Ternary Holey Carbon Nanohorn/Potassium Chloride/Polyvinylpyrrolidone Nanohybrid as Sensing Film for Resistive Humidity Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
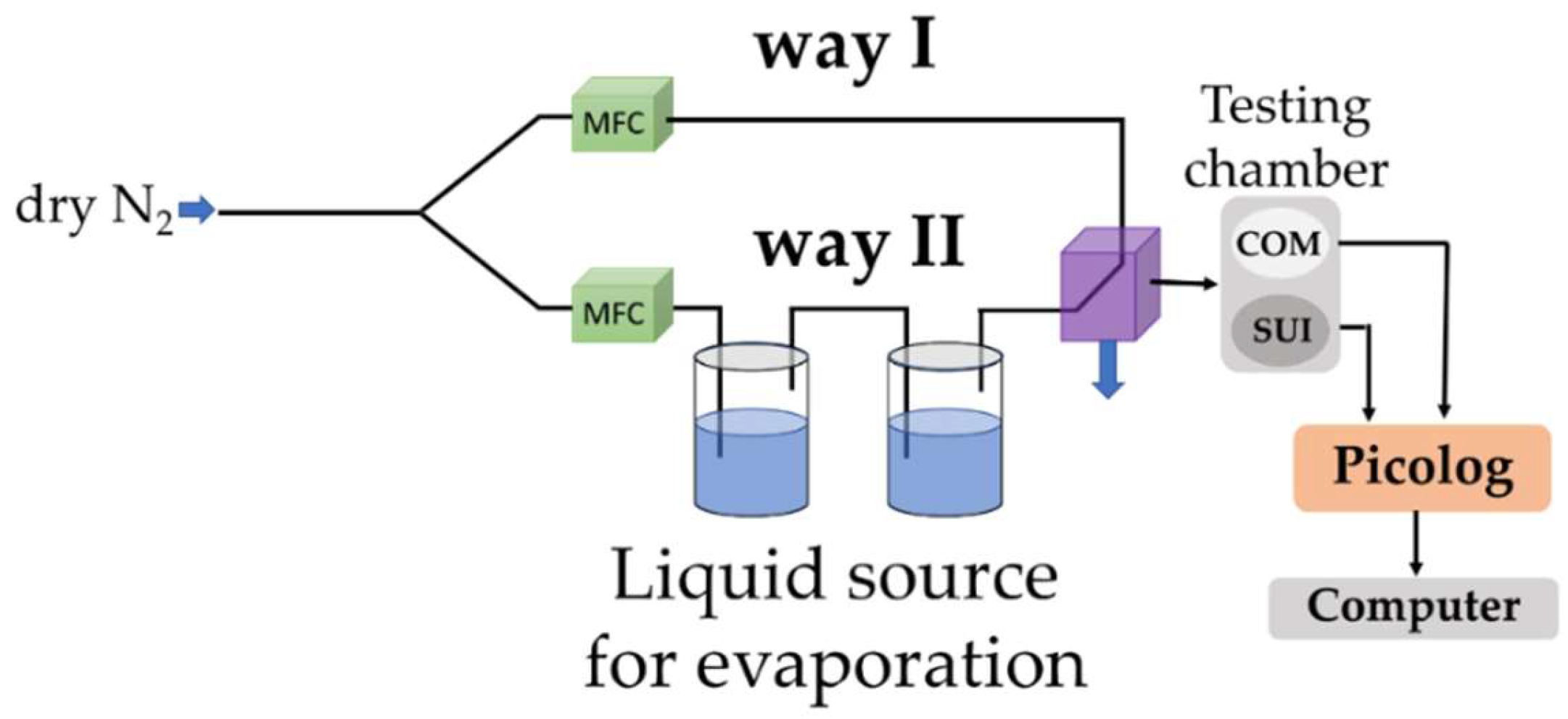
2.2. Preparation of the Quaternary Organic–Inorganic Holey CNH-Based Hybrid Sensing Films and Experimental Setup
3. Results
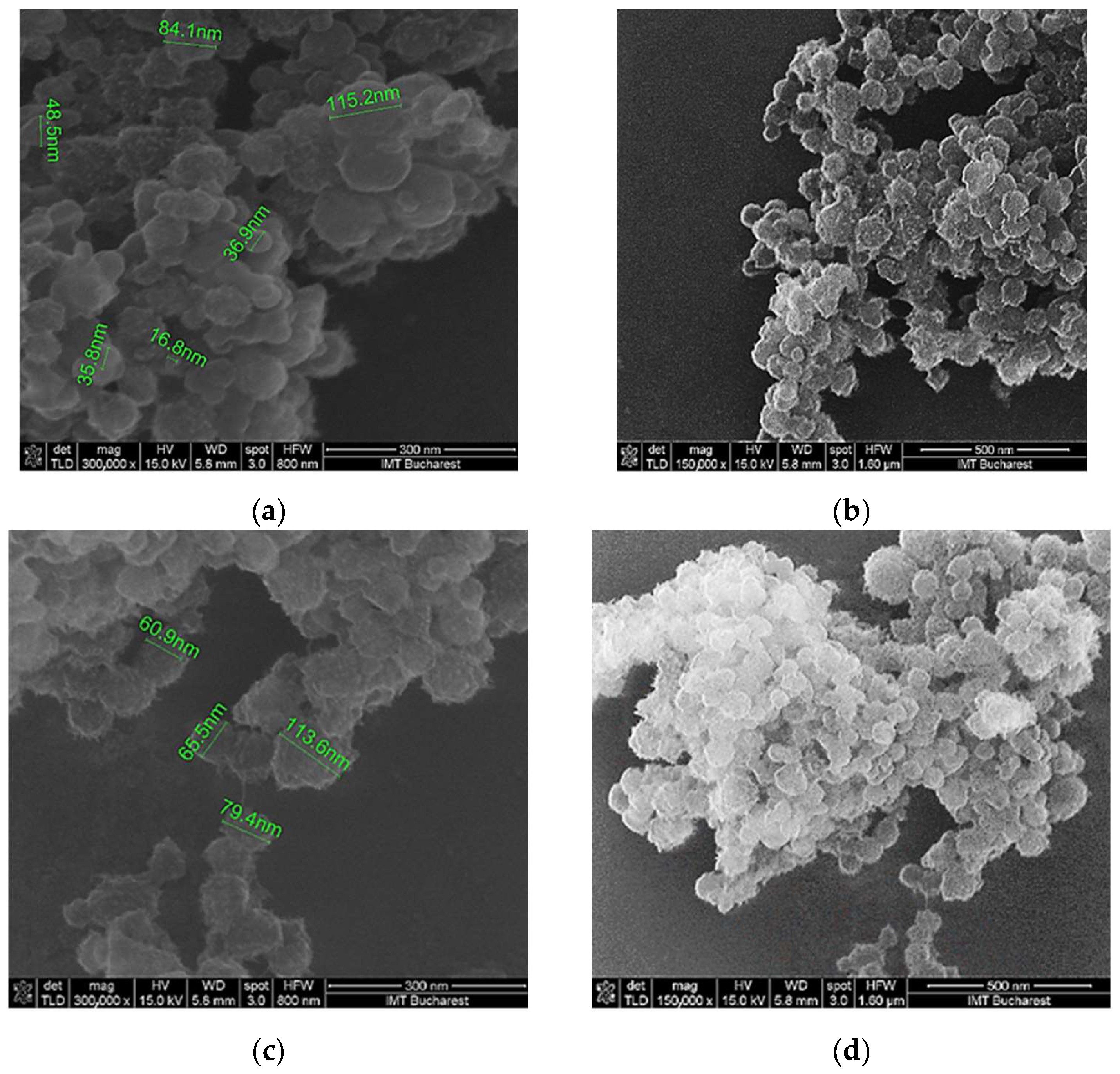
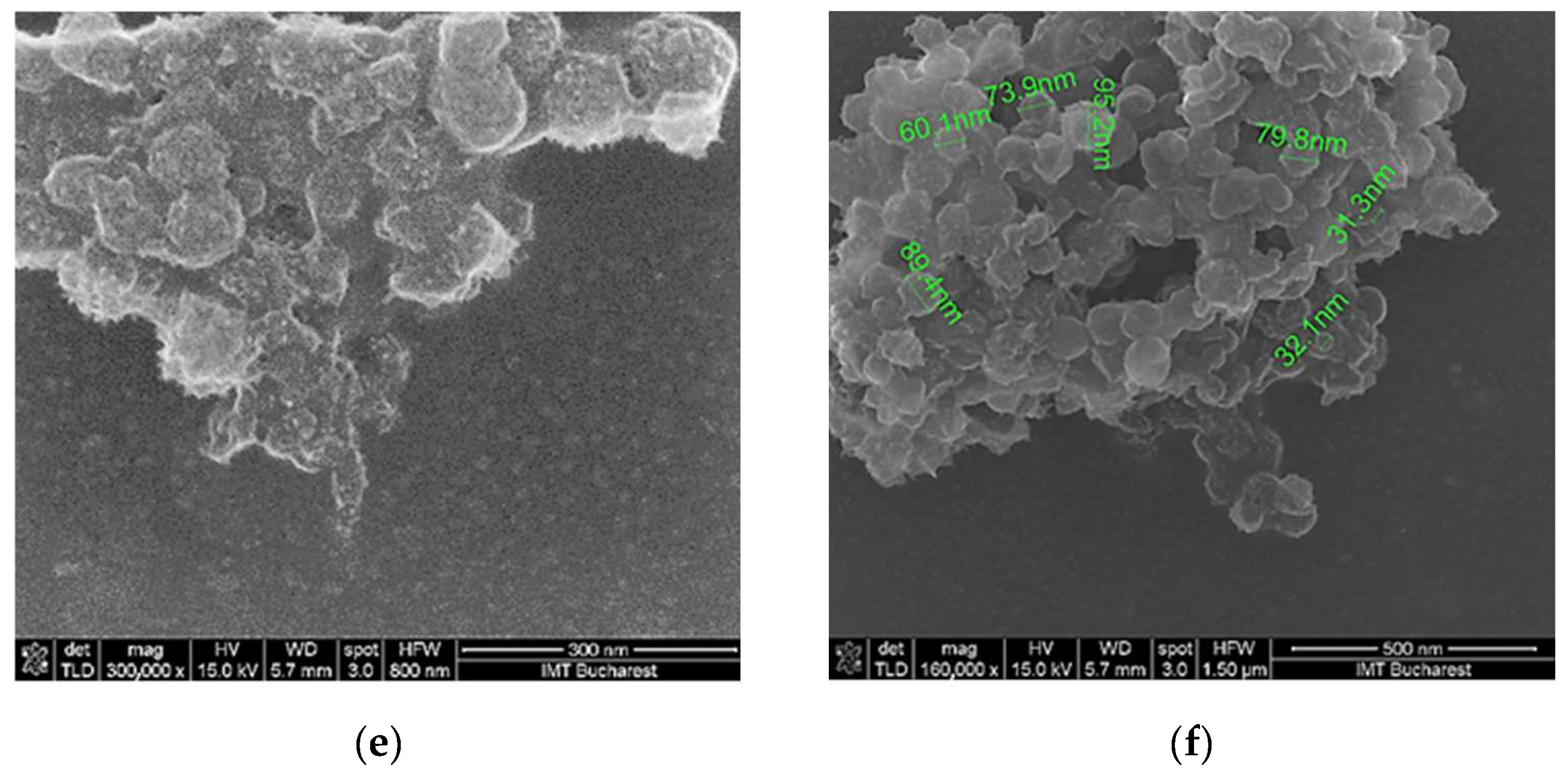
3.1. Surface Topography
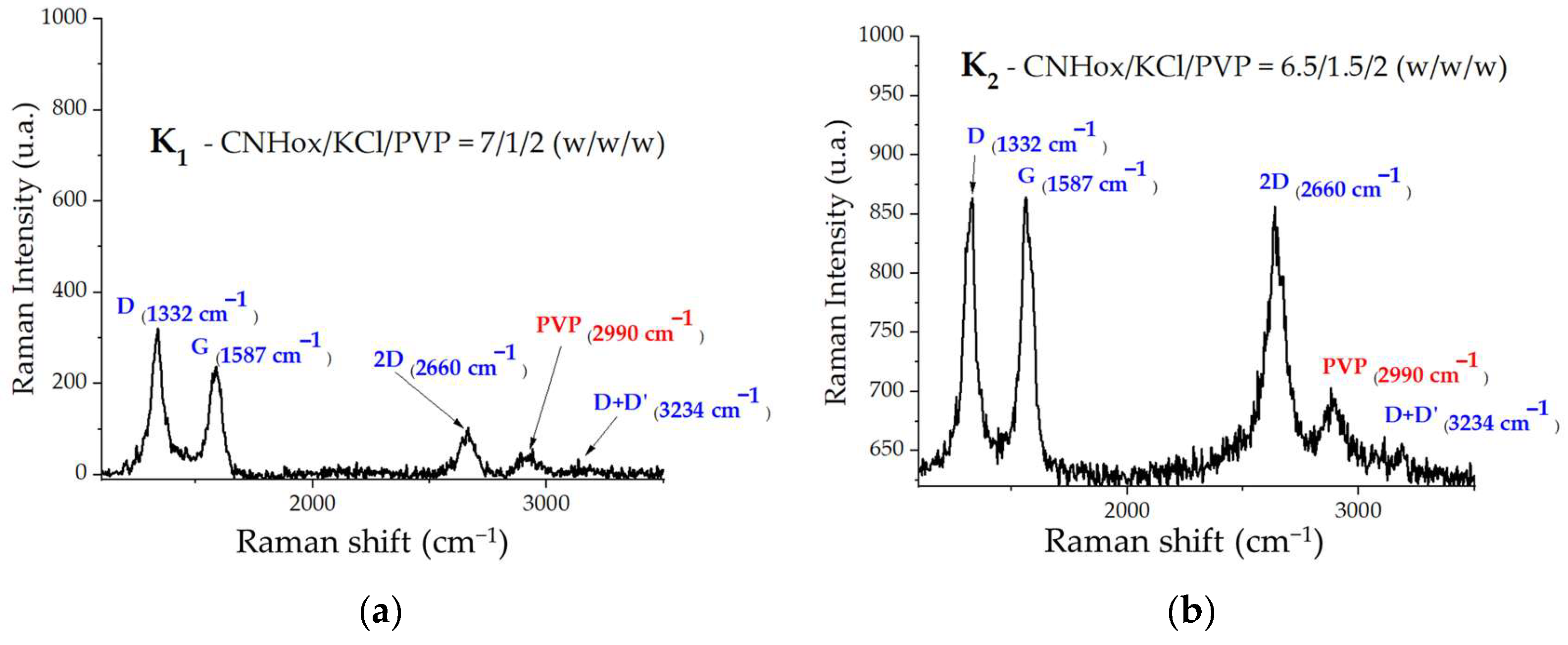
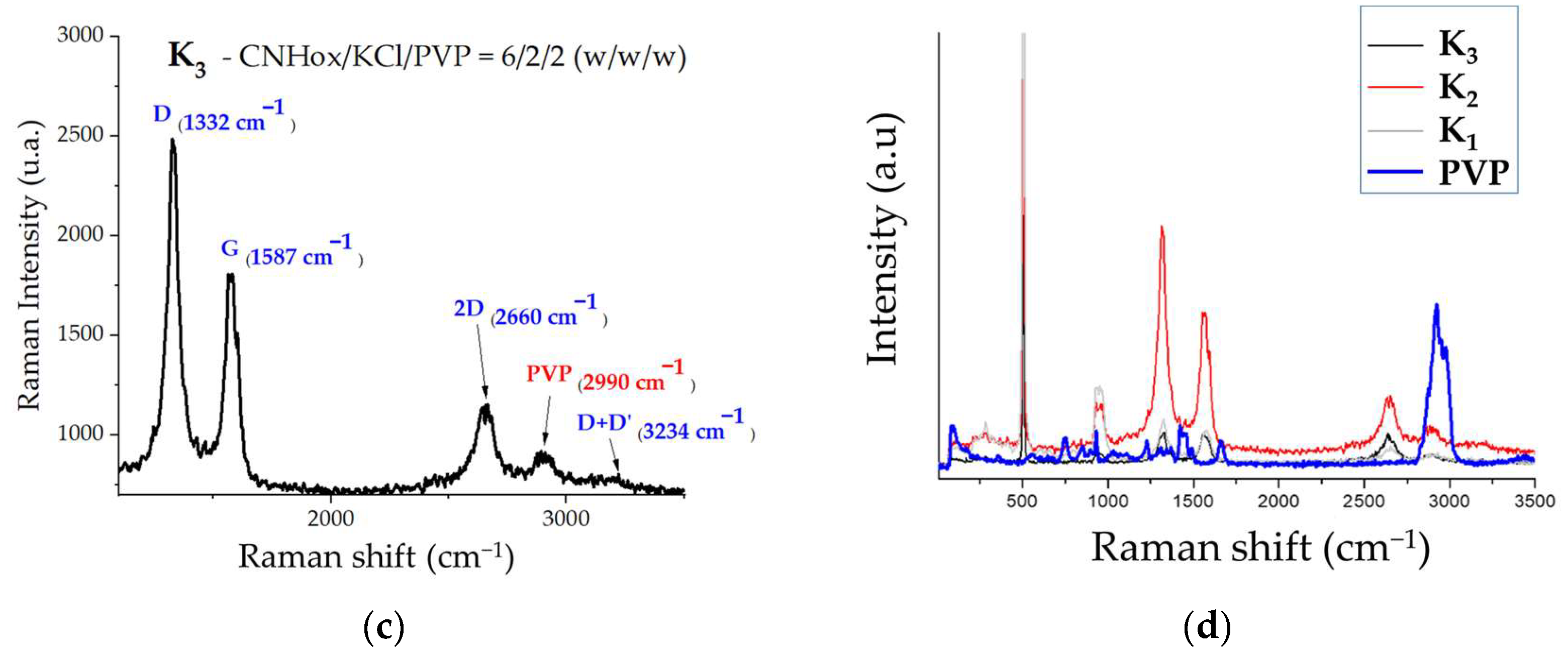
3.2. Raman Spectroscopy
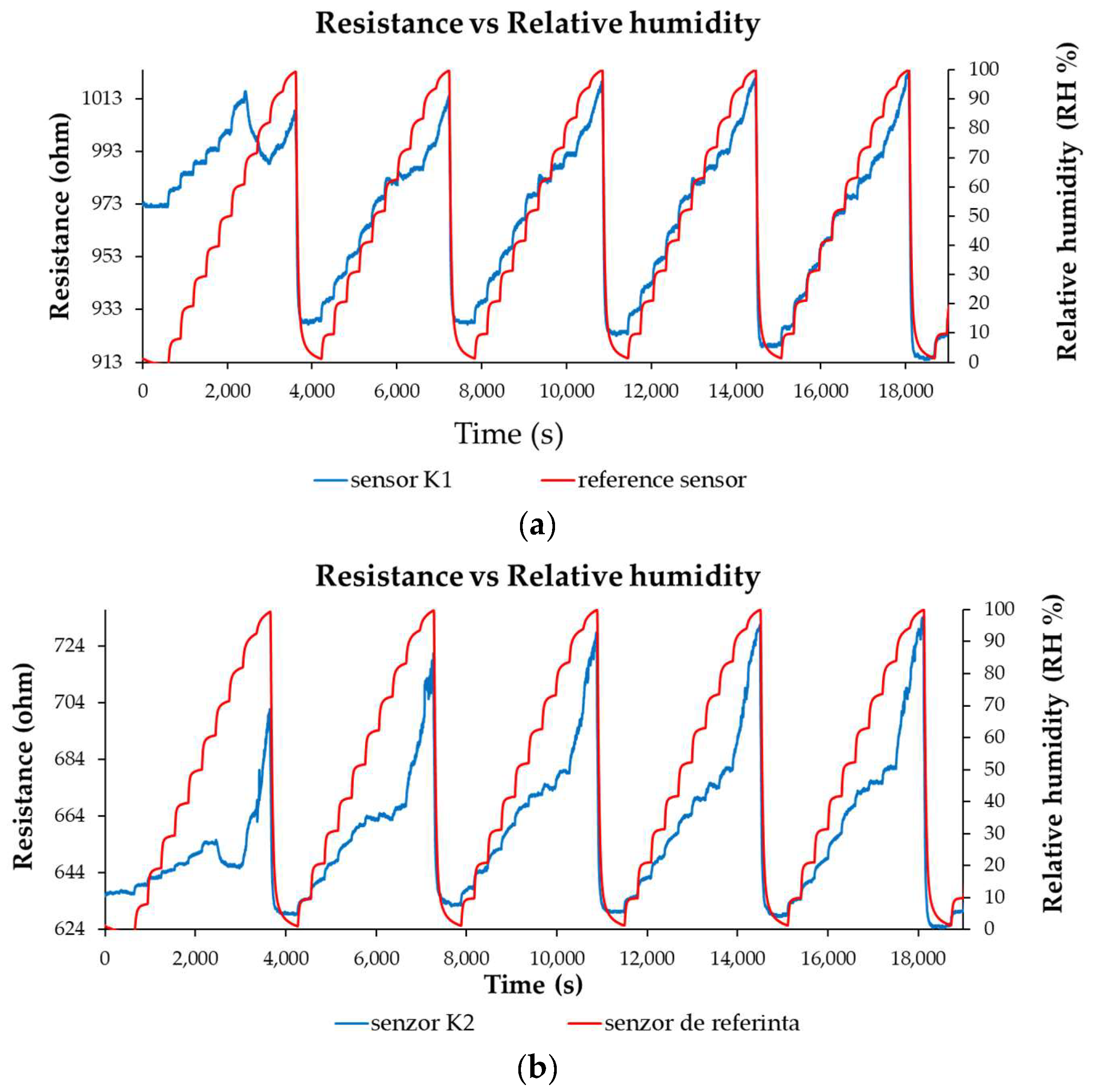
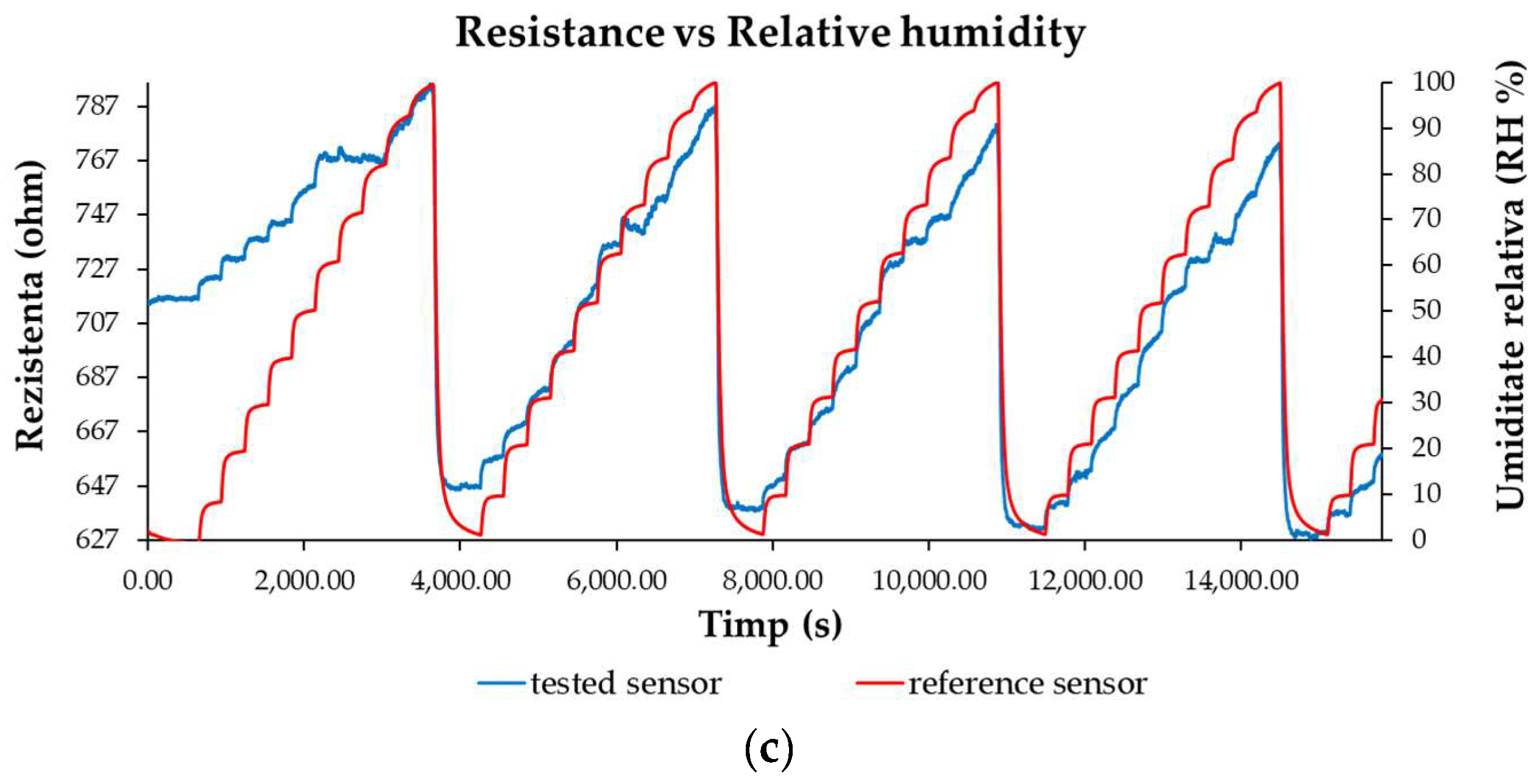
3.3. R.H. Monitoring Capability of the Ternary Nanocomposite
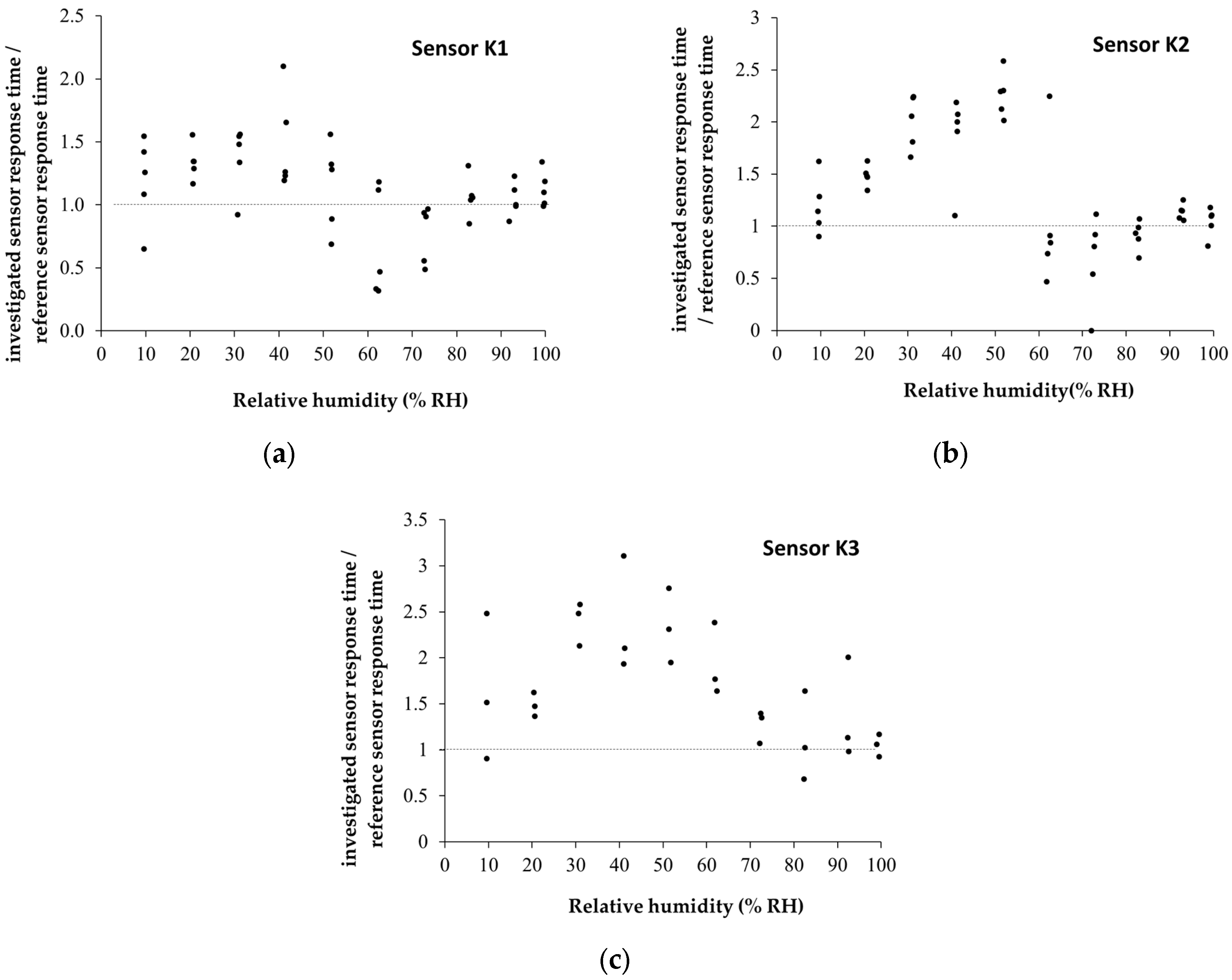
4. Analysis of the Sensing Mechanism
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Fei, T.; Zhang, T. An overview: Sensors for low humidity detection. Sens. Actuators B Chem. 2023, 376, 133039. [Google Scholar] [CrossRef]
- Ku, C.A.; Chung, C.K. Advances in Humidity Nanosensors and Their Application. Sensors 2023, 23, 2328. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jiang, M.; Liu, F. Recent Progress on Environmentally Friendly Humidity Sensor: A Mini Review. ACS Appl. Electron. Mater. 2023, 5, 4067–4079. [Google Scholar] [CrossRef]
- Farhan, K.Z.; Shihata, A.S.; Anwar, M.I.; Demirboğa, R. Temperature and humidity sensor technology for concrete health assessment: A review. Innov. Infrastruct. Solut. 2023, 8, 276. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.; Bapna, K.; Shivagan, D.D. Semiconductor-metal-oxide-based nanocomposites for humidity sensing applications. Mater. Res. Bull. 2023, 158, 112053. [Google Scholar] [CrossRef]
- Barmpakos, D.; Kaltsas, G. A review on humidity, temperature and strain printed sensors—Current trends and future perspectives. Sensors 2021, 21, 739. [Google Scholar] [CrossRef]
- Global Humidity Sensor Market—Industry Trends and Forecast to 2029. Available online: https://www.databridgemarketresearch.com/reports/global-humidity-sensor-market (accessed on 15 February 2024).
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Montes-García, V.; Samorì, P. Humidity sensing with supramolecular nanostructures. Adv. Mater. 2024, 36, 2208766. [Google Scholar] [CrossRef] [PubMed]
- Grundler, M.; Derieth, T.; Heinzel, A. Polymer compounds with high thermal conductivity. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2016; Volume 1779. [Google Scholar]
- Haq, Y.U.; Ullah, R.; Mazhar, S.; Khattak, R.; Qarni, A.A.; Haq, Z.U.; Amin, S. Synthesis and characterization of 2D MXene: Device fabrication for humidity sensing. J. Sci. Adv. Mater. Devices 2022, 7, 100390. [Google Scholar] [CrossRef]
- Yasaei, P.; Behranginia, A.; Foroozan, T.; Asadi, M.; Kim, K.; Khalili-Araghi, F.; Salehi-Khojin, A. Stable and selective humidity sensing using stacked black phosphorus flakes. ACS Nano 2015, 9, 9898–9905. [Google Scholar] [CrossRef]
- Emperhoff, S.; Eberl, M.; Dwertmann, T.; Wöllenstein, J. Humidity Impact on Thermal Conductivity Sensors. Proceedings 2024, 97, 93. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, G.; Wang, X.; Kong, X. Comparative Study of Gravimetric Humidity Sensor Platforms Based on CMUT and QCM. Micromachines 2022, 13, 1651. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhao, L.; Xu, L.; Wang, Y.; Liu, K.; Wang, Y.; Chen, G.Y.; Liu, T.; Wang, Y. Review of optical humidity sensors. Sensors 2021, 21, 8049. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Liu, Y.; Peng, L.; Pang, J.; Xu, Z.; Gao, C.; Xie, J. Surface acoustic wave humidity sensors based on uniform and thickness controllable graphene oxide thin films formed by surface tension. Microsyst. Nanoeng. 2019, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.R.; Chen, K.S. Humidity sensors using polyvinyl alcohol mixed with electrolytes. Sens. Actuators B Chem. 1998, 49, 240–247. [Google Scholar] [CrossRef]
- Tu, J.; Li, N.; Geng, W.; Wang, R.; Lai, X.; Cao, Y.; Qiu, S. Study on a type of mesoporous silica humidity sensing material. Sens. Actuators B Chem. 2012, 166, 658–664. [Google Scholar] [CrossRef]
- Memon, M.M.; Hongyuan, Y.; Pan, S.; Wang, T.; Zhang, W. Surface Acoustic Wave Humidity Sensor Based on Hydrophobic Polymer Film. J. Electron. Mater. 2022, 51, 5627–5634. [Google Scholar] [CrossRef]
- Dong, W.; Ma, Z.; Duan, Q. Preparation of stable crosslinked polyelectrolyte and the application for humidity sensing. Sens. Actuators B Chem. 2018, 272, 14–20. [Google Scholar] [CrossRef]
- Rimeika, R.; Čiplys, D.; Poderys, V.; Rotomskis, R.; Balakauskas, S.; Shur, M.S. Subsecond-response S.A.W. humidity sensor with porphyrin nanostructure deposited on bare and metallised piezoelectric substrate. Electron. Lett. 2007, 43, 1055–1057. [Google Scholar] [CrossRef]
- Monereo, O.; Claramunt, S.; de Marigorta, M.M.; Boix, M.; Leghrib, R.; Prades, J.; Cornet, A.; Merino, P.; Merino, C.; Cirera, A. Flexible sensor based on carbon nanofibers with multifunctional sensing features. Talanta 2013, 107, 239–247. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.; Feng, P.; Sheng, Y.; Zhang, J. Study of humidity sensors based on nanostructured carbon films produced by physical vapor deposition. Sens. Actuators B Chem. 2013, 178, 508–513. [Google Scholar] [CrossRef]
- Sharath Kumar, J.; Murmu, N.C.; Kuila, T. Recent Advances in Functionalized Micro and Mesoporous Carbon Nanostructures for Humidity Sensors. In Functional Nanomaterials: Advances in Gas Sensing Technologies; Spring: Berlin/Heidelberg, Germany, 2020; pp. 349–381. [Google Scholar]
- Duan, Z.; Yuan, Z.; Jiang, Y.; Liu, Y.; Tai, H. Amorphous carbon material of daily carbon ink: Emerging applications in pressure, strain, and humidity sensors. J. Mater. Chem. C 2023, 11, 5585–5600. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Salmeron, J.F.; Murru, F.; Lapresta-Fernández, A.; Rodríguez, N.; Capitan-Vallvey, L.F.; Salinas-Castillo, A. Carbon dots as sensing layer for printed humidity and temperature sensors. Nanomaterials 2020, 10, 2446. [Google Scholar] [CrossRef] [PubMed]
- Frattini, G.; Torres, S.; Silva, L.I.; Repetto, C.E.; Gómez, B.; Dobry, A. The effect of nitriding on the humidity sensing properties of hydrogenated amorphous carbon films. Phys. Scr. 2021, 96, 055701. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Yu, X.; Chen, X.; Ding, X.; Zhao, X. Flexible wearable humidity sensor based on nanodiamond with fast response. IEEE Trans. Electron Devices 2019, 66, 1911–1916. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.M.; Wu, Z.; Li, X.; Wang, N.; Tao, K.; Wang, G.P. Carbon nanocoil-based fast-response and flexible humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef]
- Chen, W.P.; Zhao, Z.G.; Liu, X.W.; Zhang, Z.X.; Suo, C.G. A capacitive humidity sensor based on multi-wall carbon nanotubes (MWCNTs). Sensors 2009, 9, 7431–7444. [Google Scholar] [CrossRef]
- Saxena, S.; Srivastava, A.K. Carbon nanotube-based sensors and their application. In Nano-Optics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 265–291. [Google Scholar]
- Tripathi, D.; Tripathi, S.; Rawat, R.K.; Chauhan, P. Highly Sensitive Humidity Sensor Based on Freestanding Graphene Oxide Sheets for Respiration and Moisture Detection. J. Electron. Mater. 2023, 52, 2396–2408. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent advances in graphene-based humidity sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef]
- Liang, T.; Hou, W.; Ji, J.; Huang, Y. Wrinkled reduced graphene oxide humidity sensor with fast response/recovery and flexibility for respiratory monitoring. Sens. Actuators A Phys. 2023, 350, 114104. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Jin, F.; Ge, H.L.; Gao, F.; Wu, Q.; Yang, H. Facile preparation of fullerenol-based humidity sensor with highly fast response. Fuller. Nanotub. Carbon Nanostructures 2023, 31, 1132–1136. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yu, X.; Chen, X.; Ding, X.; Zhao, X. A high-sensitive humidity sensor based on water-soluble composite material of fullerene and graphene oxide. IEEE Sens. J. 2017, 18, 962–966. [Google Scholar] [CrossRef]
- Selvam, K.P.; Nakagawa, T.; Marui, T.; Inoue, H.; Nishikawa, T.; Hayashi, Y. Synthesis of solvent-free conductive and flexible cellulose–carbon nanohorn sheets and their application as a water vapor sensor. Mater. Res. Express 2020, 7, 056402. [Google Scholar] [CrossRef]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Bumbac, M.; Pachiu, C.; Nicolescu, C.M. Oxidized carbon nanohorn-hydrophilic polymer nanocomposite as the resistive sensing layer for relative humidity. Anal. Lett. 2021, 54, 527–540. [Google Scholar] [CrossRef]
- Serban, B.-C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Pachiu, C.; Craciun, G.; et al. Ternary nanocomposites based on oxidized carbon nanohorns as sensing layers for room temperature resistive humidity sensing. Materials 2021, 14, 2705. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Radulescu, C.; Craciun, G.; et al. Quaternary Holey Carbon Nanohorns/SnO2/ZnO/PVP Nano-Hybrid as Sensing Element for Resistive-Type Humidity Sensor. Coatings 2021, 11, 1307. [Google Scholar] [CrossRef]
- Serban, B.C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Brezeanu, M.; Radulescu, C.; Craciun, G.; Nicolescu, C.M.; Romanitan, C.; et al. Ternary Holey Carbon Nanohorns/TiO2/PVP Nanohybrids as Sensing Films for Resistive Humidity Sensors. Coatings 2021, 11, 1065. [Google Scholar] [CrossRef]
- Song, X.; Qi, Q.; Zhang, T.; Wang, C. A humidity sensor based on KCl-doped SnO2 nanofibers. Sens. Actuators B Chem. 2009, 138, 368–373. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Wang, S.; Zheng, X. Humidity sensing properties of KCl-doped ZnO nanofibers with super-rapid response and recovery. Sens. Actuators B Chem. 2009, 137, 649–655. [Google Scholar] [CrossRef]
- Geng, W.; Yuan, Q.; Jiang, X.; Tu, J.; Duan, L.; Gu, J.; Zhang, Q. Humidity sensing mechanism of mesoporous MgO/KCl–SiO2 composites analyzed by complex impedance spectra and bode diagrams. Sens. Actuators B Chem. 2012, 174, 513–520. [Google Scholar] [CrossRef]
- Kunchakara, S.; Dutt, M.; Ratan, A.; Shah, J.; Singh, V.; Kotnala, R.K. Synthesis and characterizations of highly ordered KCl–MCM–41 porous nanocomposites for impedimetric humidity sensing. J. Porous Mater. 2019, 26, 389–398. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Liu, L.; Zhang, H.; Zheng, W.; Wang, Y.; Wang, C. Humidity sensor based on LiCl-doped ZnO electrospun nanofibers. Sens. Actuators B Chem. 2009, 141, 404–409. [Google Scholar] [CrossRef]
- Buvailo, A.I.; Xing, Y.; Hines, J.; Dollahon, N.; Borguet, E. TiO2/LiCl-based nanostructured thin film for humidity sensor applications. ACS Appl. Mater. Interfaces 2011, 3, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Duan, Z.; Fan, Z.; Yao, P.; Yuan, Z.; Jiang, Y.; Tai, H. Power generation humidity sensor based on NaCl/halloysite nanotubes for respiratory patterns monitoring. Sens. Actuators B Chem. 2023, 380, 133396. [Google Scholar] [CrossRef]
- Lazarus, N.; Bedair, S.S.; Lo, C.C.; Fedder, G.K. CMOS-MEMS capacitive humidity sensor. J. Microelectromech. Syst. 2009, 19, 183–191. [Google Scholar] [CrossRef]
- Muto, S.; Suzuki, O.; Amano, T.; Morisawa, M. A plastic optical fiber sensor for real-time humidity monitoring. Meas. Sci. Technol. 2003, 14, 746. [Google Scholar] [CrossRef]
- Penza, M.; Anisimkin, V.I. Surface acoustic wave humidity sensor using polyvinyl-alcohol film. Sens. Actuators A 1999, 76, 162–166. [Google Scholar] [CrossRef]
- Su, P.G.; Wang, C.S. Novel flexible resistive-type humidity sensor. Sens. Actuators B Chem. 2007, 123, 1071–1076. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, Z.; Zhang, B.; Yuan, Z.; Zhao, Q.; Jiang, Y.; Tai, H. Electrochemical humidity sensor enabled self-powered wireless humidity detection system. Nano Energy 2023, 115, 108745. [Google Scholar] [CrossRef]
- Arman Kuzubasoglu, B. Recent studies on the humidity sensor: A mini review. ACS Appl. Electron. Mater. 2022, 4, 4797–4807. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of humidity on gas sensing performance of carbon nanotube gas sensors operated at room temperature. IEEE Sens. J. 2020, 21, 5763–5770. [Google Scholar] [CrossRef]
- Serban, B.-C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Radulescu, C.; Craciun, G.; et al. Quaternary Oxidized Carbon Nanohorns—Based Nanohybrid as Sensing Coating for Room Temperature Resistive Humidity Monitoring. Coatings 2021, 11, 530. [Google Scholar] [CrossRef]
- Heidari, B.; Salmani, S.; Sasani Ghamsari, M.; Ahmadi, M.; Majles-Ara, M.H. Ag/PVP nanocomposite thin film with giant optical nonlinearity. Opt. Quantum Electron. 2020, 52, 86. [Google Scholar] [CrossRef]
- Le, G.T.; Lerkprasertkun, P.; Sano, N.; Wu KC, W.; Charinpanitkul, T. Carbon nanohorns with surface functionalized by plasma treatment and their applications in drug delivery systems. J. Sci. Adv. Mater. Devices 2023, 8, 100616. [Google Scholar] [CrossRef]
- Santra, S.; Hu, G.; Howe, R.C.T.; De Luca, A.; Ali, S.Z.; Udrea, F.; Gardner, J.W.; Ray, S.K.; Guha, P.K.; Hasan, T. CMOS integration of inkjet-printed graphene for humidity sensing. Sci. Rep. 2015, 5, 17374. [Google Scholar] [CrossRef] [PubMed]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhanen, T. Ultrafast graphene oxide humidity sensors. ACS Nano 2013, 7, 11166–11173. [Google Scholar] [CrossRef]
- Li, B.; Weng, X.; Sun, X.; Zhang, Y.; Lv, X.; Gu, G. Facile synthesis of Fe3O4/reduced graphene oxide/polyvinyl pyrrolidone ternary composites and their enhanced microwave absorbing properties. J. Saudi Chem. Soc. 2018, 22, 979–984. [Google Scholar] [CrossRef]
- Serban, B.-C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C.; Brezeanu, M.; Pachiu, C.; Serbanescu, M. Electrical percolation threshold and size effects in polyvinylpyrrolidone-oxidized single-wall carbon nanohorn nanocomposite: The impact for relative humidity resistive sensors design. Sensors 2021, 21, 1435. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C.; Brezeanu, M. Electrical percolation threshold in oxidized single wall carbon nanohorn-polyvinylpyrrolidone nanocomposite: A possible application for high sensitivity resistive humidity sensor. In Proceedings of the 2020 International Semiconductor Conference (CAS), Sinaia, Romania, 7–9 October 2020; IEEE: Piscataway Township, NJ, USA, 2020; pp. 239–242. [Google Scholar]
- Serban, B.; Kumar, A.S.; Cobianu, C.; Buiu, O.; Costea, S.; Bostan, C.; Varachiu, N. Selection of gas sensing materials using the Hard Soft Acid Base theory; application to Surface Acoustic Wave CO2 detection. In Proceedings of the CAS 2010 Proceedings (International Semiconductor Conference), Sinaia, Romania, 11–13 October 2010; IEEE: Piscataway Township, NJ, USA, 2010; Volume 1, pp. 247–250. [Google Scholar]
- Serban, B.C.; Brezeanu, M.; Cobianu, C.; Costea, S.; Buiu, O.; Stratulat, A.; Varachiu, N. Materials selection for gas sensing. An HSAB perspective. In Proceedings of the 2014 International Semiconductor Conference (CAS), Sinaia, Romania, 13–15 October 2014; IEEE: Piscataway Township, NJ, USA, 2014; pp. 21–30. [Google Scholar]





| Type of Humidity Sensor | Sensitive Response | Reference | |
|---|---|---|---|
| capacitive humidity sensors | the dielectric constant of the material changes, leading to a change in capacitance | polymers, carbon-based materials, metal oxide-based materials, composites, mesoporous silica, macroporous silica | [9,10,11] |
| resistive humidity sensors | changes in the electrical resistance of the sensing material | polymers, carbon-based materials, metal oxide-based materials, composites, mesoporous silica | [9,10,12] |
| thermal conductivity humidity sensors | thermal conductivity of air increases, leading to a change in temperature or heat flow within the sensor | polymers, ceramics, nanocomposites, carbon-based materials | [13,14] |
| gravimetric humidity sensors | changes in mass or weight of a humidity-sensitive material | hygroscopic polymers or salts, porous materials, quartz crystal microbalance sensors | [15] |
| optical humidity sensors | changes in optical properties, such as refractive index or absorbance | fiber optics coated with humidity-sensitive materials (photonic crystals, hygroscopic coatings, hydrogels, nanoparticles) | [16] |
| surface acoustic wave (SAW) humidity sensors | the shift in frequency or phase of the acoustic waves on the surface of a piezoelectric substrate | polymers, carbon-based materials, metal oxide-based materials, composites | [17] |
| electrolytic humidity sensors | the change of conductivity or impedance of the solution | hydrogel polymers, solid electrolytes, conductive polymer composites | [18,19,20,21] |
| Sensing Layer (Mass Ratio) | ) (Ω/%R.H.) | Reference |
|---|---|---|
| CNHox | 0.013–0.021 | [35] |
| CNHox/PVP 1/1 | 0.020–0.058 | [38] |
| CNHox/PVP 1/2 | 0.017–0.025 | [38] |
| CNHox/GO/PVP 3/1/1 | 0,043–0, 051 | [38] |
| CNHox/GO/SnO2/PVP 0.75/0.75/1/1 | 0.548–0.770 | [56] |
| CNHox/G.O./SnO2/PVP 1/1/1/1 | 0.798–0.980 | [56] |
| CNHox/KCl/PVP 7/1/2 (K1) | 0.200–1.245 | This work |
| CNHox/KCl/PVP 6.5/1.5/2 (K2) | 0.300–0.950 | This work |
| CNHox/KCl/PVP 6/2/2 (K3) | 0.650–1.940 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, B.-C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Pachiu, C.; Brezeanu, M.; Craciun, G.; Nicolescu, C.M.; Diaconescu, V.; Cobianu, C. Ternary Holey Carbon Nanohorn/Potassium Chloride/Polyvinylpyrrolidone Nanohybrid as Sensing Film for Resistive Humidity Sensor. Coatings 2024, 14, 517. https://doi.org/10.3390/coatings14040517
Serban B-C, Buiu O, Bumbac M, Dumbravescu N, Pachiu C, Brezeanu M, Craciun G, Nicolescu CM, Diaconescu V, Cobianu C. Ternary Holey Carbon Nanohorn/Potassium Chloride/Polyvinylpyrrolidone Nanohybrid as Sensing Film for Resistive Humidity Sensor. Coatings. 2024; 14(4):517. https://doi.org/10.3390/coatings14040517
Chicago/Turabian StyleSerban, Bogdan-Catalin, Octavian Buiu, Marius Bumbac, Nicolae Dumbravescu, Cristina Pachiu, Mihai Brezeanu, Gabriel Craciun, Cristina Mihaela Nicolescu, Vlad Diaconescu, and Cornel Cobianu. 2024. "Ternary Holey Carbon Nanohorn/Potassium Chloride/Polyvinylpyrrolidone Nanohybrid as Sensing Film for Resistive Humidity Sensor" Coatings 14, no. 4: 517. https://doi.org/10.3390/coatings14040517







