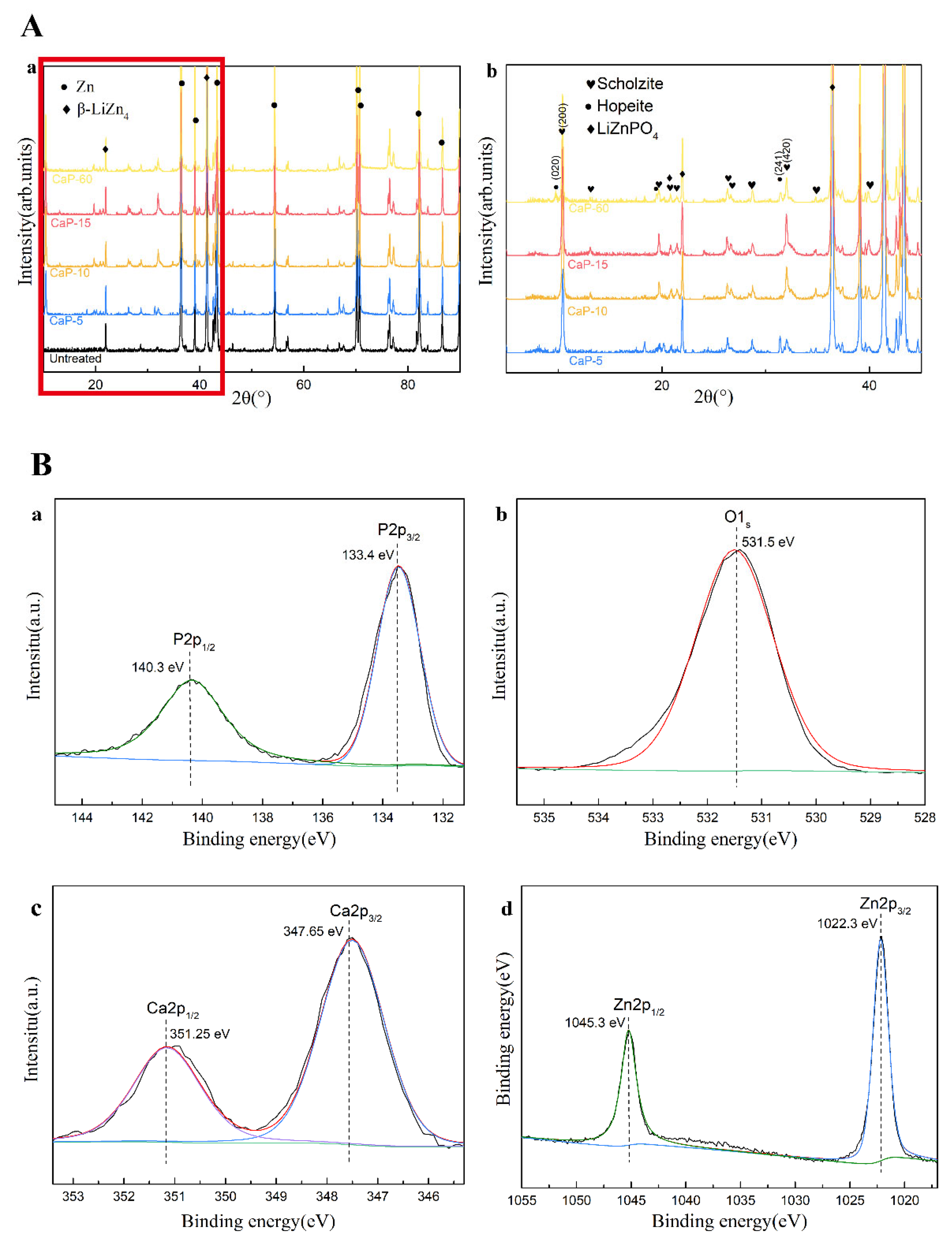
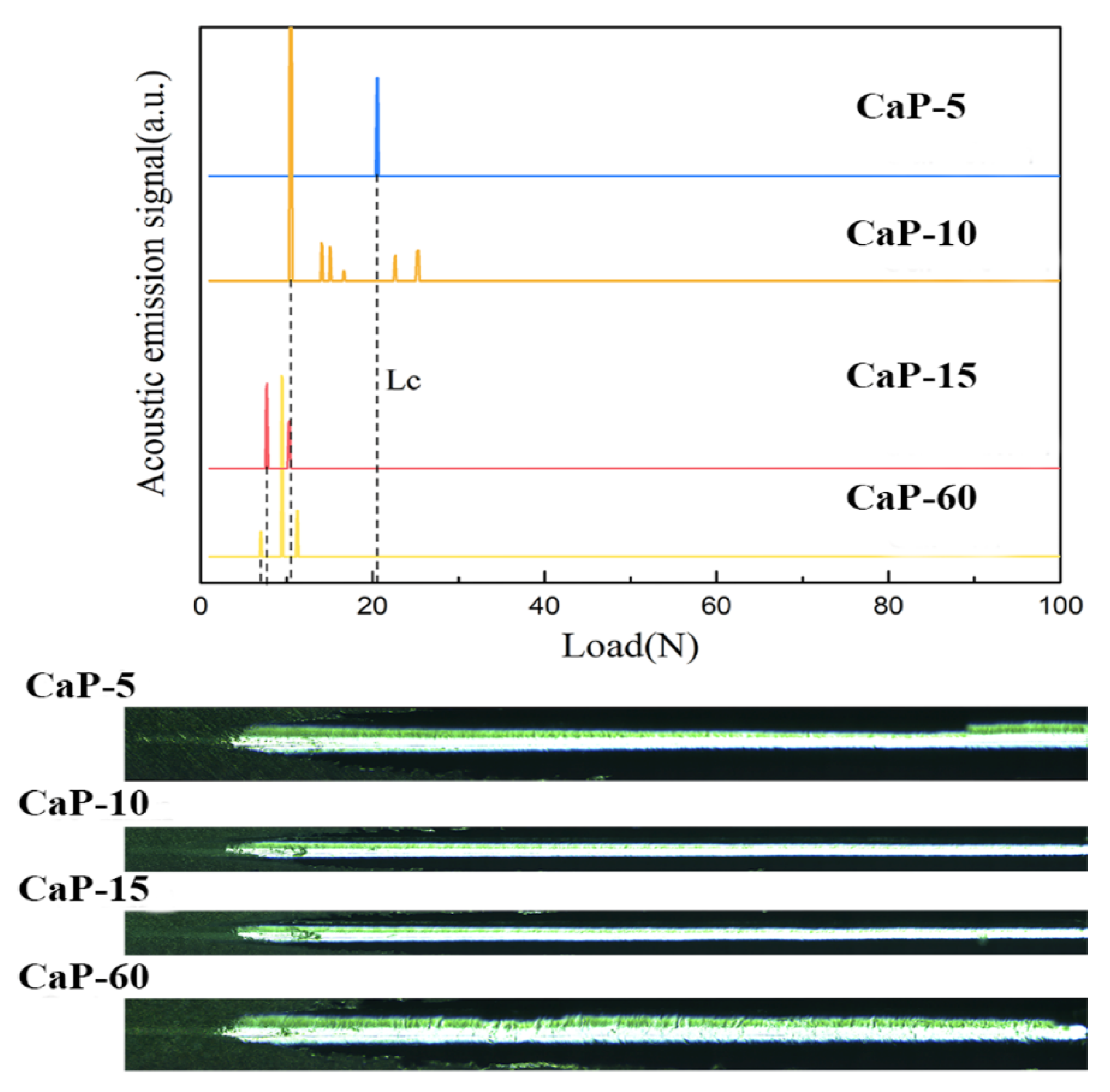
3.1. Morphology, Structure, and Properties of CaP Coatings
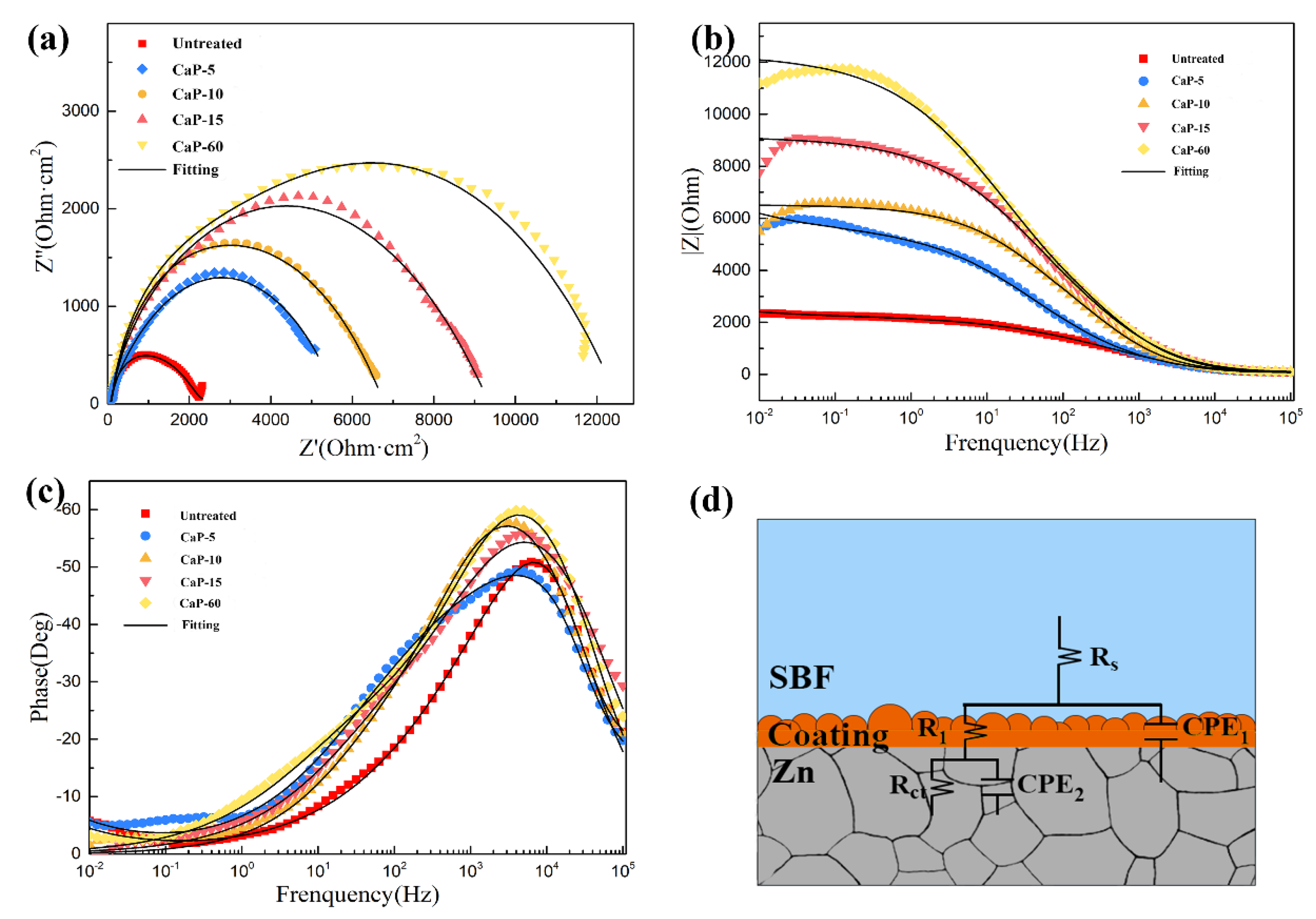
3.2. Electrochemical Test
3.3. In Vitro Degradation Behavior
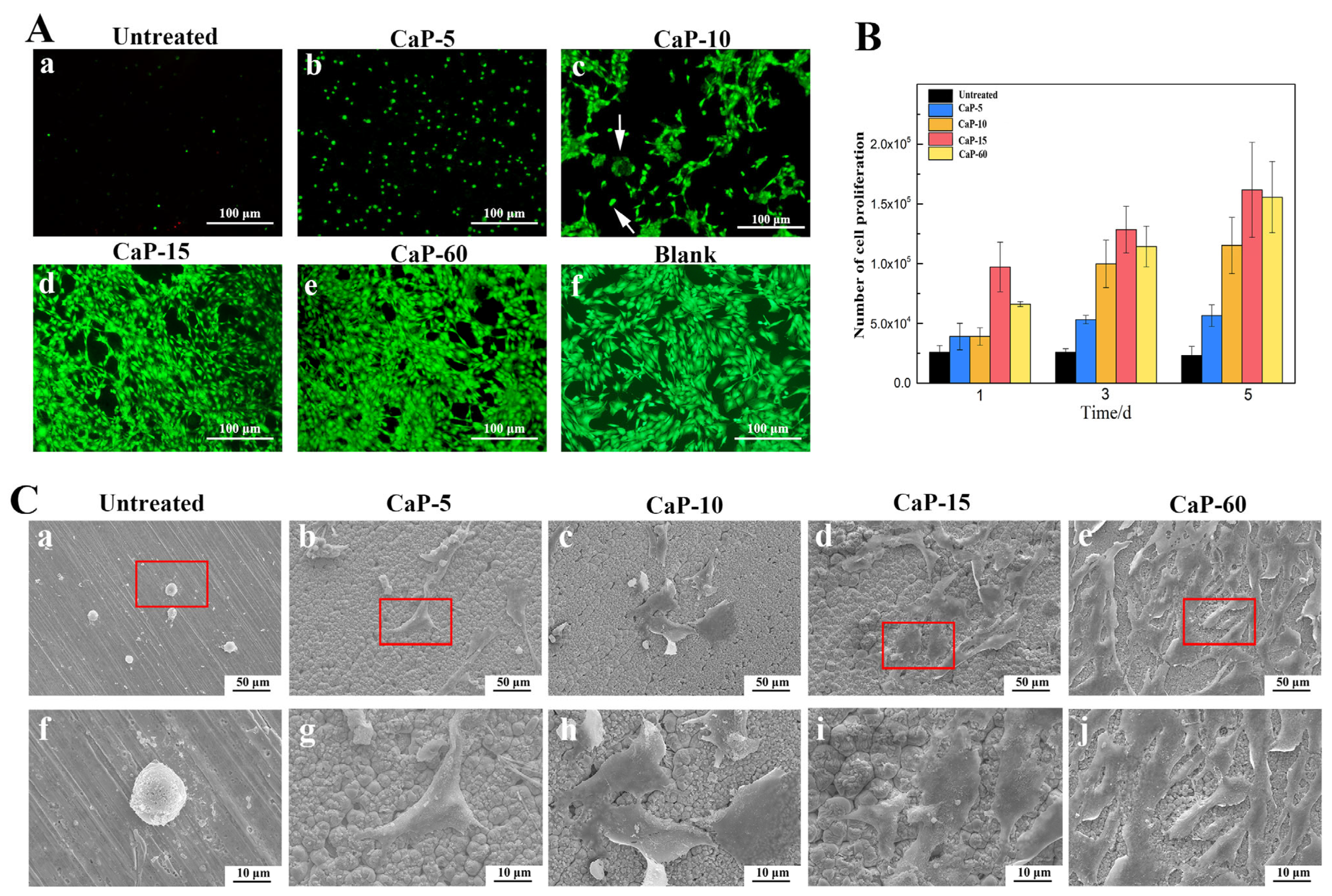
3.4. Cell Viability, Adhesion, and Proliferation
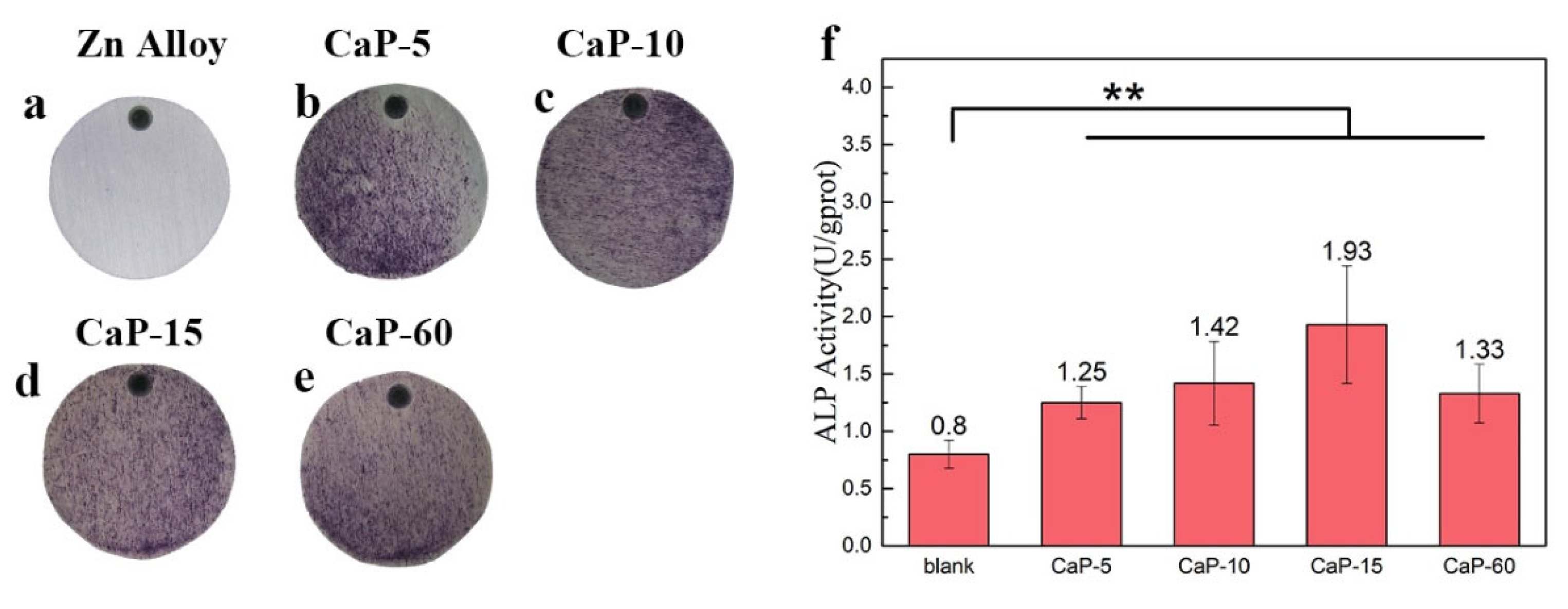
3.5. Osteogenic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Batra, U.; Kumar, K.; Ahuja, N.; Mahapatro, A. Progress in bioactive surface coatings on biodegradable Mg alloys: A critical review towards clinical translation. Bioact. Mater. 2023, 19, 717–757. [Google Scholar] [CrossRef]
- Yao, R.; Wang, H.; Shan, R.; Liu, L.; Zhao, Y.; Sun, Y.; Yao, X.; Huang, D.; Hang, R. Biodegradable porous Zn-1Mg-3βTCP scaffold for bone defect repair: In vitro and in vivo evaluation. J. Mater. Sci. Technol. 2023, 162, 189–202. [Google Scholar] [CrossRef]
- Peng, B.; Xu, H.; Song, F.; Wen, P.; Tian, Y.; Zheng, Y. Additive manufacturing of porous magnesium alloys for biodegradable orthopedic implants: Process, design, and modification. J. Mater. Sci. Technol. 2024, 182, 79–110. [Google Scholar] [CrossRef]
- Yang, H.T.; Jia, B.; Zhang, Z.C.; Qu, X.H.; Li, G.N.; Lin, W.J.; Zhu, D.H.; Dai, K.R.; Zheng, Y.F. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef]
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.-W.; Mohd Ariffin, M.K.A.; Seng Kim, C.L.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef]
- Li, H.F.; Huang, J.Y.; Lin, G.C.; Wang, P.Y. Recent advances in tribological and wear properties of biomedical metallic materials. Rare Met. 2021, 40, 3091–3106. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Dai, J.; Yang, J.; Bai, J.; Huang, Z.; Guo, C.; Xue, F.; Han, L.; Chu, C. Effects of different magnitudes of static stress on the in vitro corrosion behavior of biodegradable zinc. Corros. Sci. 2024, 227, 111763. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, F.; You, C.; Tang, C.; Zhou, B.; Zhang, S.; Feng, J.; Tian, A.; Chen, M. Preparation of porous Zn-Li alloy scaffolds for bone repair and its degradation behavior in vitro and in vivo. Mater. Today Commun. 2023, 35, 105605. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, X.; Huang, H.; Chen, C.; Jiang, J.; Niu, J.; Dargusch, M.; Yuan, G. Microstructure evolution, mechanical properties and corrosion behavior of biodegradable Zn-2Cu-0.8Li alloy during room temperature drawing. Mater. Charact. 2022, 185, 111722. [Google Scholar] [CrossRef]
- Molenda, M.; Kolmas, J. The Role of Zinc in Bone Tissue Health and Regeneration—A Review. Biol. Trace Elem. Res. 2023, 201, 5640–5651. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, D.; Jiang, Y.; Su, Z.; Pi, Y.; Luo, T.; Jiang, Q.; Yang, L.; Guo, L. Multifunctional Lithium-Doped Mesoporous Nanoparticles for Effective Dentin Regeneration in vivo. Int. J. Nanomed. 2023, 18, 5309–5325. [Google Scholar] [CrossRef]
- Duarte, P.M.; Miranda, T.S.; Marins, L.M.; da Silva, J.R.B.; de Souza Malta, F.; de Vasconcelos Gurgel, B.C.; Napimoga, M.H. Lithium chloride stimulates bone formation in extraction socket repair in rats. Oral Maxillofac. Surg. 2022, 28, 169–177. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef]
- Zhao, D.W.; Du, C.M.; Zuo, K.Q.; Zhao, Y.X.; Xu, X.Q.; Li, Y.B.; Tian, S.; Yang, H.R.; Lu, Y.P.; Cheng, L.; et al. Calcium-Zinc Phosphate Chemical Conversion Coating Facilitates the Osteointegration of Biodegradable Zinc Alloy Implants by Orchestrating Macrophage Phenotype. Adv. Healthc. Mater. 2023, 12, 2202537. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, D.; Zhang, X.; Su, Y.; Shi, Z.; Wang, K.; Lin, J.; Li, Y.; Lin, J.; Wen, C. Microstructure, mechanical properties, biocompatibility, and in vitro corrosion and degradation behavior of a new Zn-5Ge alloy for biodegradable implant materials. Acta Biomater. 2018, 82, 197–204. [Google Scholar] [CrossRef]
- Jablonská, E.; Vojtech, D.; Fousová, M.; Kubásek, J.; Lipov, J.; Fojt, J.; Ruml, T. Influence of surface pre-treatment on the cytocompatibility of a novel biodegradable ZnMg alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 198–204. [Google Scholar] [CrossRef]
- You, M.Y.; Echeverry-Rendón, M.; Zhang, L.; Niu, J.L.; Zhang, J.; Pei, J.; Yuan, G.Y. Effects of composition and hierarchical structures of calcium phosphate coating on the corrosion resistance and osteoblast compatibility of Mg alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111734. [Google Scholar] [CrossRef]
- Shi, Y.; Xue, Z.; Li, P.; Yang, S.; Zhang, D.; Zhou, S.; Guan, Z.; Li, Y.; Wang, L.-N. Surface modification on biodegradable zinc alloys. J. Mater. Res. Technol. 2023, 25, 3670–3687. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Surface Modification of Biodegradable Zinc Alloy for Biomedical Applications. BioNanoScience 2023, 13, 1381–1398. [Google Scholar] [CrossRef]
- Li, H.F.; Zheng, Y.X.; Ji, X.J. Synthesis and in vitro evaluation of Ca-P coating on biodegradable Zn alloys. J. Mater. Sci. Technol. 2023, 141, 124–134. [Google Scholar] [CrossRef]
- Gil, J.; Manero, J.M.; Ruperez, E.; Velasco-Ortega, E.; Jiménez-Guerra, A.; Ortiz-García, I.; Monsalve-Guil, L. Mineralization of Titanium Surfaces: Biomimetic Implants. Materials 2021, 14, 2879. [Google Scholar] [CrossRef]
- Rahman, M.; Li, Y.C.; Wen, C.E. Realization and characterization of double-layer Ca-P coating on WE43 Mg alloy for biomedical applications. Surf. Coat. Technol. 2020, 398, 126091. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, J.; Zhang, C.; Barbieri, D.; Yuan, H.; Moroni, L.; Feng, G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020, 106, 22–33. [Google Scholar] [CrossRef]
- Marchenko, E.S.; Baigonakova, G.A.; Dubovikov, K.M.; Kokorev, O.V.; Gordienko, I.I.; Chudinova, E.A. Properties of Coatings Based on Calcium Phosphate and Their Effect on Cytocompatibility and Bioactivity of Titanium Nickelide. Materials 2023, 16, 2581. [Google Scholar] [CrossRef]
- Ishikawa, K.; Kareiva, A. Sol-gel synthesis of calcium phosphate-based coatings—A review. Chemija 2020, 31, 25–41. [Google Scholar] [CrossRef]
- Mardali, M.; SalimiJazi, H.R.; Karimzadeh, F.; Luthringer, B.; Blawert, C.; Labbaf, S. Comparative study on microstructure and corrosion behavior of nanostructured hydroxyapatite coatings deposited by high velocity oxygen fuel and flame spraying on AZ61 magnesium based substrates. Appl. Surf. Sci. 2019, 465, 614–624. [Google Scholar] [CrossRef]
- Montesissa, M.; Borciani, G.; Rubini, K.; Valle, F.; Boi, M.; Baldini, N.; Boanini, E.; Graziani, G. Ionized Jet Deposition of Calcium Phosphates-Based Nanocoatings: Tuning Coating Properties and Cell Behavior by Target Composition and Substrate Heating. Nanomaterials 2023, 13, 1758. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Bioactive Calcium Phosphate Coatings for Bone Implant Applications: A Review. Coatings 2023, 13, 1091. [Google Scholar] [CrossRef]
- Chen, J.P.; Zhou, Y.W.; Lin, X.N.; Li, H. Macrophage Polarization Related to Biomimetic Calcium Phosphate Coatings: A Preliminary Study. Materials 2023, 16, 332. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, H.; Niu, J.; Zhu, D.; Yuan, G. Fabrication and characterization of biodegradable Zn-Cu-Mn alloy micro-tubes and vascular stents: Microstructure, texture, mechanical properties and corrosion behavior. Acta Biomater. 2022, 151, 647–660. [Google Scholar] [CrossRef]
- Liu, L.J.; Meng, Y.; Volinsky, A.A.; Zhang, H.J.; Wang, L.N. Influences of albumin on in vitro corrosion of pure Zn in artificial plasma. Corros. Sci. 2019, 153, 341–356. [Google Scholar] [CrossRef]
- Hiromoto, S.; Nozoe, E.; Hanada, K.; Yoshimura, T.; Shima, K.; Kibe, T.; Nakamura, N.; Doi, K. In vivo degradation and bone formation behaviors of hydroxyapatite-coated Mg alloys in rat femur. Mater. Sci. Eng. C 2021, 122, 111942. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, X.; Dai, K. Chapter 1—Material characteristics, surface/interface, and biological effects on the osteogenesis of bioactive materials. In Bioactive Materials for Bone Regeneration; Chang, J., Zhang, X., Dai, K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–103. [Google Scholar]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cristallini, C.; Gautier di Confiengo, G.; Barberi, J.; Cazzola, M.; Miola, M.; Vernè, E.; Spriano, S. The mechanical and chemical stability of the interfaces in bioactive materials: The substrate-bioactive surface layer and hydroxyapatite-bioactive surface layer interfaces. Mater. Sci. Eng. C 2020, 116, 111238. [Google Scholar] [CrossRef]
- He, Z.H.; Sun, S.L.; Deng, C.L. Effect of Hydroxyapatite Coating Surface Morphology on Adsorption Behavior of Differently Charged Proteins. J. Bionic Eng. 2020, 17, 345–356. [Google Scholar] [CrossRef]
- Xu, X.; Sun, X.; Tian, Y.; Zhang, L.; Liu, L. Osteogenic activity of a micro/nano hierarchical nano-hydroxyapatite coating on zirconium alloy. Mater. Charact. 2023, 205, 113356. [Google Scholar] [CrossRef]
- Mohammed Mohammed, A.H.; Shariff, K.A.; Bakar, M.H.A.; Mohamad, H. A review on the behavioral responses of osteoclast and osteoblast cells on the near-surface of the bioceramic coating: Roles of ions released, solubility, and pH. J. Aust. Ceram. Soc. 2022, 58, 1715–1727. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, H.; Tang, Y.; Fa, X.; Zhang, H.; Shi, Z.; Yao, S.; Wang, L. Enhanced Biocompatibility and Osteogenic Property of Biodegradable Zn-0.5Li Alloy through Calcium–Phosphorus Coating. Coatings 2024, 14, 350. https://doi.org/10.3390/coatings14030350
Xing H, Tang Y, Fa X, Zhang H, Shi Z, Yao S, Wang L. Enhanced Biocompatibility and Osteogenic Property of Biodegradable Zn-0.5Li Alloy through Calcium–Phosphorus Coating. Coatings. 2024; 14(3):350. https://doi.org/10.3390/coatings14030350
Chicago/Turabian StyleXing, Haotian, Yunzhi Tang, Xinying Fa, Hongyun Zhang, Zhangzhi Shi, Shenglian Yao, and Luning Wang. 2024. "Enhanced Biocompatibility and Osteogenic Property of Biodegradable Zn-0.5Li Alloy through Calcium–Phosphorus Coating" Coatings 14, no. 3: 350. https://doi.org/10.3390/coatings14030350




