In Situ Synthesis of an Epoxy Resin Microwave Absorption Coating with Anti-Ultraviolet Aging Effects
Abstract
:1. Introduction
2. Experimental Section
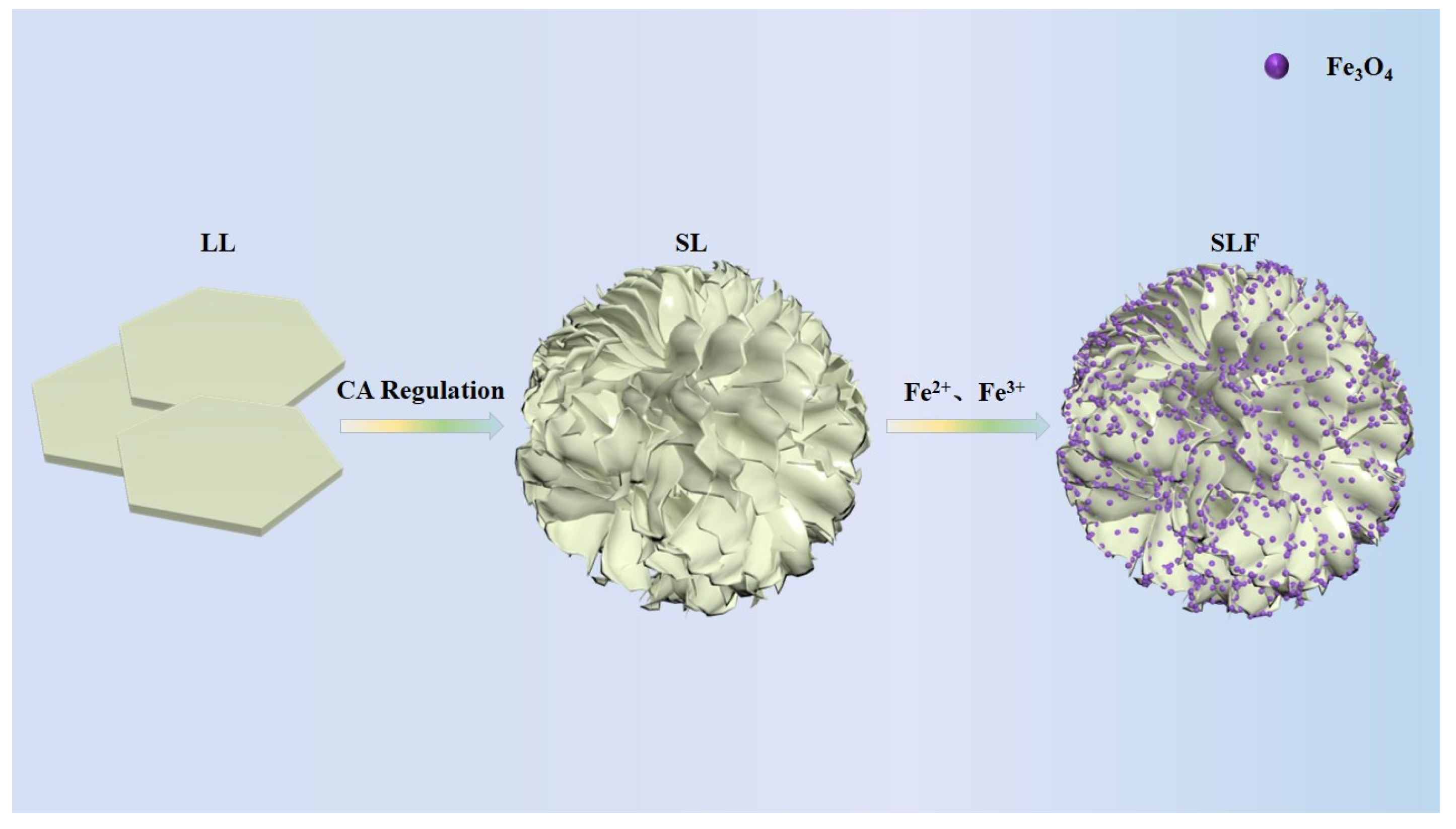
2.1. Synthesis of the SL and SLF Composites
2.2. Preparation of SLF/EP Composite Coatings
3. Results and Discussion
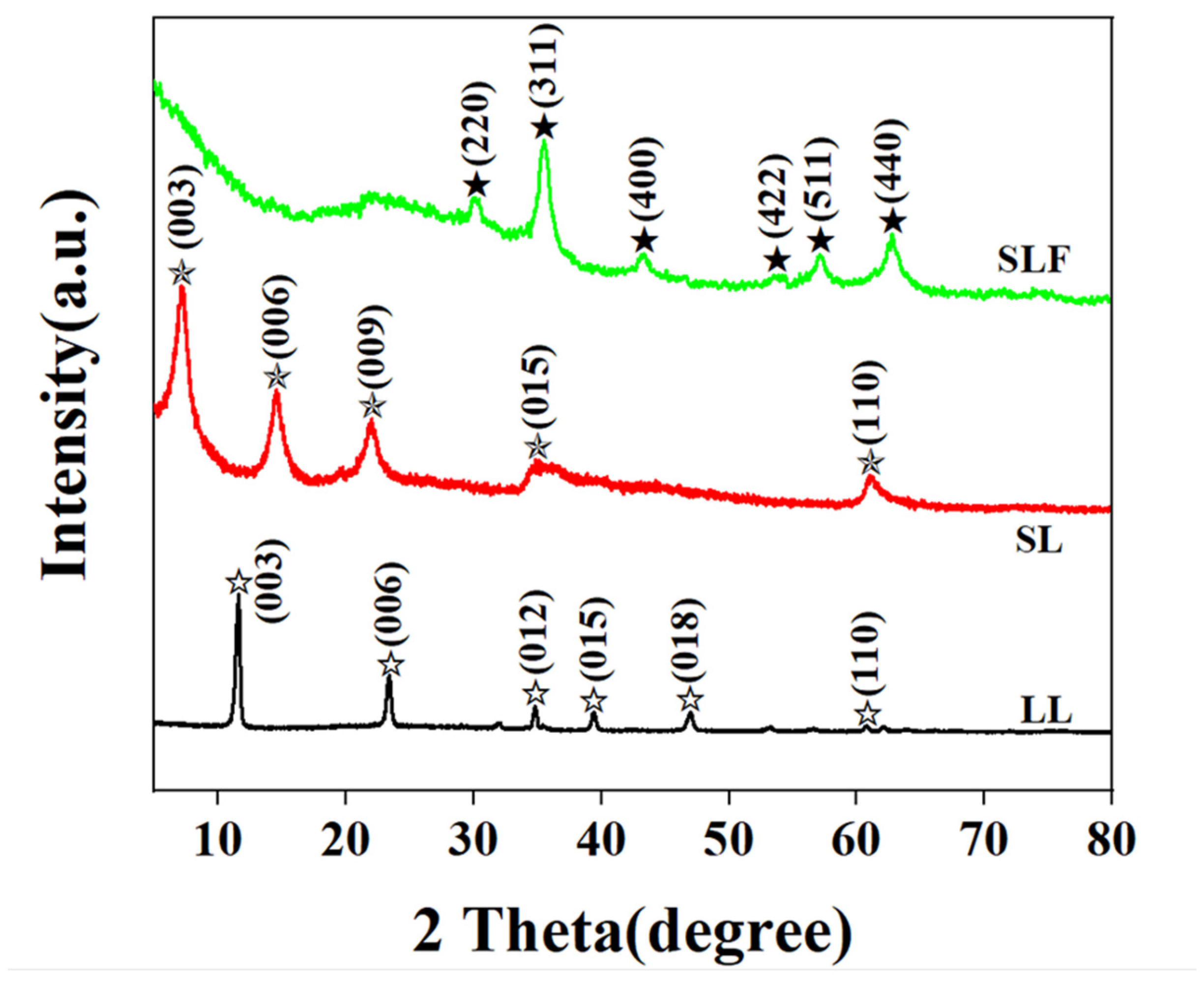
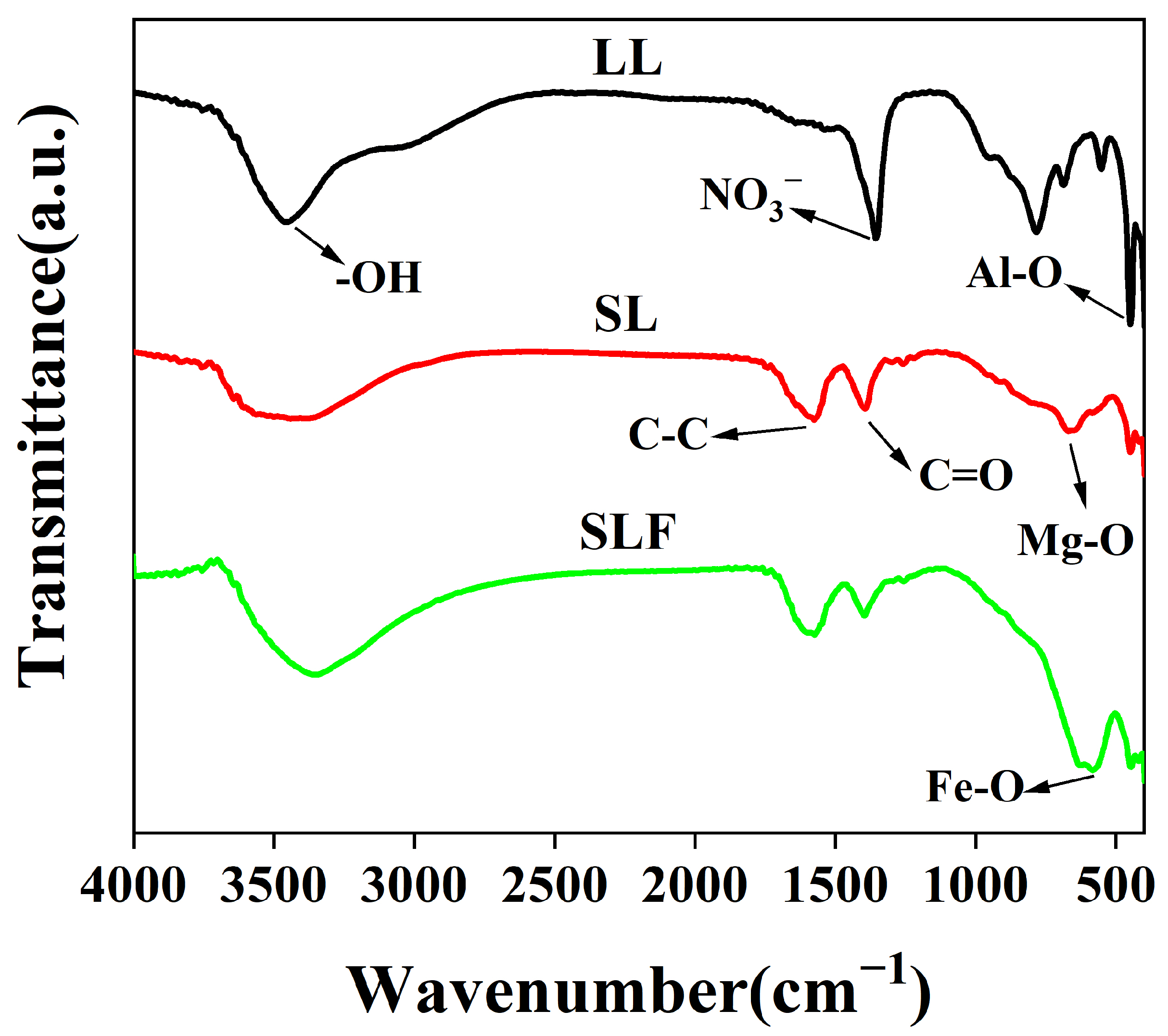
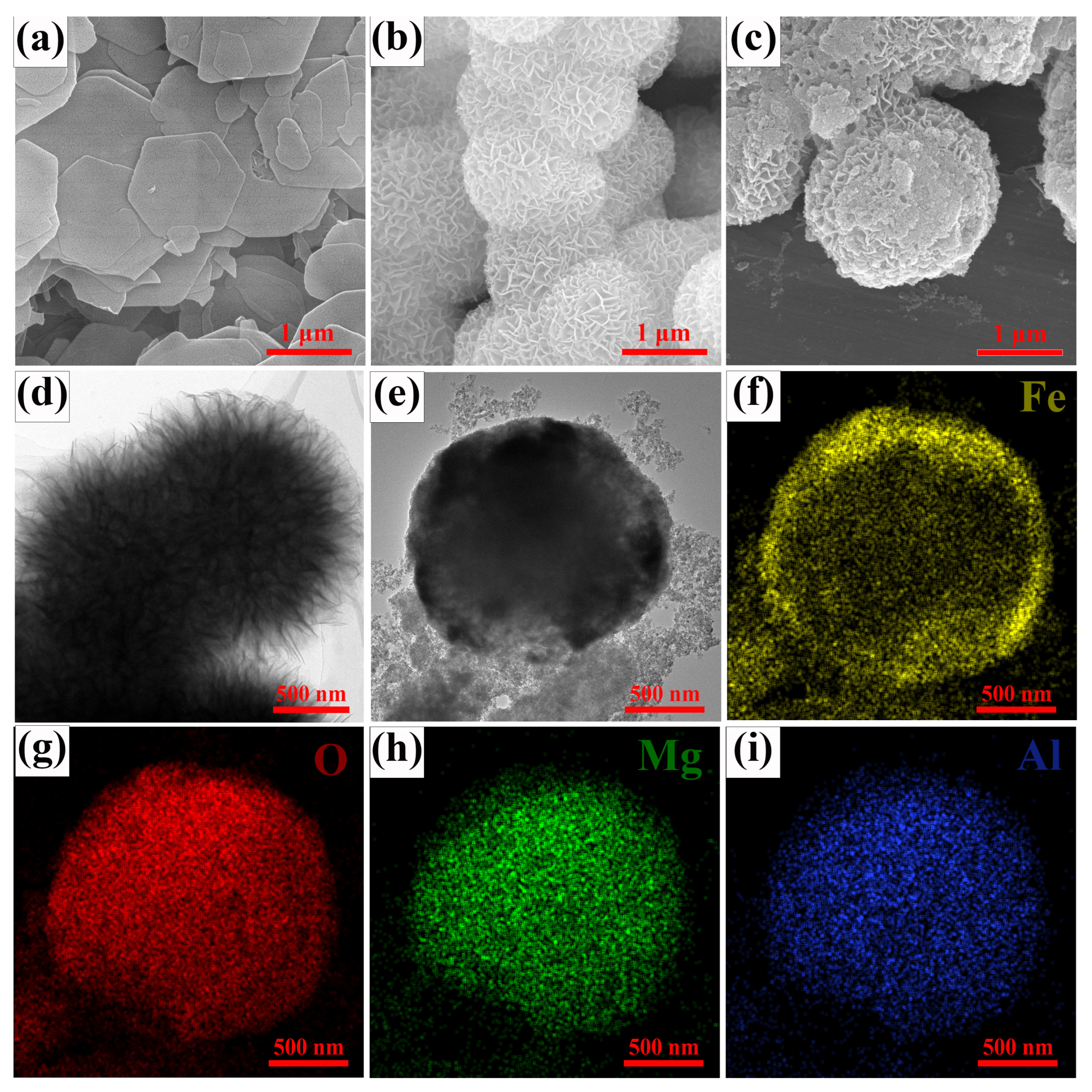
3.1. Morphological and Structural Characterization
3.2. MA Performance
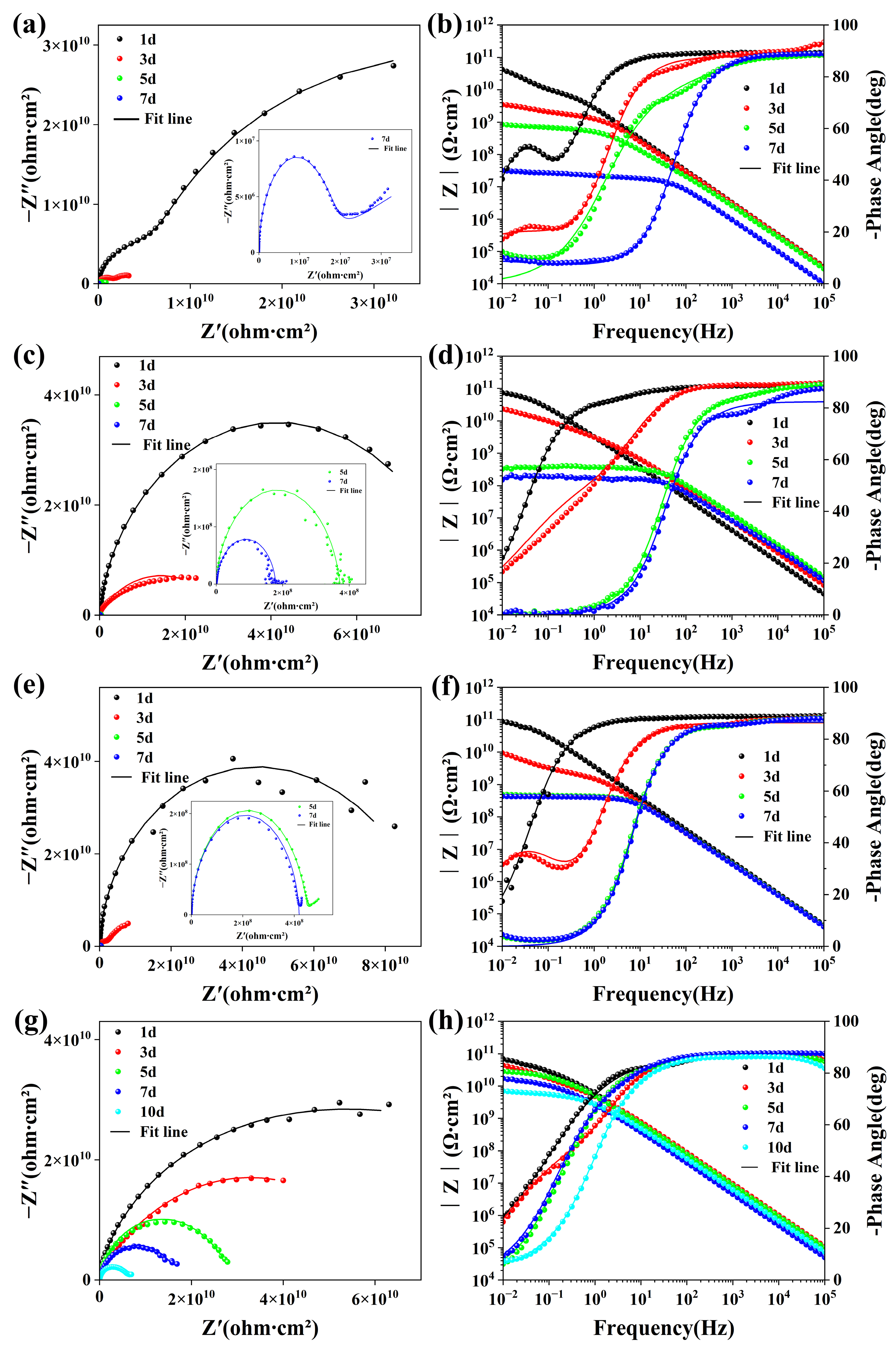
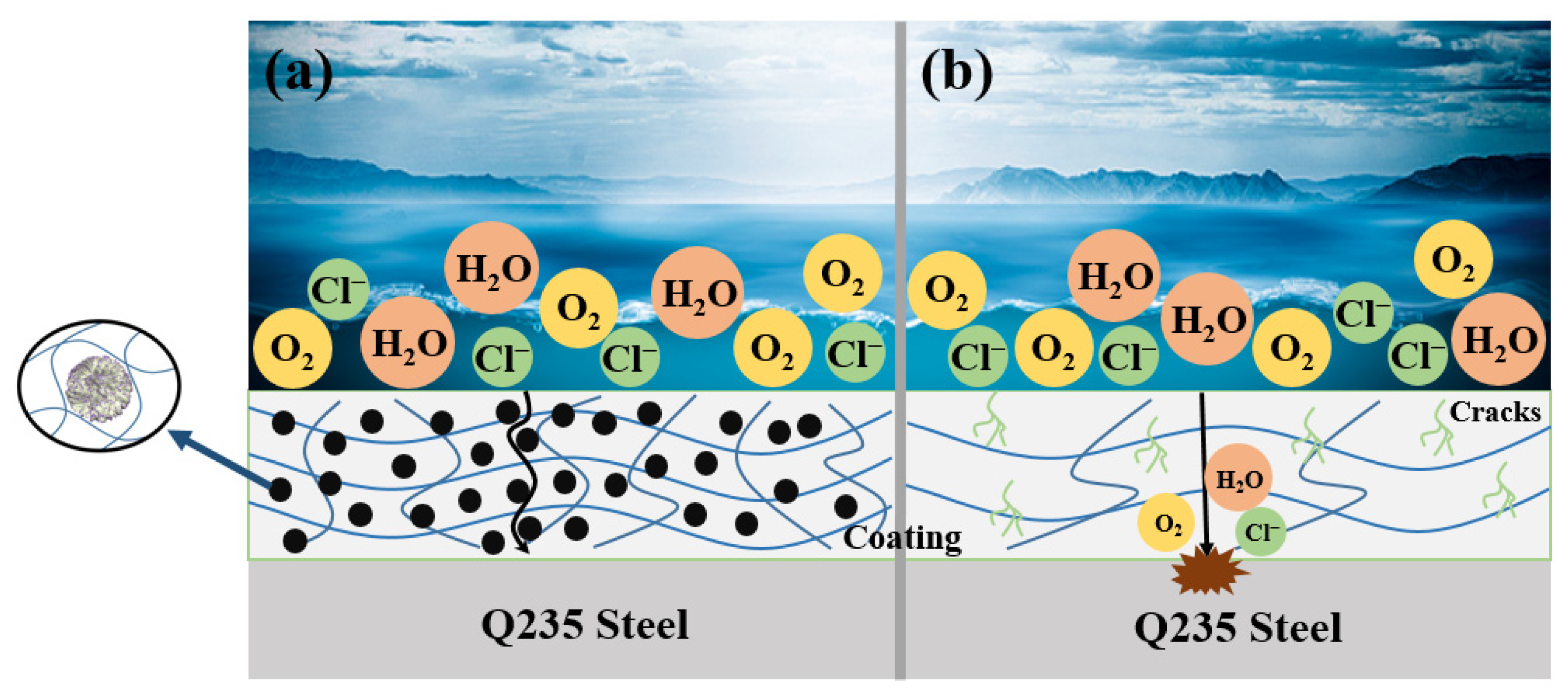
3.3. Anti-Corrosion Performance
3.4. Anti-UV Aging Performance
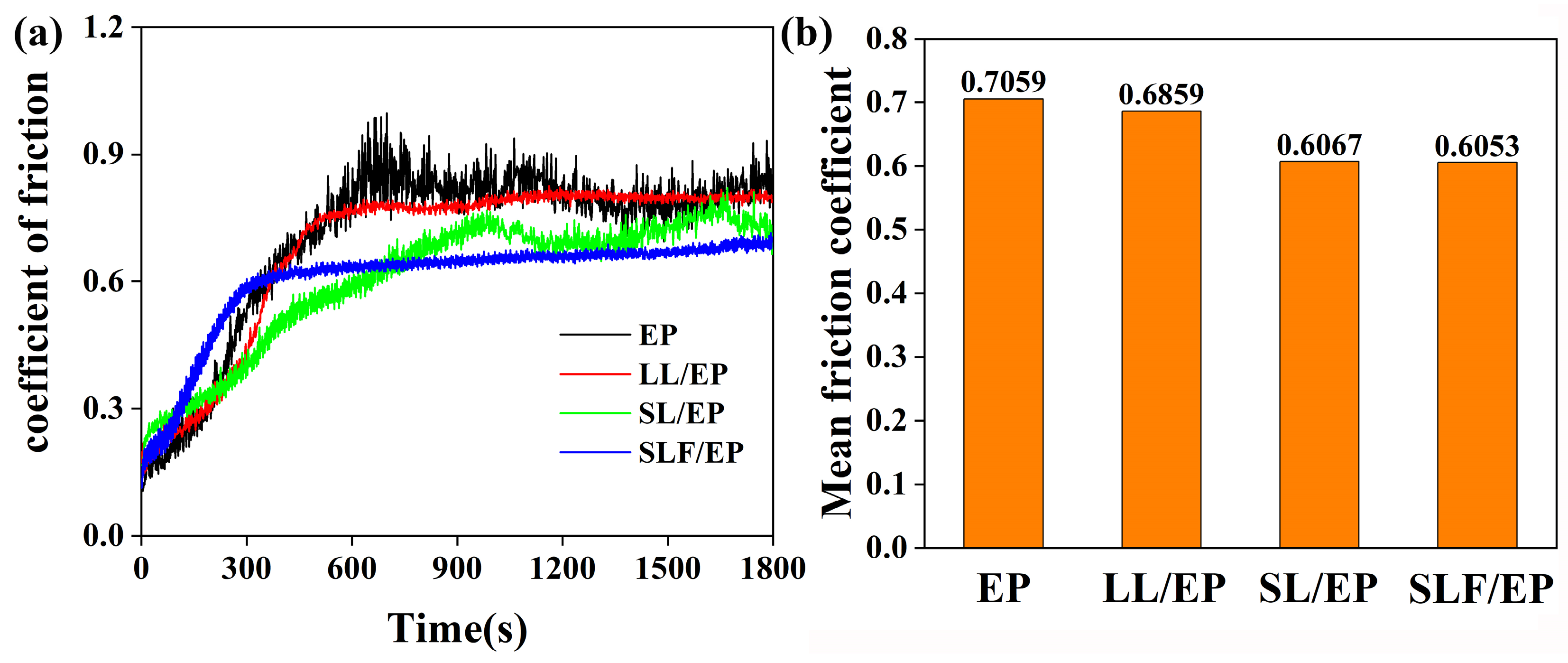
3.5. Wear Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.; Lan, D.; Zhang, L.; Wu, H. A Flexible, Mechanically Strong, and Anti-Corrosion Electromagnetic Wave Absorption Composite Film with Periodic Electroconductive Patterns. Adv. Funct. Mater. 2021, 32, 2111045. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Kong, L.; Qiao, J.; Li, B.; Ding, X.; Liu, J.; Liu, W.; Wang, F. Construction of Ni-Zn bimetal sulfides Heterostructured-hybrids for High-performance electromagnetic wave absorption. J. Colloid Interface Sci. 2022, 606, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Wu, X.; Zhang, W.; Luo, C.; Liu, P. Facile design of 3D hierarchical NiFe2O4/N-GN/ZnO composite as a high performance electromagnetic wave absorber. Chem. Eng. J. 2019, 375, 121942. [Google Scholar] [CrossRef]
- Bibi, M.; Abbas, S.; Ahmad, N.; Muhammad, B.; Iqbal, Z.; Rana, U.; Khan, S. Microwaves absorbing characteristics of metal ferrite/multiwall carbon nanotubes nanocomposites in X-band. Compos. Part B Eng. 2017, 114, 139–148. [Google Scholar] [CrossRef]
- Wei, S.; Chen, T.; Wang, Q.; Shi, Z.; Li, W.; Chen, S. Metal-organic framework derived hollow CoFe@C composites by the tunable chemical composition for efficient microwave absorption. J. Colloid Interface Sci. 2021, 593, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yan, H.; Cai, M.; Huang, Y.; Fan, X.; Zhu, M. Multilayer structural epoxy composite coating towards long-term corrosion/wear protection. Carbon 2021, 183, 42–52. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Yuan, J.; Zhu, Z.; Liu, X.; Li, D.; He, L.; Xiao, F. Furfural-based P/N/S flame retardant towards high-performance epoxy resins with flame retardancy, toughness, low dielectric properties and UV resistance. Polym. Degrad. Stab. 2023, 212, 110343. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, F.; Lin, D.; Peng, J.; Zhu, Y.; Wang, H. A smart anti-corrosion coating based on triple functional fillers. Chem. Eng. J. 2022, 446, 137078. [Google Scholar] [CrossRef]
- Wang, Y.; Di, X.; Chen, J.; She, L.; Pan, H.; Zhao, B.; Che, R. Multi-dimensional C@NiCo-LDHs@Ni aerogel: Structural and componential engineering towards efficient microwave absorption, anti-corrosion and thermal-insulation. Carbon 2022, 191, 625–635. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, J.; Kim, K. Microwave absorption properties of graphene oxide capsulated carbonyl iron particles. Appl. Surf. Sci. 2019, 475, 1065–1069. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, M.; Zhao, W.; Hu, J.; Fu, H. Anti-corrosion epoxy/modified graphene oxide/glass fiber composite coating with dual physical barrier network. Prog. Org. Coat. 2022, 167, 106823. [Google Scholar] [CrossRef]
- Fan, G.; Xiong, T.; Mouldi, A.; Bouallegue, B.; Tran, N.; Mahmoud, M. Enhanced electromagnetic interference shielding effectiveness of h-BN decorated micro cube-like CaTiO3/Cu nanocomposite. Ceram. Int. 2022, 48, 8529–8539. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Zhang, M.; Sun, Y.; Cui, Y.; Zhu, Y.; Wang, H. Fluoro-substituted polyaniline deeply incorporation with inert h-BN for interface improvement in anti-corrosion. Prog. Org. Coat. 2022, 170, 106993. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Qiang, Y.; Chen, Z.; Wang, L.; Gao, X.; Fang, Z. π-π interaction between fluorinated reduced graphene oxide and acridizinium ionic liquid: Synthesis and anti-corrosion application. Carbon 2020, 159, 292–302. [Google Scholar] [CrossRef]
- Ma, L.; Qiang, Y.; Zhao, W. Designing novel organic inhibitor loaded MgAl-LDHs nanocontainer for enhanced corrosion resistance. Chem. Eng. J. 2021, 408, 127367. [Google Scholar] [CrossRef]
- Quan, B.; Liang, X.; Ji, G.; Lv, J.; Dai, S.; Xu, G.; Du, Y. Laminated graphene oxide-supported high-efficiency microwave absorber fabricated by an in situ growth approach. Carbon 2018, 129, 310–320. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; You, W.; Xing, L.; Yu, X.; Li, Y.; Che, R. Morphology-controlled synthesis and excellent microwave absorption performance of ZnCo2O4 nanostructures via a self-assembly process of flake units. Nanoscale 2019, 11, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Du, Y.; Li, Z.; Wang, Y.; Xu, P.; Zhao, H.; Wang, F.; Li, C.; Han, X. Facile synthesis of 3D flower-like Ni microspheres with enhanced microwave absorption properties. J. Mater. Chem. C 2018, 6, 9615–9623. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, G.; Fan, B.; Zhao, W.; Xie, Y.; Zhang, R. Synthesis of flower-like CuS hollow microspheres based on nanoflakes self-assembly and their microwave absorption properties. J. Mater. Chem. A 2015, 3, 10345–10352. [Google Scholar] [CrossRef]
- Chen, X.; Yu, H.; Liu, Y.; Zhou, H.; Zhang, B.; Zhao, N.; Qi, F.; Ouyang, X. Exploration on the multi-structural morphology regulation and microwave absorption performance of LDHs. Mater. Lett. 2023, 352, 135187. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, D.; Yang, Y.; Kang, F.; Gu, J. Fe3O4/carbon composite nanofiber absorber with enhanced microwave absorption performance. Mater. Sci. Eng. B 2013, 178, 1–9. [Google Scholar] [CrossRef]
- Du, Y.; Liu, W.; Qiang, R.; Wang, Y.; Han, X.; Ma, J.; Xu, P. Shell thickness-dependent microwave absorption of core-shell Fe3O4@C composites. ACS Appl. Mater. Interfaces 2014, 6, 12997–13006. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; Yu, Z.; Ma, Y.; Li, F.; Lv, L.; Pan, Y.; Lin, Y.; Liu, Y.; He, Y. Graphene oxide decorated with Fe3O4 nanoparticles with advanced anticorrosive properties of epoxy coatings. J. Taiwan Inst. Chem. Eng. 2016, 64, 244–251. [Google Scholar] [CrossRef]
- Yang, W.; Tian, H.; Liao, J.; Wang, Y.; Liu, L.; Zhang, L.; Lu, A. Flexible and strong Fe3O4/cellulose composite film as magnetic and UV sensor. Appl. Surf. Sci. 2020, 507, 145092. [Google Scholar] [CrossRef]
- Sun, L.; Hu, C. Facile synthesis via a solvothermal route and characterization of Mg-Al layered double hydroxide (LDH) 3D micro-nano structures. Mater. Res. Bull. 2011, 46, 1922–1927. [Google Scholar] [CrossRef]
- Liu, W.; Xu, S.; Liang, R.; Wei, M.; Evans, D.; Duan, X. In situ synthesis of nitrogen-doped carbon dots in the interlayer region of a layered double hydroxide with tunable quantum yield. J. Mater. Chem. C 2017, 5, 3536–3541. [Google Scholar] [CrossRef]
- Du, B.; Shi, X.; Zhu, H.; Xu, J.; Bai, Y.; Wang, Q.; Wang, X.; Zhou, J. Preparation and characterization of bifunctional wolfsbane-like magnetic Fe3O4 nanoparticles-decorated lignin-based carbon nanofibers composites for electromagnetic wave absorption and electrochemical energy storage. Int. J. Biol. Macromol. 2023, 246, 125574. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Lu, S.; Gao, Y.; Zhang, R.; Tan, X.; Chen, C. Fabrication of hierarchical core-shell polydopamine@MgAl-LDHs composites for the efficient enrichment of radionuclides. Appl. Surf. Sci. 2017, 396, 1726–1735. [Google Scholar] [CrossRef]
- Liu, W.; Gao, J.; Huang, Z.; Zhang, H.; Li, M.; Zhang, X.; Ma, X.; Xu, Z. Layered double hydroxide modified polyamide reverse osmosis membrane for improved permeability. Desalination Water Treat. 2020, 203, 35–46. [Google Scholar] [CrossRef]
- Feng, B.; Hong, R.; Wang, L.; Guo, L.; Li, H.; Ding, J.; Zheng, Y.; Wei, D. Synthesis of Fe3O4/APTES/PEG diacid functionalized magnetic nanoparticles for MR imaging. Colloids Surf. A Physicochem. Eng. Asp. 2008, 328, 52–59. [Google Scholar] [CrossRef]
- Zhu, Z.; Xiang, M.; Li, P.; Shan, L.; Zhang, P. Surfactant-modified three-dimensional layered double hydroxide for the removal of methyl orange and rhodamine B: Extended investigations in binary dye systems. J. Solid State Chem. 2020, 288, 121448. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, S.; Liu, Y.; Huangfu, H.; Guo, X.; Zhang, J. Enhancement of corrosion resistance of AZ31B magnesium alloy by preparing MgAl-LDHs coatings modified with imidazolium based dicationic ionic liquids. Surf. Coat. Technol. 2022, 440, 128504. [Google Scholar] [CrossRef]
- Khalilifard, M.; Javadian, S. Magnetic superhydrophobic polyurethane sponge loaded with Fe3O4@oleic acid@graphene oxide as high performance adsorbent oil from water. Chem. Eng. J. 2021, 408, 127369. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, X.; Wu, Z.; Zeng, G.; Jiang, L.; Peng, X.; Li, H. Adsorption behavior and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon spheres on the removal of Pb(II) and Cu(II). J. Colloid Interface Sci. 2019, 536, 440–455. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Yu, H.; Yan, L.; Du, B.; Pei, Z. Removal of Cu2+, Cd2+ and Pb2+ from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide. J. Colloid Interface Sci. 2018, 532, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, X.; Pan, F.; Cai, L.; Jiang, H.; Cheng, J.; Shi, Z.; Xiang, Z.; Lu, W. Implanting NiCo2O4 equalizer with designable nanostructures in agaric aerogel-derived composites for efficient multiband electromagnetic wave absorption. Carbon 2022, 190, 68–79. [Google Scholar] [CrossRef]
- Guo, Z.; Ren, P.; Zhang, F.; Duan, H.; Chen, Z.; Jin, Y.; Ren, F.; Li, Z. Magnetic coupling N self-doped porous carbon derived from biomass with broad absorption bandwidth and high-efficiency microwave absorption. J. Colloid Interface Sci. 2022, 610, 1077–1087. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.; Li, Q.; Wang, Y.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Wrinkled Fe3O4@C magnetic composite microspheres: Regulation of magnetic content and their microwave absorbing performance. J. Colloid Interface Sci. 2021, 601, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Y.; Sun, X.; Qiang, R.; Xu, Y.; Ma, Y.; Zhang, E.; Li, Y. Lightweight, Flexible, and Thermal Insulating Carbon/SiO2@CNTs Composite Aerogel for High-Efficiency Microwave Absorption. Small 2024, e2311657. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Zhang, Y.; Feng, R.; Chen, X.; Xiong, C.; Dong, L. Broadband and Lightweight Microwave Absorber Constructed by in Situ Growth of Hierarchical CoFe2O4/Reduced Graphene Oxide Porous Nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 13860–13868. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, W.; Shao, G.; Fan, B.; Zhang, R. Morphology-Control Synthesis of a Core-Shell Structured NiCu Alloy with Tunable Electromagnetic-Wave Absorption Capabilities. ACS Appl. Mater. Interfaces 2015, 7, 12951–12960. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, Y.; He, M.; Liao, Q.; Yang, H.; Li, H.; Xu, R.; Ding, Q. Hollow Ni-Co layered double hydroxides-derived NiCo-alloy@g-C3N4 microtubule with high-performance microwave absorption. Mater. Lett. 2018, 231, 171–174. [Google Scholar] [CrossRef]
- Shi, Y.; Li, S.; Tian, Z.; Yang, X.; Dong, Y.; Zhu, Y.; Fu, Y. Self-assembled lightweight three-dimensional hierarchically porous Ti3C2Tx MXene@polyaniline hybrids for superior microwave absorption. J. Alloys Compd. 2022, 892, 162194. [Google Scholar] [CrossRef]
- Zhou, A.; Yu, H.; Tang, J.; Zhang, B.; Qi, F.; Zhou, Y.; Zhao, N.; Ouyang, X. N-PMI modified PAZ nanocomposite coatings with self-healing function for anticorrosion and antifouling applications. Prog. Org. Coat. 2023, 180, 107589. [Google Scholar] [CrossRef]
- Wan, P.; Zhao, N.; Qi, F.; Zhang, B.; Xiong, H.; Yuan, H.; Liao, B.; Ouyang, X. Synthesis of PDA-BN@f-Al2O3 hybrid for nanocomposite epoxy coating with superior corrosion protective properties. Prog. Org. Coat. 2020, 146, 105713. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Li, F.; Di, H.; Zhang, L.; Zhan, Y. h-BN decorated with Fe3O4 nanoparticles through mussel-inspired chemistry of dopamine for reinforcing anticorrosion performance of epoxy coatings. J. Alloys Compd. 2016, 685, 743–751. [Google Scholar] [CrossRef]
- An, K.; Sui, Y.; Qing, Y.; Yang, C.; Long, C.; Wang, L.; Liu, C. Synergistic reinforcement coating with anti-corrosion and UV aging resistance by filling modified CeO2 nanoflakes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126904. [Google Scholar] [CrossRef]
- Tian, H.; Wang, C.; Guo, M.; Cui, Y.; Gao, J.; Tang, Z. Microstructures and high-temperature self-lubricating wear-resistance mechanisms of graphene-modified WC-12Co coatings. Friction 2020, 9, 315–331. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Zhang, Z.; Wang, Q. Preparation of ammonium polyphosphate and dye co-intercalated LDH/polypropylene composites with enhanced flame retardant and UV resistance properties. Chemosphere 2021, 277, 130370. [Google Scholar] [CrossRef]
- Li, C.; Wu, S.; Shu, B.; Li, Y.; Chen, Z. Microwave absorption and anti-aging properties of modified bitumen contained SiC attached layered double hydroxides. Constr. Build. Mater. 2019, 227, 116714. [Google Scholar] [CrossRef]
- Jia, K.; Zhao, R.; Zhong, J.; Liu, X. Preparation and microwave absorption properties of loose nanoscale Fe3O4 spheres. J. Magn. Magn. Mater. 2010, 322, 2167–2171. [Google Scholar] [CrossRef]
- Ni, S.; Wang, X.; Zhou, G.; Yang, F.; Wang, J.; He, D. Designed synthesis of wide range microwave absorption Fe3O4-carbon sphere composite. J. Alloy. Compd. 2010, 489, 252–256. [Google Scholar] [CrossRef]
- Sun, L.; Zhan, L.; Shi, Y.; Chu, L.; Ge, G.; He, Z. Microemulsion synthesis and electromagnetic wave absorption properties of monodispersed Fe3O4/polyaniline core-shell nanocomposites. Synthetic met. 2014, 187, 102–107. [Google Scholar] [CrossRef]
- Tian, N.; Wang, J.; Li, F.; Mei, Z.; Lu, Z.; Ge, L.; You, C. Enhanced microwave absorption of Fe flakes with magnesium ferrite cladding. J. Magn. Magn. Mater. 2012, 324, 4175–4178. [Google Scholar] [CrossRef]
- Li, C.; Zeng, G.; Zhou, M.; Fang, Y.; Chen, Z.; Xu, Y.; Ding, S.; Yuan, M.; Li, H.; Wu, S. Controllable synthesis of SiC wrapped LDHs to reinforce microwave absorption and exothermic properties of styrene-butadiene-styrene (SBS) polymer modified asphalt. Mater. Res. Express 2021, 8, 035501. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Chen, X.; Zhang, A.; Tang, J. In Situ Synthesis of an Epoxy Resin Microwave Absorption Coating with Anti-Ultraviolet Aging Effects. Coatings 2024, 14, 514. https://doi.org/10.3390/coatings14040514
Yan S, Chen X, Zhang A, Tang J. In Situ Synthesis of an Epoxy Resin Microwave Absorption Coating with Anti-Ultraviolet Aging Effects. Coatings. 2024; 14(4):514. https://doi.org/10.3390/coatings14040514
Chicago/Turabian StyleYan, Shujun, Xin Chen, Angui Zhang, and Jun Tang. 2024. "In Situ Synthesis of an Epoxy Resin Microwave Absorption Coating with Anti-Ultraviolet Aging Effects" Coatings 14, no. 4: 514. https://doi.org/10.3390/coatings14040514




