Utilizing Metal Oxide Thin Films for Device Engineering of Solution-Processed Organic Multi-Junction Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
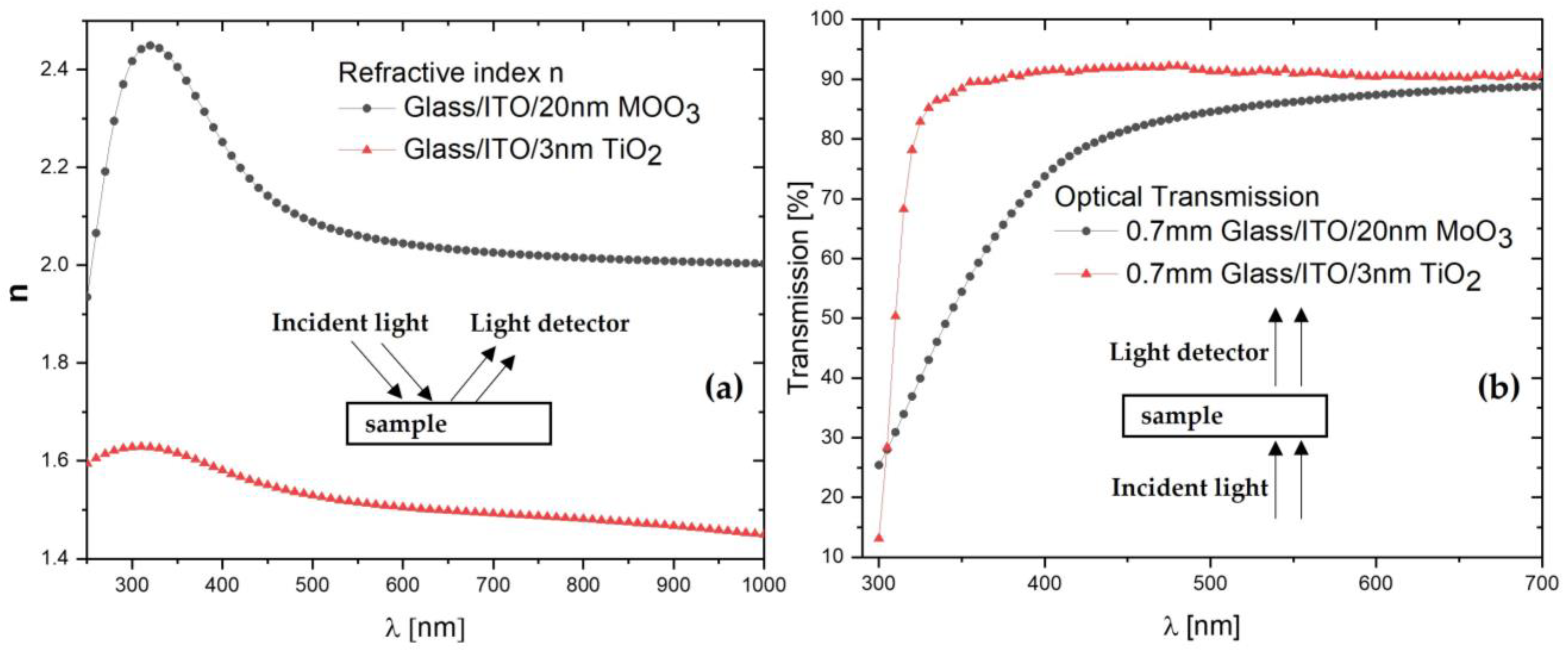
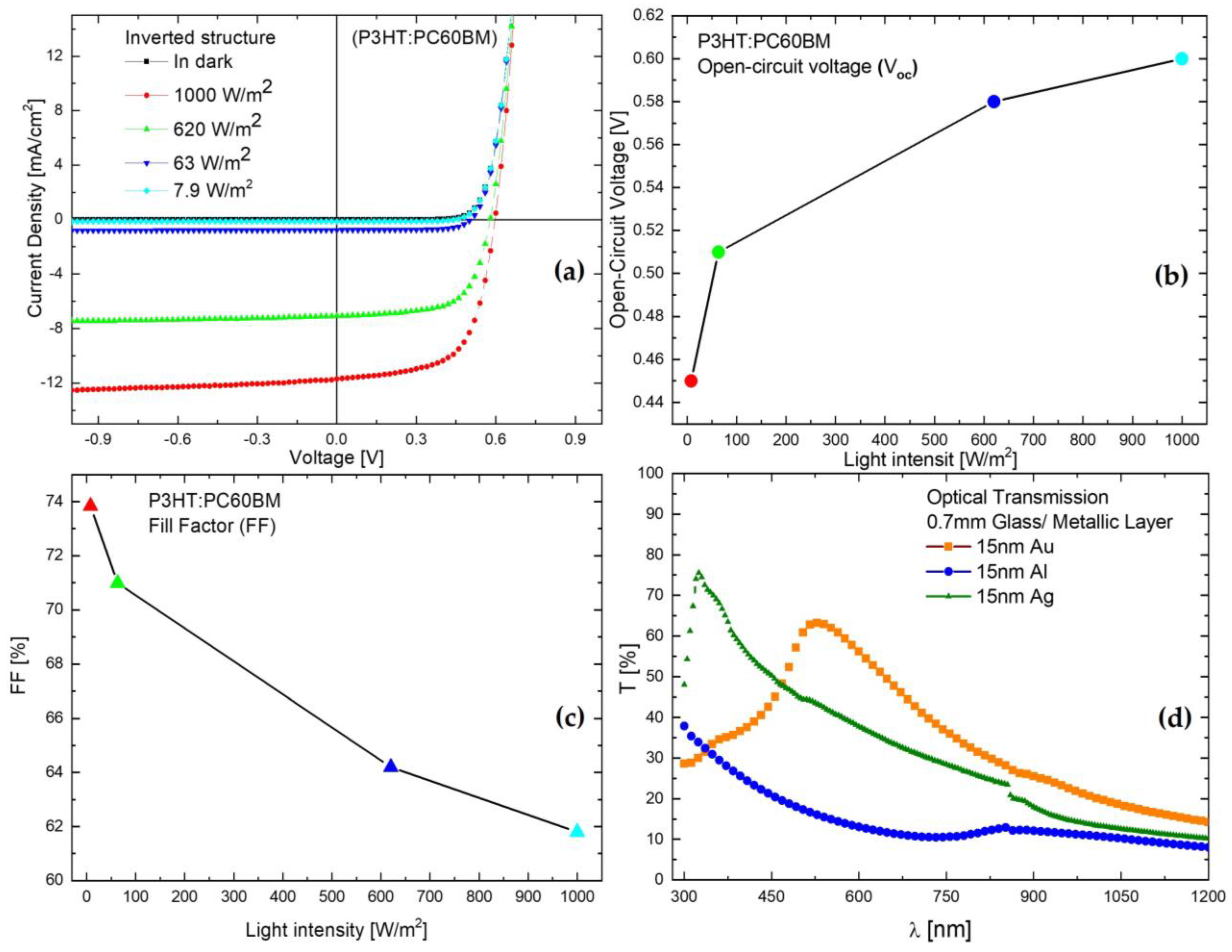
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, C.D.; Li, L.; Wang, B.; Du, Z.-X.; Liu, Z.; Shen, P.; Wang, X.; Zhang, J.; Cai, S.; Cheng, C.; et al. Cold-Aging and Solvent Vapor Mediated Aggregation Control toward 18% Efficiency Binary Organic Solar Cells. Adv. Energy Mater. 2021, 11, 2102000. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Cai, Y.; Li, Y.; Wang, R.; Wu, H.; Chen, Z.; Zhang, J.; Ma, Z.; Hao, X.; Zhao, Y.; Zhang, C.; et al. A well-mixed phase formed by two compatible non-fullerene acceptors enables ternary organic solar cells with efficiency over 18.6%. Adv. Mater. 2021, 33, 2101733. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Zhang, S.; Chen, Z.; Xu, Y.; Cui, Y.; Zhang, T.; Ren, J.; Qin, J.; Hong, L.; Hao, X.; et al. Reduced non-radiative charge recombination enables organic photovoltaic cell approaching 19% efficiency. Joule 2021, 5, 2408–2419. [Google Scholar] [CrossRef]
- Khandelwal, K.; Biswas, S.; Mishra, A.; Ganesh, D.; Sharma, G.D. Semitransparent organic solar cells: From molecular design to structure–performancerelationships. J. Mater. Chem. C 2022, 10, 13. [Google Scholar] [CrossRef]
- Ballif, C.; Perret-Aebi, L.; Lufkin, S.; Rey, E. Integrated thinking for photovoltaics in buildings. Nat. Energy 2018, 3, 438–442. [Google Scholar] [CrossRef]
- Mishra, A.; Bauerle, P. Small molecule organic semiconductors on the move. Angew. Chem. Int. Ed. 2012, 51, 2020–2067. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A. Material perceptions and advances in molecular heteroacenes for organic solar cells. Energy Environ. Sci. 2020, 13, 4738–4793. [Google Scholar] [CrossRef]
- Jiang, Y.; Bai, Y.; Wang, S. Orgaic solar cells: From fundamental to application. Energies 2023, 16, 2262. [Google Scholar] [CrossRef]
- Cui, Y.; Hong, L.; Hou, J. Organic photovoltaics cells for indoor applications: Opportunities and challenges. Appl. Mater. Interfaces 2020, 12, 38815–38828. [Google Scholar] [CrossRef]
- Fukuda, K.; Yu, K.; Someya, T. The future of flexible organic solar cells. Adv. Energy Mater. 2020, 10, 2000765. [Google Scholar] [CrossRef]
- Xu, X.; Yu, L.; Peng, Q. Recent advances in wide bandgap polymer donors and their applications in organic solar cells. Chin. J. Chem. 2021, 39, 243–254. [Google Scholar] [CrossRef]
- Suman Banerjee, S.; Gupta, S.K.; Singh, A.; Garg, A. Buffer layers in inverted organic solar cells and their impact on the interface and device characteristics: An experimental and modeling analysis. Org. Electron. 2016, 37, 228–238. [Google Scholar] [CrossRef]
- Bishnoi, S.; Datt, R.; Arya, S.; Gupta, S.; Gupta, R.; Chung Tsoi, W.; Sharma, S.N.; Patole, S.P.; Gupta, V. Engineered Cathode Buffer Layers for Highly Efficient Organic Solar Cells: A Review. Adv. Mater. Interfaces 2022, 9, 2101693. [Google Scholar] [CrossRef]
- Sreejith, S.; Ajayan, J.; Uma Reddy, N.V.; Manikandan, M.; Radhika, J.M. Critical Review on Various Buffer Layers used to Enhance the Photovoltaic Performance of Organic Solar Cells. Curr. Nanosci. 2023, 20, 801–819. [Google Scholar] [CrossRef]
- Mihailtchi, V.D.; Blom, P.W.M.; Hummelen, J.C.; Janssen, R.A.J.; Kroon, J.M.; Rispens, M.T. Cathode dependence of the open-circuit voltage of polymer-fullerene bulk heterojunction solar cells. J. Appl. Phys. 2003, 94, 6849. [Google Scholar] [CrossRef]
- Mihailtchi, V.D.; Koster, L.J.A.; Blom, P.W.M. Effect of metal electrodes on the performance of polymer-fullerene bulk heterojunction solar cells. Appl. Phys. Lett. 2004, 85, 970. [Google Scholar] [CrossRef]
- Hadipour, A.; De Boer, B.; Wildeman, J.; Kooistra, F.B.; Hummelen, J.C.; Turbiez, M.G.R.; Wienk, M.M.; Janssen, R.A.J.; Blom, P.W.M. Solution-processed Organic Tandem Solar Cells. Adv. Funct. Mater. 2006, 16, 1897–1903. [Google Scholar] [CrossRef]
- Hadipour, A.; De Boer, B.; Blom, B.P.W. Solution-processed Organic Tandem Solar Cells with Imbedded Optical Spacers. J. Appl. Phys. 2007, 102, 074506. [Google Scholar] [CrossRef]
- Hadipour, A.; de Boer, B.; Blom, P.W.M. Organic Tandem and Multi-Junction Solar Cells. Adv. Funct. Mater. 2008, 18, 169–181. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Bi, P.; Ren, J.; Wang, Y.; Yang, Y.; Liu, X.; Zhang, S.; Hou, J. Tandem Organic Solar Cell with 20.2% Efficiency. Joule 2022, 6, 171–184. [Google Scholar] [CrossRef]
- Sha Liu, S.H.; Yuan, J.; Deng, W.; Luo, M.; Xie, Y.; Liang, Q.; Zou, Y.; He, Z.; Wu, H.; Cao, Y. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics 2020, 14, 300–305. [Google Scholar]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Hadipour, A.; De Boer, B.; Blom, P.W.M. Device Operation of Organic Tandem Solar Cells. Org. Electron. 2008, 9, 617–624. [Google Scholar] [CrossRef]
- Sergeant, N.P.; Hadipour, A.; Niesen, B.; Cheyns, D.; Heremans, P.; Peumans, P.; Rand, B.P. Design of Transparent Anodes for Resonant Cavity Enhanced Light Harvesting in Organic Solar Cells. Adv. Mater. 2012, 24, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, A.; Cheyns, D.; Rand, B.P.; Heremans, P. Electrode Considerations for the Optical Enhancement of Organic Bulk Heterojunction Solar cells. Adv. Energy Mater. 2011, 1, 930–935. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, H.; Zhan, X. Comparison of conventional and inverted structures in fullerene-free organic solar cells. J. Energy Chem. 2015, 24, 744–749. [Google Scholar] [CrossRef]
- Salim, M.B.; Nekovei, R.; Jeyakumar, R. Oragnic tandem solar cells with 18.6% efficiency. Sol. Energy 2020, 198, 160–166. [Google Scholar] [CrossRef]
- Li, M.; Gao, K.; Wan, X.; Zhang, Q.; Kan, B.; Xia, R.; Liu, F. Solution-processed organic tandem solar cells with power conversion efficiency > 12%. Nat. Photonics 2017, 11, 85–90. [Google Scholar] [CrossRef]
- Ullah, F.; Chen, C.C.; Choy, W.C.H. Recent developments in organic tandem solar cells toward high efficiency. Adv. Energy Sustain. Res. 2021, 2, 2000050. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef]
- Zuo, L.; Yu, J.; Shi, X.; Lin, F.; Tang, W. High-efficiency nonfullerene organic solar cells with a parallel tandem configuration. Adv. Mater. 2017, 29, 1702547. [Google Scholar] [CrossRef]
- Hadipour, A.; Muller, R.; Heremans, P. Room Temperature Solution-processed electron transport layer for organic solar cells. Org. Electron. 2013, 14, 2379–2386. [Google Scholar] [CrossRef]
- Chen, G.; Ling, Z.; Wei, B.; Zhang, J.; Hong, Z.; Sasabe, H.; Kido, J. Comparison of the solution and vacuum-processed Squaraine:Fullerene small molecule bulk heterojunction solar cells. Front. Chem. 2018, 6, 412. [Google Scholar] [CrossRef]
- Pan, T.; Li, J.; Lin, Y.; Zue, Z.; Di, Z.; Yin, M.; Wang, J.; Lu, L.; Yang, L.; Li, D. Structural and optical studies of molybdenum oxides thin films obtained by thermal evaporation and atomic layer deposition methods for photovoltaic application. J. Mater. Sci. Mater. Electron. 2021, 32, 3475–3486. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Woudenberh, T.; Tanase, C.; Mihailtchi, V.D.; de Boer, B. Charge transport in polymeric opto-electronic devices. Polym. Prepr. 2003, 44, 342. [Google Scholar]
- Mihailtchi, V.D.; Koster, L.J.A.; Hummelen, J.C.; Blom, P.W.M. Photocurrent generation in polymer-fullerene bulk heterojunctions. Phys. Rev. Lett. 2004, 93, 216601. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Mihailtchi, V.D.; Blom, P.W.M. Extraction of photogenerated charge carriers from polymer-fullerene bulk heterojunction solar cells. Proc. SPIE 2004, 5464, 239. [Google Scholar]
- Mihailtchi, V.D.; Wildeman, J.; Blom, P.W.M. Space-charge limited photocurrent. Phys. Rev. Lett. 2005, 94, 126602. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Smits, E.C.P.; Mihailtchi, V.D.; Blom, P.W.M. Device model for the operation of polymer-fullerene bulk heterojunction solar cells. Phys. Rev. B 2005, 72, 085205. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Mehailtchi, V.D.; Blom, P.W.M. Biomolecular recombination in polymer-fullerene bulk heterojunction solar cells. Appl. Phys. Lett. 2006, 88, 052104. [Google Scholar] [CrossRef]
- Ryu, S.; Ha, N.Y.; Ahn, Y.H.; Park, J.; Lee, S. Light intensity dependence of organic solar cell operation and dominance switching between Shockley–Read–Hall and bimolecular recombination losses. Sci. Rep. 2021, 11, 16781. [Google Scholar] [CrossRef] [PubMed]
- Koster, L.J.A.; Mihailtchi, V.D.; Ramaker, R.; Xie, H.; Blom, P.W.M. Light intensity dependence of open-circuit voltage of polymer-fullerene solar cells. Appl. Phys. Lett. 2005, 86, 123509. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Mihailtchi, V.D.; Xie, H.; Blom, P.W.M. Origin of the light intensity dependence of the short-circuit current of polymer-fullerene solar cells. Appl. Phys. Lett. 2005, 87, 203502. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadipour, A. Utilizing Metal Oxide Thin Films for Device Engineering of Solution-Processed Organic Multi-Junction Solar Cells. Coatings 2024, 14, 525. https://doi.org/10.3390/coatings14050525
Hadipour A. Utilizing Metal Oxide Thin Films for Device Engineering of Solution-Processed Organic Multi-Junction Solar Cells. Coatings. 2024; 14(5):525. https://doi.org/10.3390/coatings14050525
Chicago/Turabian StyleHadipour, Afshin. 2024. "Utilizing Metal Oxide Thin Films for Device Engineering of Solution-Processed Organic Multi-Junction Solar Cells" Coatings 14, no. 5: 525. https://doi.org/10.3390/coatings14050525




