Isothermal Oxidation Behavior of Zr-Y Coating on γ-TiAl by Double Glow Plasma Surface Metal Alloying Technique
Abstract
:1. Introduction
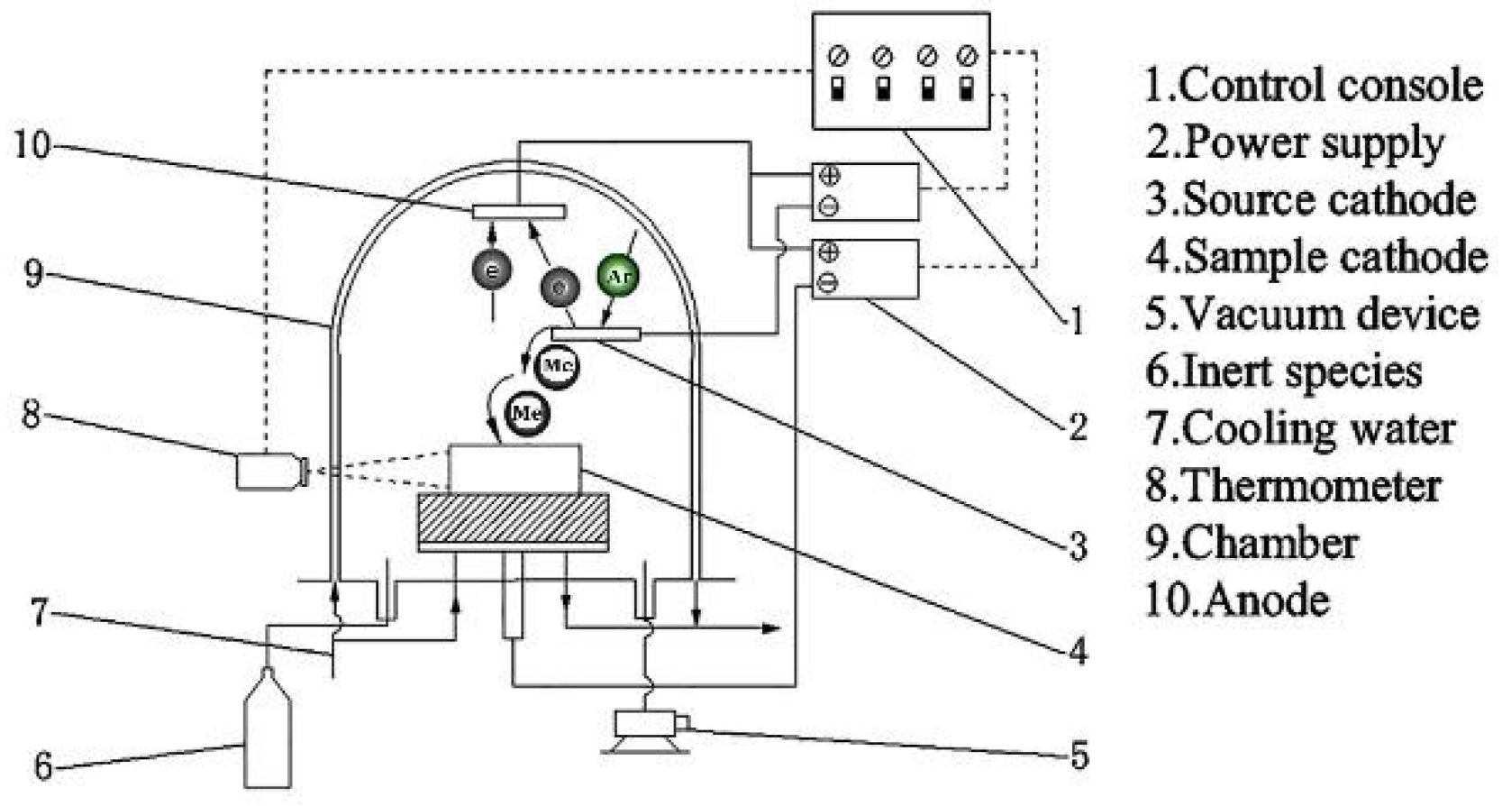
2. Experimental Section
2.1. Specimen Preparation
2.2. Oxidation Resistance Measurement
2.3. Characterization
3. Results and Discussion
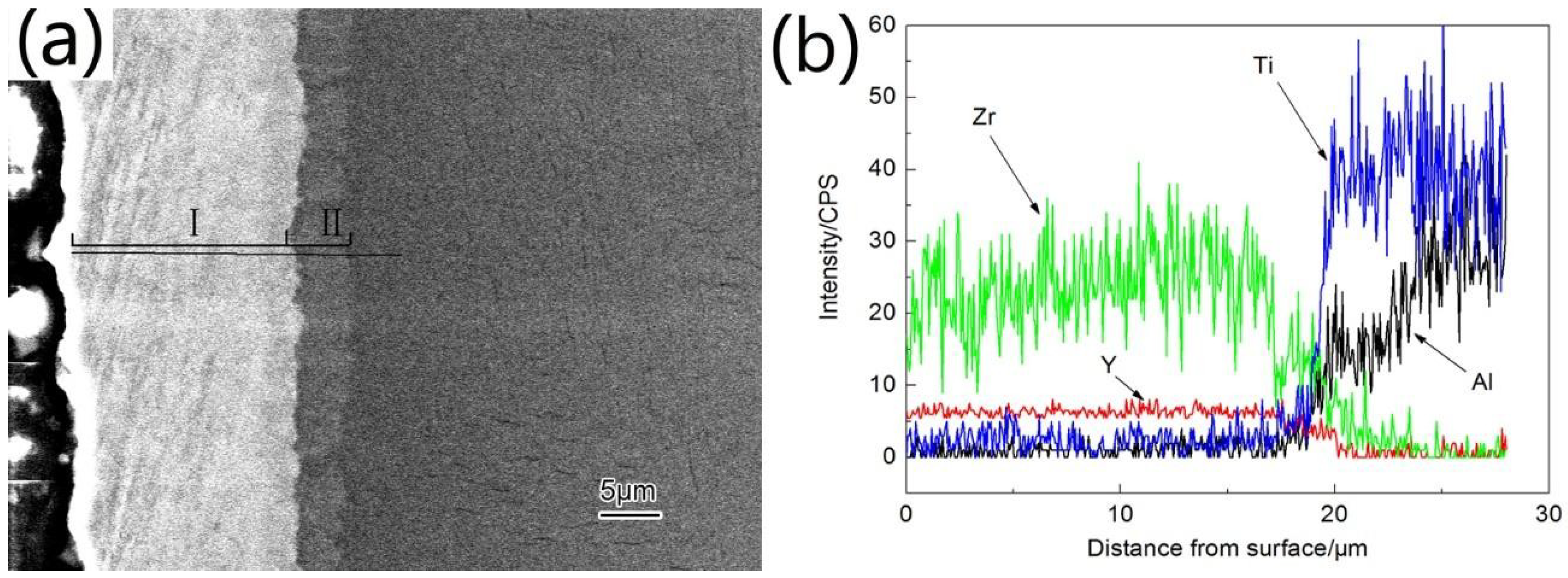
3.1. Microstructure and Phase Distribution of Coatings
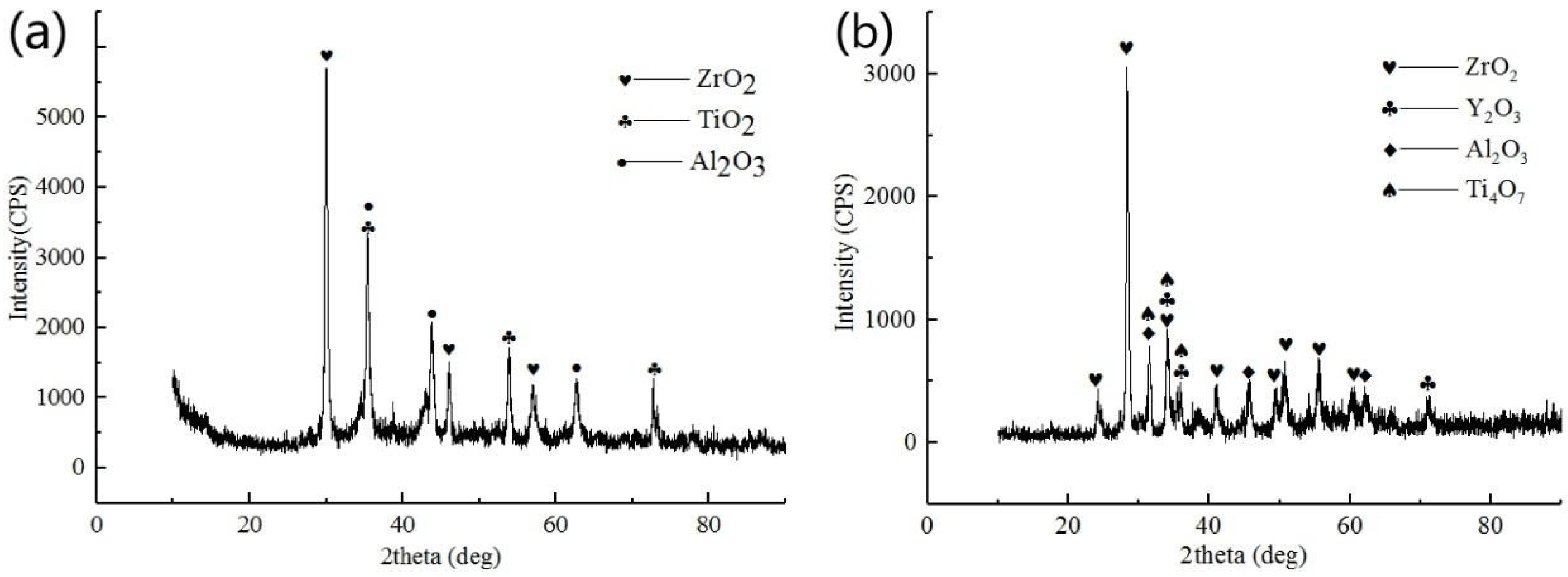
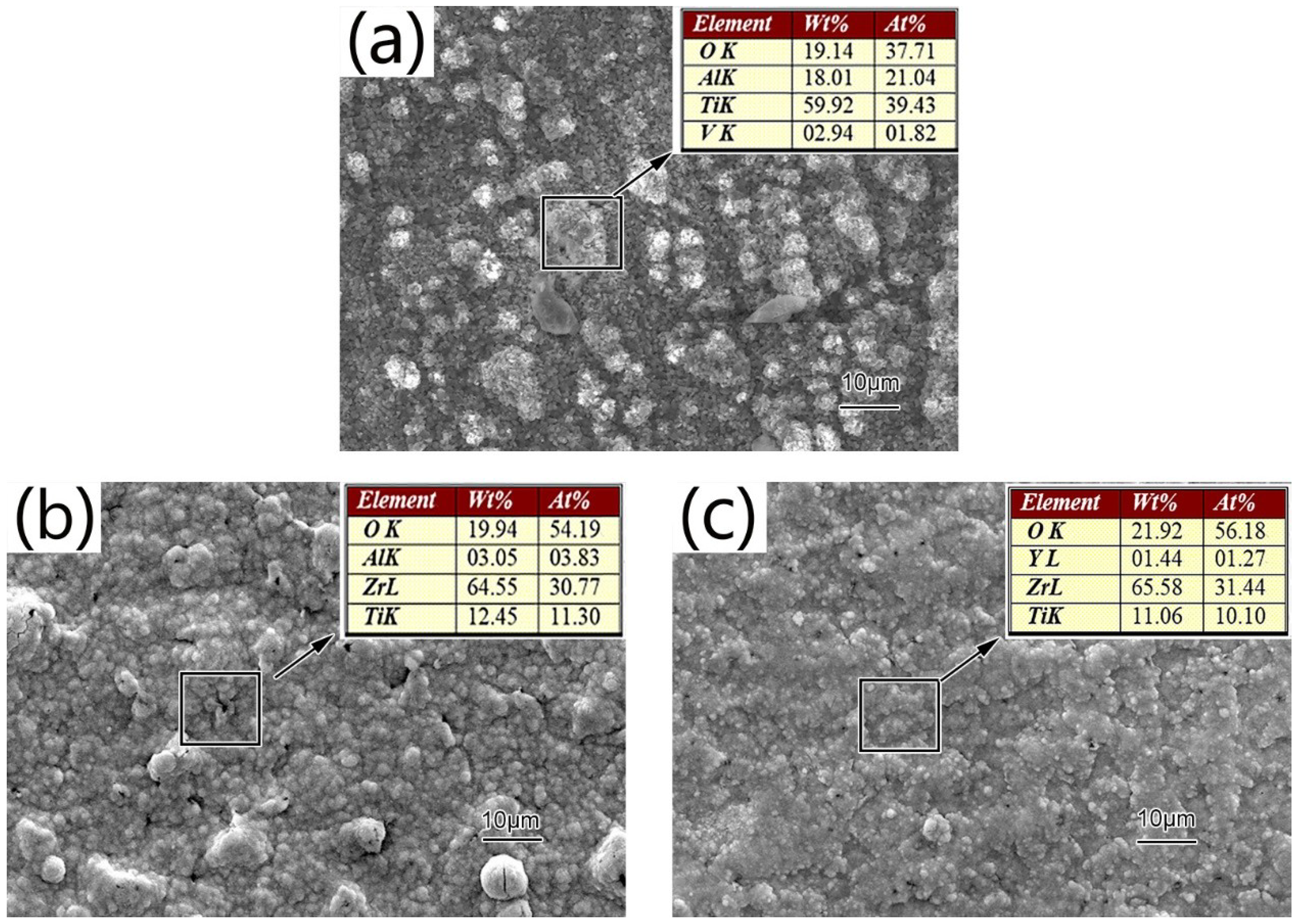
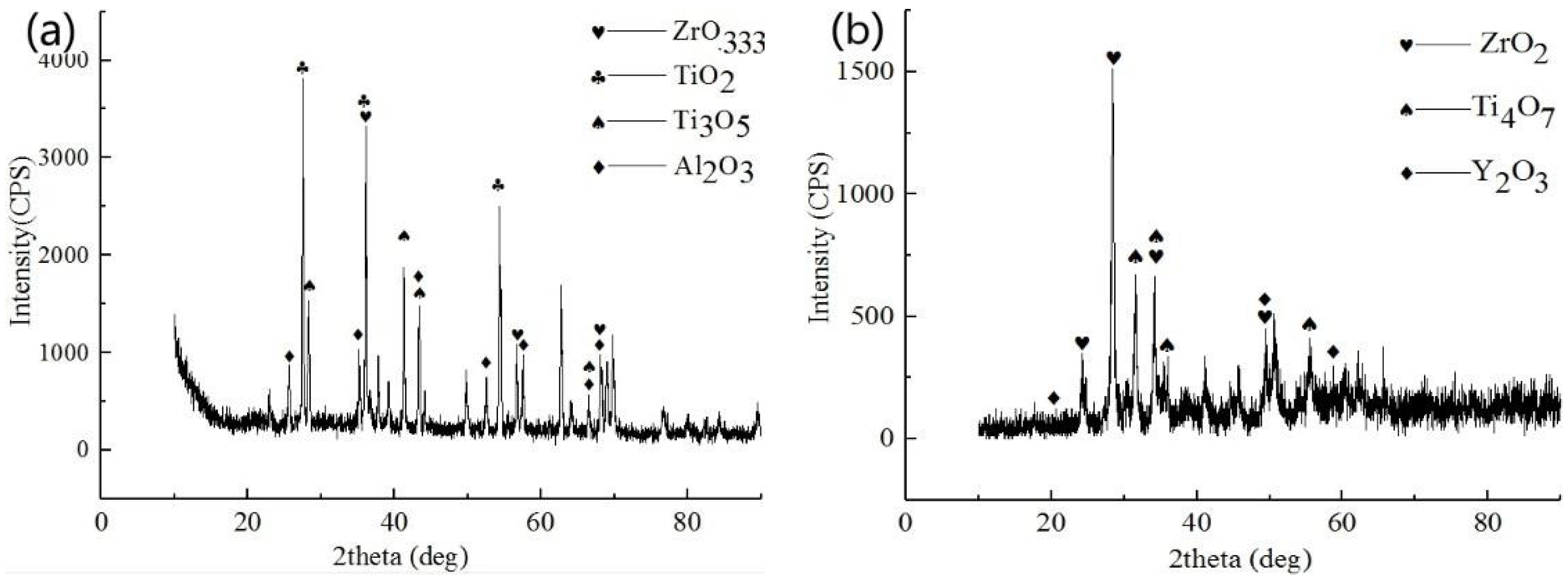
3.2. Structure and Morphologies of the Oxidation Film
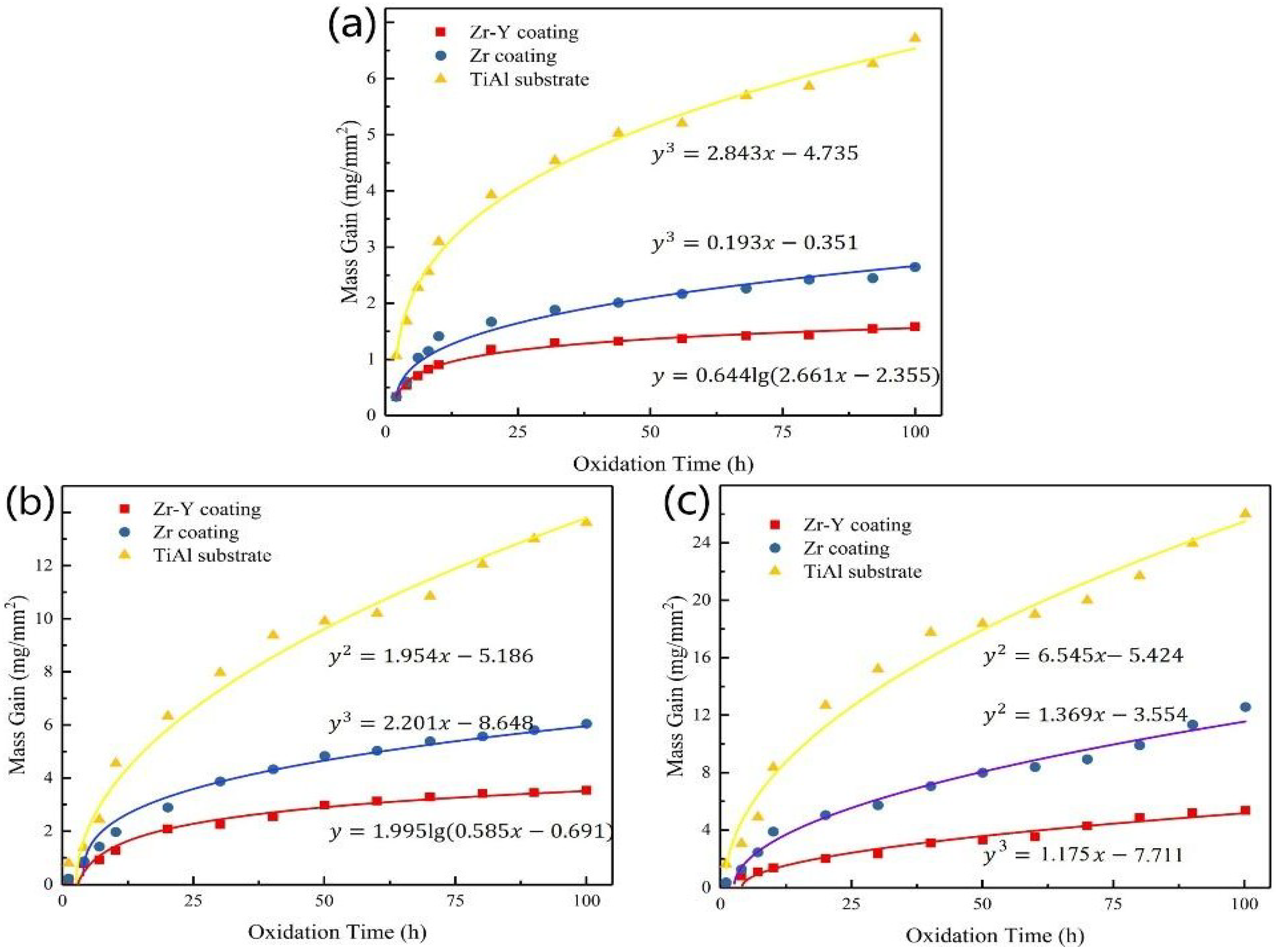
3.3. Isothermal Oxidation Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leyens, C.; Peters, M. Titanium and Titanium Alloys; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Braun, R.; Fröhlich, M.; Leyens, C.; Renusch, D. Oxidation Behaviour of TBC Systems on gamma-TiAl Based Alloy Ti-45Al-8Nb. Oxid. Met. 2009, 71, 295–318. [Google Scholar] [CrossRef]
- Kumpfert, J.; Kim, Y.W.; Dimiduk, D.M. Effect of microstructure on fatigue and tensile properties of the gamma TiAl alloy Ti-46.5Al-3.0Nb-2.1Cr-0.2W. Mater. Sci. Eng. A 1995, 192–193, 465–473. [Google Scholar] [CrossRef]
- Dettenwanger, F.; Schumann, E.; Ruhle, M.; Rakowski, J.; Meier, G. Microstructural Study of Oxidized γ-TiAl. Oxid. Met. 1998, 50, 269–307. [Google Scholar] [CrossRef]
- Yoshihara, M.; Kim, Y.W. Oxidation behavior of gamma alloys designed for high temperature applications. Intermetallics 2005, 13, 952–958. [Google Scholar] [CrossRef]
- Allen, S.M.; Pelloux, R.M.; Widmer, R. Advanced High-Temperature Alloys: Processing and Properties; American Society for Metals: Metals Park, OH, USA, 1986. Available online: http://www.osti.gov/scitech/servlets/purl/6718810 (accessed on 2 October 2018).
- Brady, M.P.; Brindley, W.J.; Smialek, J.L.; Locci, I.E. The oxidation and protection of gamma titanium aluminides. JOM 1996, 48, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Chu, P.K. Progress in direct-current plasma immersion ion implantation and recent applications of plasma immersion ion implantation and deposition. Surf. Coat. Technol. 2013, 229, 2–11. [Google Scholar] [CrossRef]
- Zhang, X.J.; Li, Q.; Zhao, S.Y.; Gao, C.X.; Wang, L.; Zhang, J. Improvement in the oxidation resistance of a γ-TiAl-based alloy by sol-gel derived Al₂O₃ film. Appl. Surf. Sci. 2008, 225, 1860–1864. [Google Scholar] [CrossRef]
- Ait-Djafer, A.Z.; Saoula, N.; Aknouche, H.; Guedouar, B.; Madaoui, N. Deposition and characterization of titanium aluminum nitride coatings prepared by RF magnetron sputtering. Appl. Surf. Sci. 2015, 350, 6–9. [Google Scholar] [CrossRef]
- Brady, M.P.; Smialek, J.L.; Humphrey, D.L.; Smith, J. The role of Cr in promoting protective alumina scale formation by γ-based Ti_Al_Cr alloys—II. Oxidation behavior in air. Acta Mater. 1997, 45, 2371–2382. [Google Scholar] [CrossRef]
- Schiller, S.; Goedicke, K.; Reschke, J.; Kirchhoff, V.; Schneider, S.; Milde, F. Pulsed magnetron sputter technology. Surf. Coat. Technol. 1993, 61, 331–337. [Google Scholar] [CrossRef]
- Thiele, E.S.; Wang, L.S.; Mason, T.O.; Barnett, S.A. Deposition and properties of yttria-stabilized zirconia thin films using reactive direct current magnetron sputtering. J. Vac. Sci. Technol. A Vac. Surf. Films 1991, 9, 3054–3060. [Google Scholar] [CrossRef]
- Musil, J.; Poláková, H. Hard nanocomposite Zr-Y-N coatings, correlation between hardness and structure. Surf. Coat. Technol. 2000, 127, 99–106. [Google Scholar] [CrossRef]
- Salvadori, M.C.; Teixeira, F.S.; Sgubin, L.G.; Cattani, M.; Brown, I.G. Surface modification by metal ion implantation forming metallic nanoparticles in an insulating matrix. Appl. Surf. Sci. 2014, 310, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Conrad, J.R.; Radtke, J.L.; Dodd, R.A.; Worzala, F.J.; Tran, N.C. Plasma source ion-implantation technique for surface modification of materials. J. Appl. Phys. 1987, 62, 4591–4596. [Google Scholar] [CrossRef]
- Luo, X.X.; Yao, Z.J.; Zhang, P.Z.; Miao, Q.; Liang, W.P.; Wei, D.B.; Chen, Y. A study on high temperature oxidation behavior of double glow plasma surface metallurgy Fe-Al-Cr alloyed layer on Q235 steel. Appl. Surf. Sci. 2014, 305, 259–266. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, P.; Yao, Z.; Zhou, J.; Wei, X.; Chen, X. Double glow plasma chromizing of Ti6Al4V alloys: Impact of working time, substrate-target distance, argon pressure and surface temperature of substrate. Vacuum 2015, 121, 81–87. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, Z.; Zhang, G.; He, Z. Surface plasma chromized burn-resistant titanium alloy. Surf. Coat. Technol. 2007, 201, 4884–4887. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Zhang, P.; Zhang, Y.; Zhang, G.; He, Z. Double glow plasma surface alloying and plasma nitriding. Surf. Coat. Technol. 2007, 201, 4822–4825. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Brady, M.P.; Sachenko, P. Effects of Fe on the oxidation/internal nitridation behavior and tensile properties of Cr and oxide dispersion ductilized Cr. Scr. Mater. 2005, 52, 809–814. [Google Scholar] [CrossRef]
- Wanjara, P.; Jahazi, M.; Monajati, H.; Yue, S.; Immarigeon, J.P. Hot working behavior of near-α alloy IMI834. Mater. Sci. Eng. A 2005, 396, 50–60. [Google Scholar] [CrossRef]
- Fuhui, W.; Hanyi, L.; Linxiang, B.; Weitao, W. Hot corrosion of yttrium-modified aluminide coatings. Mater. Sci. Eng. A 1989, 120–121, 387–389. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.G.; Li, W.; Tian, B.; Hu, S.W.; Qin, Q.D. Oxidation behavior of the Y₂O₃-modified aluminide coating on Ti-6Al-4V alloy. Mater. Sci. Eng. A 2007, 458, 34–38. [Google Scholar] [CrossRef]
- Wang, L.D.; Wang, Y.H.; Li, W.Z.; Li, H.D.; Takeo, O.K. Reaction behaviour of ZrO₂/Ti interface injointing zirconia ceramics and stainless steel 304 with Ti foil. Acta Metall. Sin. 1997, 7, 756–762. [Google Scholar]
- Chen, Y.; Kong, F.; Han, J.; Chen, Z.; Tian, J. Influence of yttrium on microstructure, mechanical properties and deformability of Ti-43Al-9V alloy. Intermetallics 2005, 13, 263–266. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, G.M.; Kim, C.J. Oxidation behavior of TiAl-X (X = Cr, V, Si, Mo or Nb) intermetallics at elevated temperature. Scr. Metall. Mater. 1995, 33, 1117–1125. [Google Scholar] [CrossRef]








| Ti | Al | V | Cr | Nb | O | C | N |
|---|---|---|---|---|---|---|---|
| The rest | 46.5 | ≤1.5 | ≤1 | ≤0.20 | ≤0.01 | ≤0.1 | ≤0.05 |
| Parameters | Value |
|---|---|
| Source voltage/V | 600–650 |
| Cathode voltage/V | 450–500 |
| Processing time/h | 3 |
| Work-piece pressure/Pa | 36 |
| Processing temperature/°C | 900–950 |
| Distance between source and cathode/mm | 18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, F.; Zhang, P.; Wei, D.; Chen, X.; Wang, S.; Wang, Z.; Zhu, Y. Isothermal Oxidation Behavior of Zr-Y Coating on γ-TiAl by Double Glow Plasma Surface Metal Alloying Technique. Coatings 2018, 8, 361. https://doi.org/10.3390/coatings8100361
Ding F, Zhang P, Wei D, Chen X, Wang S, Wang Z, Zhu Y. Isothermal Oxidation Behavior of Zr-Y Coating on γ-TiAl by Double Glow Plasma Surface Metal Alloying Technique. Coatings. 2018; 8(10):361. https://doi.org/10.3390/coatings8100361
Chicago/Turabian StyleDing, Feng, Pingze Zhang, Dongbo Wei, Xiaohu Chen, Shiyuan Wang, Zhangzhong Wang, and Yimin Zhu. 2018. "Isothermal Oxidation Behavior of Zr-Y Coating on γ-TiAl by Double Glow Plasma Surface Metal Alloying Technique" Coatings 8, no. 10: 361. https://doi.org/10.3390/coatings8100361





