In Vitro Evaluation of Optimized PEEK Surfaces for Enhanced Osseointegration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
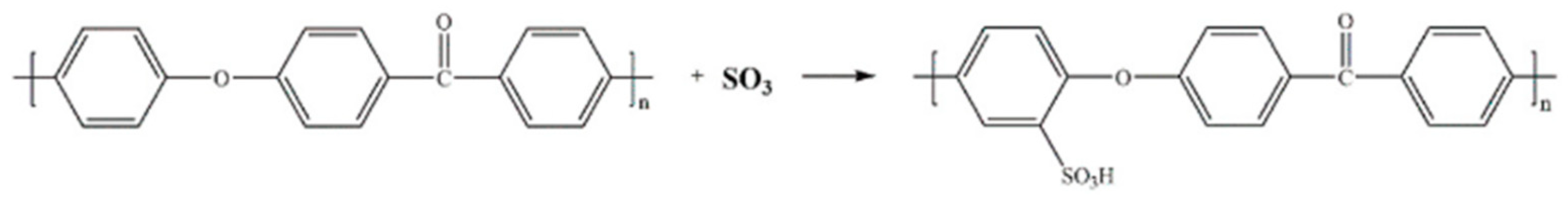
2.3. Experimental Design—Optimizing the Sulfonated Surface
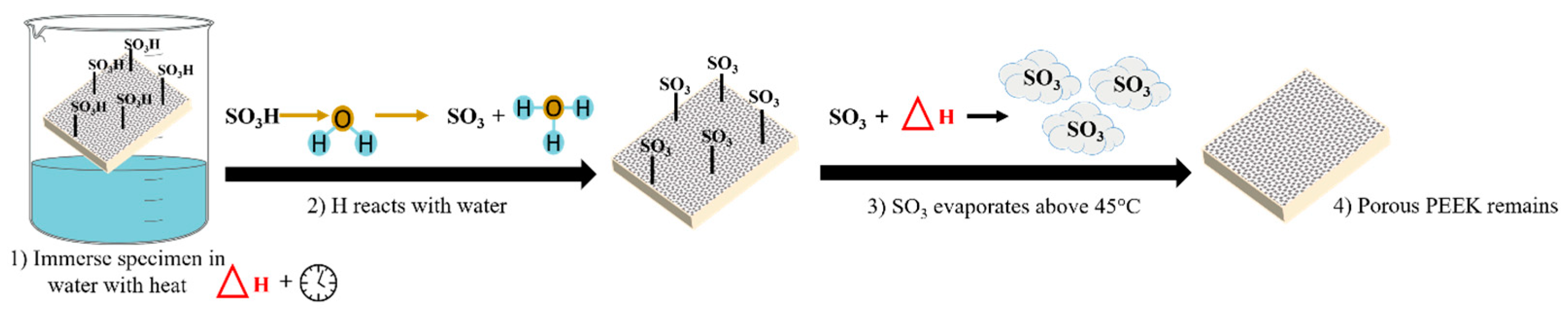
2.4. Optimizing the Hydrothermal Treatment
2.5. Surface and Physical Characterization
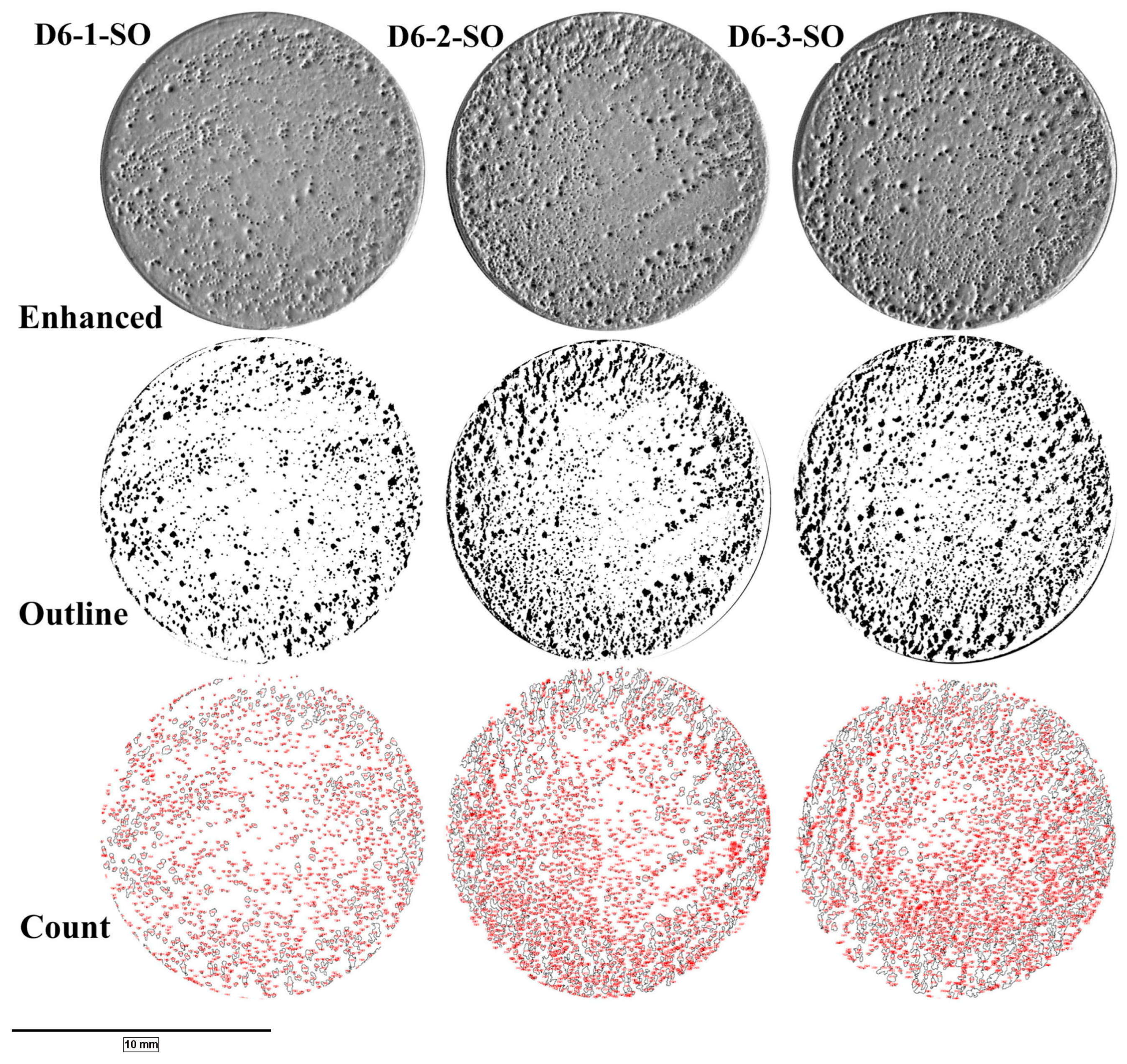
2.5.1. Digital Imaging and Pore Size Measurements
2.5.2. Atomic Force Microscopy
2.5.3. Contact Angle
2.6. In Vitro Characterization
2.6.1. Cell Culture
2.6.2. Cell Viability
2.6.3. Biochemical Analysis
2.6.4. Cell Proliferation
2.6.5. Cell Differentiation
2.6.6. Cell Mineralization
2.7. Statistical Analysis
3. Results
3.1. Optimized Sulfonated Surface
3.2. Hydrothermal Treatment
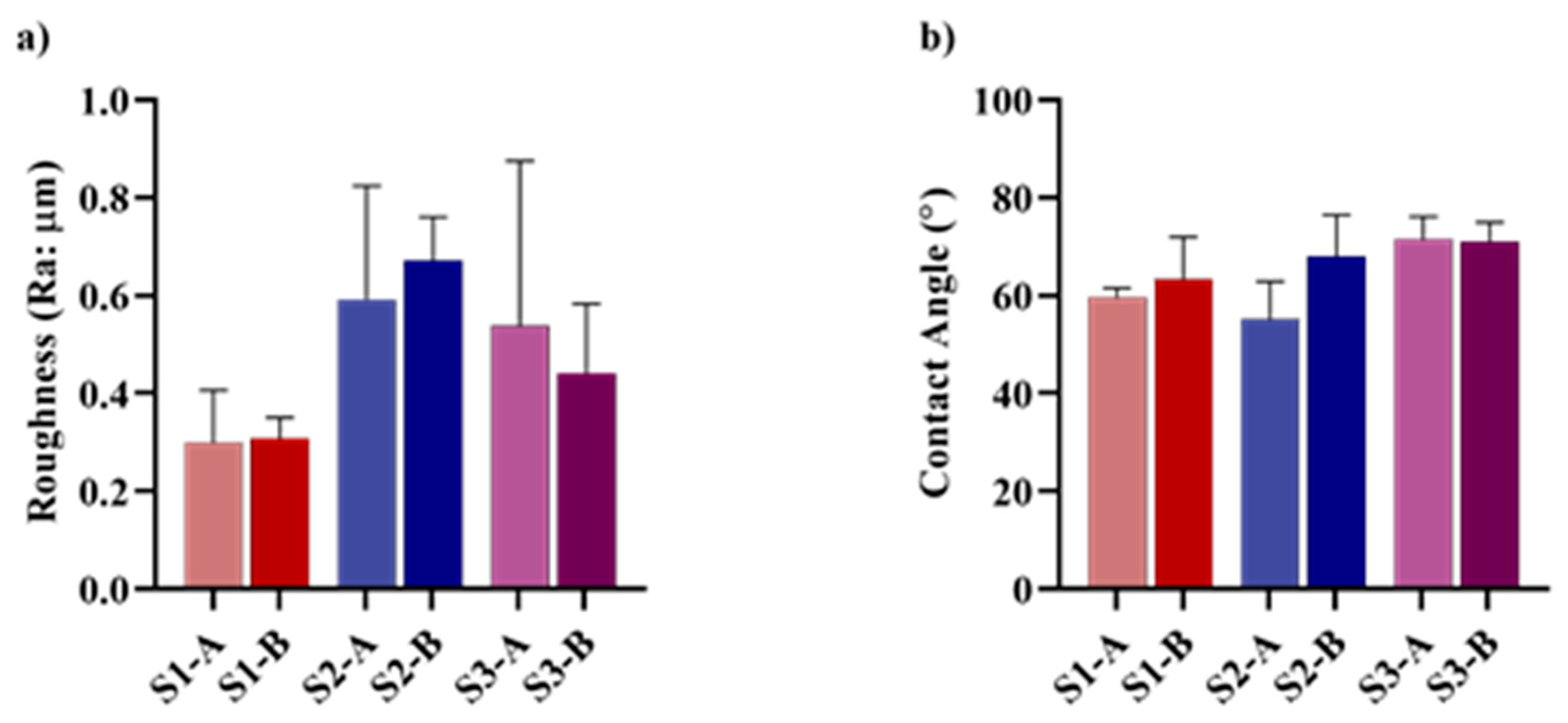
3.3. Atomic Force Microscopy
3.4. Contact Angle Analysis
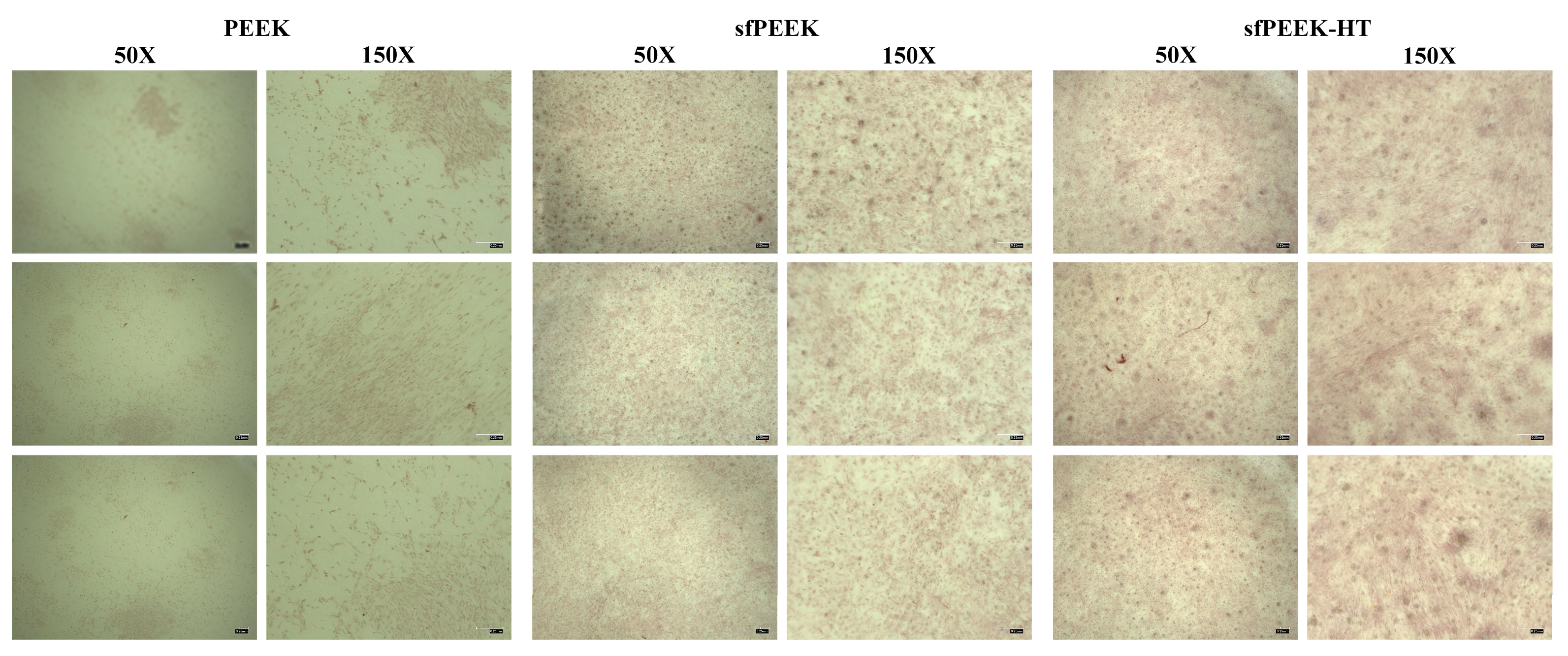
3.5. In Vitro Evaluation
3.5.1. Cell Viability
3.5.2. Cell Proliferation and Differentiation
3.5.3. Cell Mineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watkins-Castillo, S.; Andersson, G. United States Bone and Joint Initiative: The Burden of Musculoskeletal Dieseases in the United States (BMUSA). 2014. Available online: http://www.boneandjointburden.org (accessed on 21 March 2024).
- Afewerki, S.; Bassous, N.; Harb, S.; Palo-Nieto, C.; Ruiz-Esparza, G.U.; Marciano, F.R.; Webster, T.J.; Furtado, A.S.A.; Lobo, A.O. Advances in dual functional antimicrobial and osteoinductive biomaterials for orthopaedic applications. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102143. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediat. Inflamm. 2014, 2014, 185150. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An update. J. Indian Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Civantos, A.; Domínguez, C.; Pino, R.J.; Setti, G.; Pavón, J.J.; Martínez-Campos, E.; Garcia, F.J.G.; Rodríguez, J.A.; Allain, J.P.; Torres, Y. Designing bioactive porous titanium interfaces to balance mechanical properties and in vitro cells behavior towards increased osseointegration. Surf. Coat. Technol. 2019, 368, 162–174. [Google Scholar] [CrossRef]
- Zheng, J.-P.; Chen, L.-J.; Chen, D.-Y.; Shao, C.-S.; Yi, M.-F.; Zhang, B. Effects of pore size and porosity of surface-modified porous titanium implants on bone tissue ingrowth. Trans. Nonferr. Met. Soc. China 2019, 29, 2534–2545. [Google Scholar] [CrossRef]
- Hahn, B.-D.; Park, D.-S.; Choi, J.-J.; Ryu, J.; Yoon, W.-H.; Choi, J.-H.; Kim, J.-W.; Ahn, C.-W.; Kim, H.-E.; Yoon, B.-H.; et al. Osteoconductive hydroxyapatite coated PEEK for spinal fusion surgery. Appl. Surf. Sci. 2013, 283, 6–11. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Buck, E.; Manimunda, P.; Farivar, R.; Chromik, R.; Murshed, M.; Cerruti, M. Surface phosphonation enhances hydroxyapatite coating adhesion on polyetheretherketone and its osseointegration potential. Acta Biomater. 2017, 47, 149–158. [Google Scholar] [CrossRef]
- Mishra, S.; Chowdhary, R. PEEK materials as an alternative to titanium in dental implants: A systematic review. Clin. Implant. Dent. Relat. Res. 2019, 21, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, W.; Chen, J.; Yu, J.; Zhang, J.; Chen, J. The application of polyetheretherketone (PEEK) implants in cranioplasty. Brain Res. Bull. 2019, 153, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.T.; Torstrick, F.B.; Lee, C.S.; Dupont, K.M.; Safranski, D.L.; Chang, W.A.; Macedo, A.E.; Lin, A.S.; Boothby, J.M.; Whittingslow, D.C.; et al. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015, 13, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Medina, F.; Lopez, H.; Martinez, E.; Machado, B.I.; Hernandez, D.H.; Martinez, L.; Lopez, M.I.; Wicker, R.B.; et al. Next-generation biomedical implants using additive manufacturing of complex, cellular and functional mesh arrays. Philos. Trans. A Math Phys. Eng. Sci. 2010, 368, 1999–2032. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, F.; Zhao, P.; Lin, C.; Wen, X.; Ye, X. Layer-by-layer self-assembled multilayers on PEEK implants improve osseointegration in an osteoporosis rabbit model. Nanomedicine 2017, 13, 1423–1433. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Tan, J.; Lv, D.; Song, W.; Su, R.; Li, L.; Liu, X.; Ouyang, L.; Liao, Y. Strontium ranelate incorporated 3D porous sulfonated PEEK simulating MC3T3-E1 cell differentiation. Regen. Biomater. 2021, 8, rbaa043. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Li, Y.; Shi, J.; Zhou, J.; Zhang, L.; Deng, Y.; Yang, W. Strontium/adiponectin co-decoration modulates the osteogenic activity of nano-morphologic polyetheretherketone implant. Colloids Surf. B Biointerfaces 2019, 176, 38–46. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Z.; Jiao, Z.; Wu, Z.; Guo, M.; Wang, Y.; Liu, J.; Zhang, P. Enhancing antibacterial capability and osseointegration of polyetheretherketone (PEEK) implants by dual-functional surface modification. Mater. Des. 2021, 205, 109733. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Necula, B.; Apachitei, I.; Fratila-Apachitei, L.; van Langelaan, E.; Duszczyk, J. Titanium bone implants with superimposed micro/nano-scale porosity and antibacterial capability. Appl. Surf. Sci. 2013, 273, 310–314. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Torstrick, F.B.; Lin, A.S.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Zhao, G.; Raines, A.; Wieland, M.; Schwartz, Z.; Boyan, B. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007, 28, 2821–2829. [Google Scholar] [CrossRef]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.B.; Washburn, N.R.; Simon, C.G., Jr.; Amis, E.J. Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials 2006, 27, 3817–3824. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Guadarrama Bello, D.; Nanci, A. Surface nanoporosity has a greater influence on osteogenic and bacterial cell adhesion than crystallinity and wettability. Appl. Surf. Sci. 2018, 445, 255–261. [Google Scholar] [CrossRef]
- Buck, E.; Li, H.; Cerruti, M. Surface Modification Strategies to Improve the Osseointegration of Poly(etheretherketone) and Its Composites. Macromol. Biosci. 2020, 20, e1900271. [Google Scholar] [CrossRef] [PubMed]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shi, L.; He, W.-X.; Han, D.; Yan, Y.; Niu, Z.-Y.; Shi, S.-G. Bioinspired micro/nano fabrication on dental implant–bone interface. Appl. Surf. Sci. 2013, 265, 480–488. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Shao, P.; Burns, C.M.; Feng, X. Sulfonation of poly(ether ether ketone)(PEEK): Kinetic study and characterization. J. Appl. Polym. Sci. 2001, 82, 2651–2660. [Google Scholar] [CrossRef]
- Khomein, P.; Ketelaars, W.; Lap, T.; Liu, G. Sulfonated aromatic polymer as a future proton exchange membrane: A review of sulfonation and crosslinking methods. Renew. Sustain. Energy Rev. 2021, 137, 110471. [Google Scholar] [CrossRef]
- Zaidi, S.M.J. Polymer sulfonation—A versatile route to prepare proton-conducting membrane material for advanced technologies. Arab. J. Sci. Eng. Sect. B Eng. 2003, 28, 183–194. [Google Scholar]
- He, M.; Huang, Y.; Xu, H.; Feng, G.; Liu, L.; Li, Y.; Sun, D.; Zhang, L. Modification of polyetheretherketone implants: From enhancing bone integration to enabling multi-modal therapeutics. Acta Biomater. 2021, 129, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ouyang, L.; Ma, X.; Qiao, Y.; Liu, X. Controllable and durable release of BMP-2-loaded 3D porous sulfonated polyetheretherketone (PEEK) for osteogenic activity enhancement. Colloids Surf. B Biointerfaces 2018, 171, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.; Yan, C.H.; Yeung, K.W.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Jiao, Z.; Guo, M.; Wang, Z.; Wan, Y.; Lin, K.; Liu, Q.; Zhang, P. Gaseous sulfur trioxide induced controllable sulfonation promoting biomineralization and osseointegration of polyetheretherketone implants. Bioact. Mater. 2020, 5, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Zhao, Y.; Jin, G.; Lu, T.; Li, J.; Qiao, Y.; Ning, C.; Zhang, X.; Chu, P.K.; Liu, X. Influence of sulfur content on bone formation and antibacterial ability of sulfonated PEEK. Biomaterials 2016, 83, 115–126. [Google Scholar] [CrossRef]
- Qin, W.; Li, Y.; Ma, J.; Liang, Q.; Cui, X.; Jia, H.; Tang, B. Osseointegration anvd biosafety of graphene oxide wrapped porous CF/PEEK composites as implantable materials: The role of surface structure and chemistry. Dent. Mater. 2020, 36, 1289–1302. [Google Scholar] [CrossRef]



| Design Space | Pre-Surface Condition (Grit) | Soak Condition | Acid Conc. | Soak Time (min) | Soak Temp. (°C) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | S/NS/SO/SS | % | Low | Center | High | Low | Center | High | |
| Design #1 | 220 | 1200 | S/NS | 80–100 | 1 | - | 10 | 22 | - | 60 |
| Design #2 | 220 | - | NS | 100 | 1 | 2.5 | 4 | 50 | 65 | 80 |
| Design #3 | 220 | - | NS | 100 | 2.5 | 3.75 | 5 | 55 | 65 | 80 |
| Design #4 | 320 | - | SO | 100 | 1 | 2.5 | 5 | 55 | 60 | 65 |
| Design #5 | 320 | - | SO/SS | 100 | 1 | 1.45 | 2.5 | 55 | 60 | 65 |
| Design #6 | 320 | - | SO/SS | 100 | 1 | - | - | 65 | - | - |
| Specimen ID | Soak Time (min) | Soak Temp. (°C) | |
|---|---|---|---|
| Design #1 | H1 | 90 | 45 |
| H2 | 60 | 80 | |
| H3 a | 0 | 0 | |
| H4 | 75 | 63 | |
| H5 | 90 | 80 | |
| H6 b | 60 | 45 | |
| Design #2 | sfPEEK—2 h HT | 120 (2 h) | 45 |
| sfPEEK—3 h HT | 180 (3 h) | ||
| sfPEEK—4 h HT | 240 (4 h) | ||
| sfPEEK—5 h HT | 300 (5 h) |
| Specimen ID | Pore Coverage (% Area) | Pore Count | Pore Diameter (Avg: µm) |
|---|---|---|---|
| D6-1-SS | 13 | 2124 | 103 ± 128 |
| D6-2-SS | 17 | 4179 | 88 ± 97 |
| D6-3-SS | 11 | 2531 | 94 ± 92 |
| D6-1-SO | 11 | 1574 | 120 ± 109 |
| D6-2-SO | 18 | 2558 | 115 ± 151 |
| D6-3-SO | 21 | 2671 | 118 ± 160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobles, K.; Janorkar, A.V.; Roach, M.D.; Walker, L.; Williamson, R.S. In Vitro Evaluation of Optimized PEEK Surfaces for Enhanced Osseointegration. Coatings 2024, 14, 518. https://doi.org/10.3390/coatings14050518
Nobles K, Janorkar AV, Roach MD, Walker L, Williamson RS. In Vitro Evaluation of Optimized PEEK Surfaces for Enhanced Osseointegration. Coatings. 2024; 14(5):518. https://doi.org/10.3390/coatings14050518
Chicago/Turabian StyleNobles, Kadie, Amol V. Janorkar, Michael D. Roach, Lawrence Walker, and Randall Scott Williamson. 2024. "In Vitro Evaluation of Optimized PEEK Surfaces for Enhanced Osseointegration" Coatings 14, no. 5: 518. https://doi.org/10.3390/coatings14050518







