Growth without Postannealing of Monoclinic VO2 Thin Film by Atomic Layer Deposition Using VCl4 as Precursor
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Acknowledgment
Conflicts of Interest
References
- Morin, F.J. Oxides which show a metal-to-insulator transition at the Neel temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Barker, A.S.; Verleur, H.W.; Guggenheim, H.J. Infrared optical properties of vanadium dioxide above and below the transition temperature. Phys. Rev. Lett. 1966, 17, 1286–1289. [Google Scholar] [CrossRef]
- Park, J.H.; Coy, J.M.; Kasirga, T.S.; Huang, C.; Fei, Z.; Hunter, S.; Cobdem, D.H. Measurement of a solid-state triple point at the metal-insulator transition in VO2. Nature 2013, 500, 431–434. [Google Scholar] [CrossRef] [PubMed]
- O’Callahan, B.T.; Jones, A.C.; Park, J.H.; Cobden, D.H.; Atkin, J.M.; Raschke, M.B. Inhomogeneity of the ultrafast insulator-to-metal transition dynamics of VO2. Nat. Commun. 2015, 6, 6849. [Google Scholar] [CrossRef] [PubMed]
- Cueff, S.; Li, D.; Zhou, Y.; Wong, F.J.; Kurvits, J.A.; Ramanathan, S.; Zia, R. Dynamic control of light emission faster than the lifetime limit using VO2 phase-change. Nat. Commun. 2015, 6, 8636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ko, C.; Ramanathan, S. Oxide electronics utilizing ultrafast metal-insulator transitions. Annu. Rev. Mater. Res. 2011, 41, 337–367. [Google Scholar] [CrossRef]
- Nakano, M.; Shibuya, K.; Okuyama, D.; Hatano, T.; Ono, S.; Kawasaki, M.; Iwasa, Y.; Tokura, Y. Collective bulk carrier delocalization driven by electrostatic surface charge accumulation. Nature 2012, 487, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, S.; Zeng, X.T.; Magdassi, S.; Long, Y. Mg/W-codoped vanadium dioxide thin films with enhanced visible transmittance and low phase transition temperature. J. Mater. Chem. C 2015, 3, 6771–6777. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, I.S.; Lauhon, L.J. Stoichiometry engineering of monoclinic to rutile phase transition in suspended single crystalline vanadium dioxide nanobeams. Nano Lett. 2011, 11, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Petraru, A.; Soni, R.; Kohlstedt, H. Voltage controlled biaxial strain in VO2 films grown on 0.72Pb(Mg1/3Nb2/3)-0.28PbTiO3 crystals and its effect on the transition temperature. Appl. Phys. Lett. 2014, 105, 092902. [Google Scholar] [CrossRef]
- Jian, J.; Chen, A.; Zhang, W.; Wang, H. Sharp semiconductor-to-metal transition of VO2 thin films on glass substrates. J. Appl. Phys. 2013, 114, 244301. [Google Scholar] [CrossRef]
- Jeong, J.; Aetukuri, N.; Graf, T.; Schladt, T.D.; Samant, M.G.; Parkin, S.S.P. Suppression of metal-insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science 2013, 339, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Aetukuri, N.B.; Gary, A.X.; Drouard, M.; Cossale, M.; Gao, L.; Reid, A.H.; Kukreja, R.; Ohldag, H.; Jenkins, C.A.; Arenholz, E.; et al. Control of the metal–insulator transition in vanadium dioxide by modifying orbital occupancy. Nat. Phys. 2013, 9, 661–666. [Google Scholar] [CrossRef]
- Wu, J.M.; Liou, L.B. Room temperature photo-induced phase transitions of VO2 nanodevices. J. Mater. Chem. 2011, 21, 5499–5504. [Google Scholar] [CrossRef]
- Xu, F.; Cao, X.; Luo, H.; Jin, P. Recent advances in VO2-based thermochromic composites for smart windows. J. Mater. Chem. C 2018, 6, 1903–1919. [Google Scholar] [CrossRef]
- Shukla, N.; Parihar, A.; Freeman, E.; Paik, H.; Stone, G.; Narayanan, V.; Wen, H.; Cai, Z.; Gopalan, V.; Engel-Herbert, R.; et al. Synchronized charge oscillations in correlated electron systems. Sci. Rep. 2014, 4, 4964. [Google Scholar] [CrossRef]
- Ruzmetov, D.; Gopalakrishnan, G.; Ko, C.; Narayanamurti, V.; Ramanathan, S. Three-terminal field effect devices utilizing thin film vanadium oxide as the channel layer. J. Appl. Phys. 2010, 107, 114516. [Google Scholar] [CrossRef] [Green Version]
- Yajima, T.; Nishimura, T.; Toriumi, A. Positive-bias gate-controlled metal–insulator transition in ultrathin VO2 channels with TiO2 gate dielectrics. Nat. Commun. 2015, 6, 10104. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.M.; Pryce, I.M.; Atwater, H.A. Compact silicon photonic waveguide modulator based on the vanadium dioxide metal-insulator phase transition. Opt. Express 2010, 18, 11192–11201. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Falk, A.; Wu, J.Q.; Ouyang, L.; Park, H. Current-driven phase oscillation and domain-wall propagation in WxV1-xO2 nanobeams. Nano Lett. 2007, 7, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Dicken, M.J.; Aydin, K.; Pryce, I.M.; Sweatlock, L.A.; Boyd, E.M.; Walavalkar, S.; Ma, J.; Atwater, H.A. Frequency tunable near-infrared metamaterials based on VO2 phase transition. Opt. Express 2009, 17, 18330–18339. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.; Kim, H.T.; Chae, B.G.; Di Ventra, M.; Basov, D.N. Phase-transition driven memristive system. Appl. Phys. Lett. 2009, 95, 043503. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Lee, Y.W.; Chae, B.G.; Yun, S.J.; Oh, S.Y.; Kim, H.T. Temperature dependence of the first-order metal-insulator transition in VO2 and programmable critical temperature sensor. Appl. Phys. Lett. 2007, 90, 023515. [Google Scholar] [CrossRef]
- Strelcov, E.; Lilach, Y.; Kolmakov, A. Gas sensor based on metal-insulator transition in VO2 nanowire thermistor. Nano Lett. 2009, 9, 2322–2326. [Google Scholar] [CrossRef] [PubMed]
- Heckman, E.M.; Gonzalez, L.P.; Guha, S.; Barnes, J.O.; Carpenter, A. Electrical and optical switching properties of ion implanted VO2 thin films. Thin Solid Films 2009, 518, 265–268. [Google Scholar] [CrossRef]
- Ureña-Begara, F.; Crunteanu, A.; Raskin, J.P. Raman and XPS characterization of vanadium oxide thin films with temperature. Appl. Surf. Sci. 2017, 403, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Chiu, T.W.; Tonooka, K.; Kikuchi, N. Growth of b-axis oriented VO2 thin films on glass substrates using ZnO buffer layer. Appl. Surf. Sci. 2010, 256, 6834–6837. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, K.; Deng, Q.; You, Q.; Zhang, J.; Wu, J.; Hu, Z.; Chu, J. Manipulations from oxygen partial pressure on the higher energy electronic transition and dielectric function of VO2 films during a metal-insulator transition process. J. Mater. Chem. C 2015, 3, 5033–5040. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zhang, L.; Mukherjee, D.; Zheng, Y.X.; Haislmaier, R.C.; Alem, N.; Engel-Herbert, R. Wafer-scale growth of VO2 thin films using a combinatorial approach. Nat. Commun. 2015, 6, 8475. [Google Scholar] [CrossRef] [PubMed]
- Vernardou, D.; Pemble, M.E.; Sheel, D.W. The growth of thermochromic VO2 films on glass by atmospheric-pressure CVD: A comparative study of precursors, CVD methodology, and substrates. Chem. Vap. Depos. 2006, 12, 263–274. [Google Scholar] [CrossRef]
- Vernardou, D.; Paterakis, P.; Drosos, H.; Spanakis, E.; Povey, I.M.; Pemble, M.E.; Koudoumas, E.; Katsarakis, N. A study of the electrochemical performance of vanadium oxide thin films grown by atmospheric pressure chemical vapour deposition. Sol. Energy Mater. Sol. Cells 2011, 95, 2842–2847. [Google Scholar] [CrossRef]
- Vernardou, D. Using an atmospheric pressure chemical vapor deposition process for the development of V2O5 as an electrochromic material. Coatings 2017, 7, 24. [Google Scholar] [CrossRef]
- Makarevich, A.M.; Sadykov, I.I.; Sharovarov, D.I.; Amelichev, V.A.; Adamenkov, A.A.; Tsymbarenko, D.M.; Plokhih, A.V.; Esaulkov, M.N.; Solyankin, P.M.; Kaul, A.R. Chemical synthesis of high quality epitaxial vanadium dioxide films with sharp electrical and optical switch properties. J. Mater. Chem. C 2015, 3, 9197–9205. [Google Scholar] [CrossRef]
- Blackburn, B.; Powell, M.J.; Knapp, C.E.; Bear, J.C.; Carmalt, C.J.; Parkin, I.P. [{VOCl2(CH2(COOEt)2)}4] as a molecular precursor for thermochromic monoclinic VO2 thin films and nanoparticles. J. Mater. Chem. C 2016, 4, 10453–10463. [Google Scholar] [CrossRef]
- Rampelberg, G.; Schaekers, M.; Martens, K.; Xie, Q.; Deduytsche, D.; De Schutter, B.; Blasco, N.; Kittl, J.; Detavernier, C. Semiconductor-metal transition in thin VO2 films grown by ozone based atomic layer deposition. Appl. Phys. Lett. 2011, 98, 162902. [Google Scholar] [CrossRef]
- Premkumar, P.A.; Toeller, M.; Radu, I.P.; Adelmann, C.; Schaekers, M.; Meersschaut, J.; Conard, T.; Elshocht, S.V. Process study and characterization of VO2 thin films synthesized by ALD using TEMAV and O3 precursors. ECS J. Solid State Sci. Technol. 2012, 1, P169–P174. [Google Scholar] [CrossRef]
- Tangirala, M.; Zhang, K.; Nminibapiel, D.; Pallem, V.; Dussarrat, C.; Cao, W.; Adam, T.N.; Johnson, C.S.; Elsayed-Ali, H.E.; Baumgart, H. Physical analysis of VO2 films grown by atomic layer deposition and RF magnetron sputtering. ECS J. Solid State Sci. Technol. 2014, 3, N89–N94. [Google Scholar] [CrossRef]
- Cerbu, F.; Chou, H.S.; Radu, I.P.; Martens, K.; Peter, A.P.; Afanas’ev, V.V.; Stesmans, A. Band alignment and effective work function of atomic-layer deposited VO2 and V2O5 films on SiO2 and Al2O3. Phys. Status Solidi C 2015, 12, 238–241. [Google Scholar] [CrossRef]
- Zhang, K.; Tangirala, M.; Nminibapiel, D.; Cao, W.; Pallem, V.; Dussarrat, C.; Baumgart, H. Synthesis of VO2 thin films by atomic layer deposition with TEMAV as precursor. ECS Trans. 2013, 50, 175–182. [Google Scholar] [CrossRef]
- Blanquart, T.; Niinistö, J.; Gavagnin, M.; Longo, V.; Heikkilä, M.; Puukilainen, E.; Pallem, V.R.; Dussarrat, C.; Ritala, M.; Leskelä, M. Atomic layer deposition and characterization of vanadium oxide thin films. RSC Adv. 2013, 3, 1179–1185. [Google Scholar] [CrossRef]
- Kozen, A.C.; Joress, H.; Currie, M.; Anderson, V.R.; Eddy, C.R., Jr.; Wheeler, V.D. Structural characterization of atomic layer deposited vanadium dioxide. J. Phys. Chem. C 2017, 121, 19341–19347. [Google Scholar] [CrossRef]
- Park, H.H.; Larrabee, T.J.; Ruppalt, L.B.; Culbertson, J.C.; Prokes, S.M. Tunable electrical properties of vanadium oxide by hydrogen-plasma-treated atomic layer deposition. ACS Omega 2017, 2, 1259–1264. [Google Scholar] [CrossRef]
- Musschoot, J.; Deduytsche, D.; Poelman, H.; Haemers, J.; Van Meirhaeghe, R.L.; Van den Berghe, S.; Detavernier, C. Comparison of thermal and plasma-enhanced ALD/CVD of vanadium pentoxide. J. Electrochem. Soc. 2009, 156, P122–P126. [Google Scholar] [CrossRef]
- Boukhalfa, S.; Evanoff, K.; Yushin, G. Atomic layer deposition of vanadium oxide on carbon nanotubes for high-power supercapacitor electrodes. Energy Environ. Sci. 2012, 5, 6872–6879. [Google Scholar] [CrossRef]
- Singh, T.; Wang, S.; Aslam, N.; Zhang, H.; Hoffmann-Eifert, S.; Mathur, S. Atomic layer deposition of transparent VOx thin films for resistive switching applications. Chem. Vap. Depos. 2014, 20, 291–297. [Google Scholar] [CrossRef]
- Daubert, J.S.; Lewis, N.P.; Gotsch, H.N.; Mundy, J.Z.; Monroe, D.N.; Dickey, E.C.; Losego, M.D.; Parsons, G.N. Effect of meso- and micro-porosity in carbon electrodes on atomic layer deposition of pseudocapacitive V2O5 for high performance supercapacitors. Chem. Mater. 2015, 27, 6524–6534. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Golabkan, V.; Pereira-Ramos, J.P.; Mantoux, A.; Lincot, D. A Raman study of the lithium insertion process in vanadium pentoxide thin films deposited by atomic layer deposition. J. Raman Spectrosc. 2002, 33, 631–638. [Google Scholar] [CrossRef]
- Badot, J.C.; Mantoux, A.; Baffier, N.; Dubrunfaut, O.; Lincot, D. Electrical properties of V2O5 thin films obtained by atomic layer deposition (ALD). J. Mater. Chem. 2004, 14, 3411–3415. [Google Scholar] [CrossRef]
- Chen, X.; Pomerantseva, E.; Banerjee, P.; Gregorczyk, K.; Ghodssi, R.; Rubloff, G. Ozone-based atomic layer deposition of crystalline V2O5 films for high performance electrochemical energy storage. Chem. Mater. 2012, 24, 1255–1261. [Google Scholar] [CrossRef]
- Badot, J.C.; Ribes, S.; Yousfi, E.B.; Vivier, V.; Pereira-Ramos, J.P.; Baffier, N.; Lincot, D. Atomic layer epitaxy of vanadium oxide thin films and electrochemical behavior in presence of lithium ions. Electrochem. Solid-State Lett. 2000, 3, 485–488. [Google Scholar] [CrossRef]
- Kim, H.; Maeng, W.J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Films 2009, 517, 2563–2580. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Gelde, L.; Cuevas, A.L.; Martínez de Yuso, M.D.V.; Benavente, J.; Vega, V.; Gonzalez, A.S.; Prida, V.M.; Hernando, B. Influence of TiO2-coating layer on nanoporous alumina membranes by ALD technique. Coatings 2018, 8, 60. [Google Scholar] [CrossRef]
- Cheng, H.E.; Lee, W.J. Properties of TiN films grown by atomic-layer chemical vapor deposition with a modified gaseous-pulse sequence. Mater. Chem. Phys. 2006, 97, 315–320. [Google Scholar] [CrossRef]
- Lee, W.J.; Hon, M.H. Space-limited crystal growth mechanism of TiO2 films by atomic layer deposition. J. Phys. Chem. C 2010, 114, 6917–6921. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Dai, J.; Yang, J.; Wu, Z.; Wei, S.; Xie, Y. Direct hydrothermal synthesis of monoclinic VO2(M) single-domain nanorods on large scale displaying magnetocaloric effect. J. Mater. Chem. 2011, 21, 4509–4517. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Martin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron. Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Hryha, E.; Rutqvist, E.; Nyborg, L. Stoichiometric vanadium oxides studied by XPS. Surf. Interface Anal. 2012, 44, 1022–1025. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, W.; Zhang, P. Hole-lattice coupling and photoinduced insulator-metal transition in VO2. Phys. Rev. B 2013, 88, 035119. [Google Scholar] [CrossRef]
- Zaghrioui, M.; Sakai, J.; Azhan, N.H.; Su, K.; Okimura, K. Polarized Raman scattering of large crystalline domains in VO2 films on sapphire. Vib. Spectrosc. 2015, 80, 79–85. [Google Scholar] [CrossRef]
- Shibuya, K.; Sawa, A. Polarized Raman scattering of epitaxial vanadium dioxide films with low-temperature monoclinic phase. J. Appl. Phys. 2017, 122, 015307. [Google Scholar] [CrossRef]
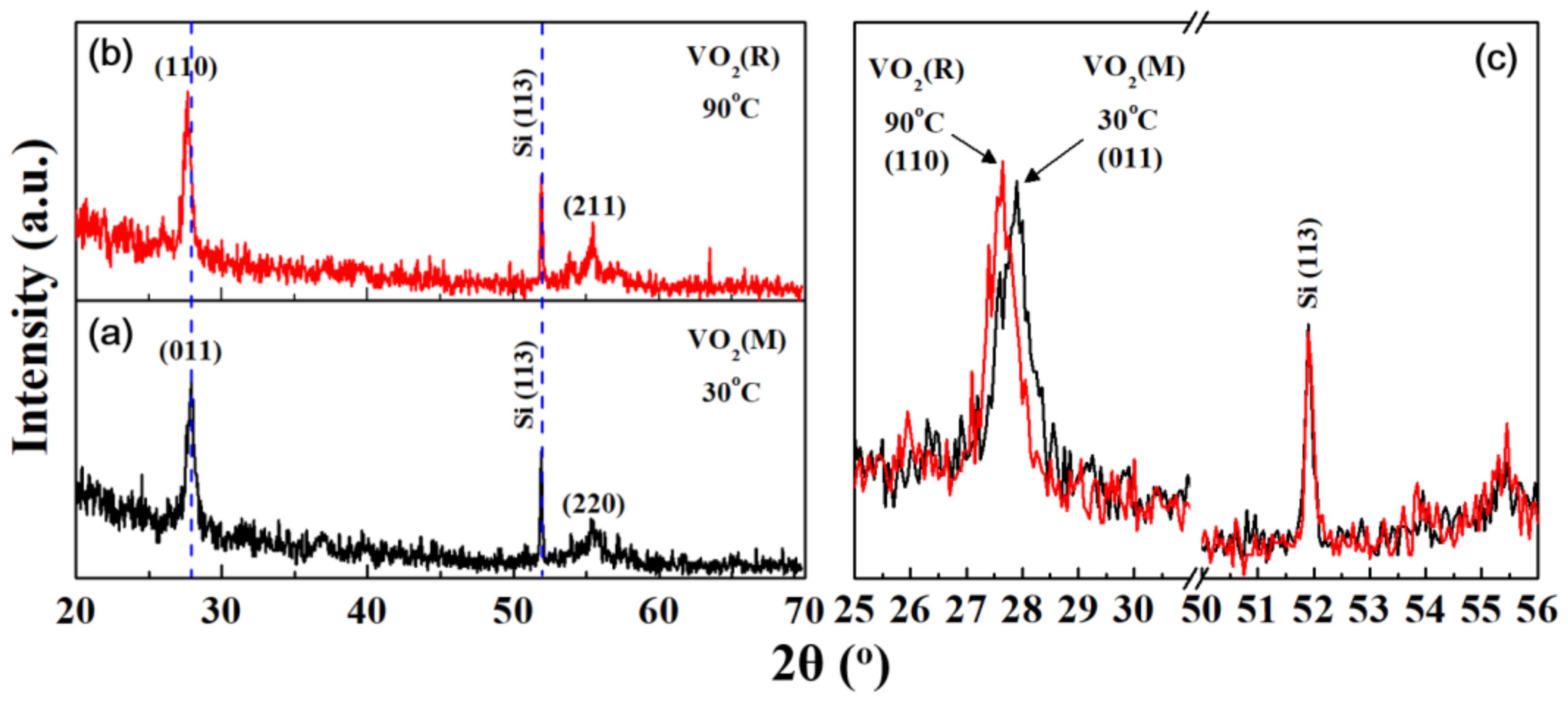
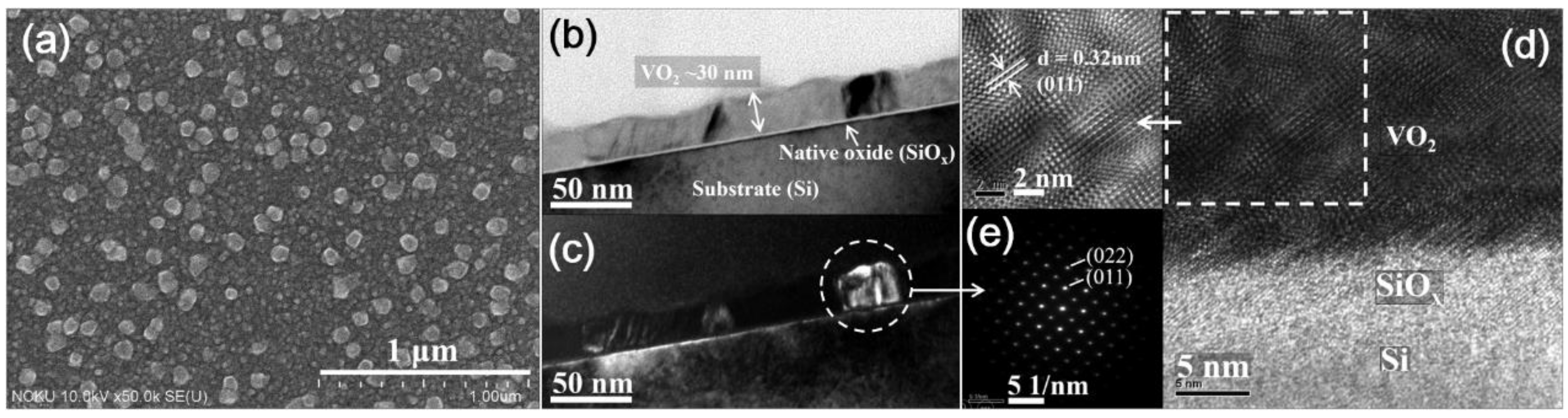
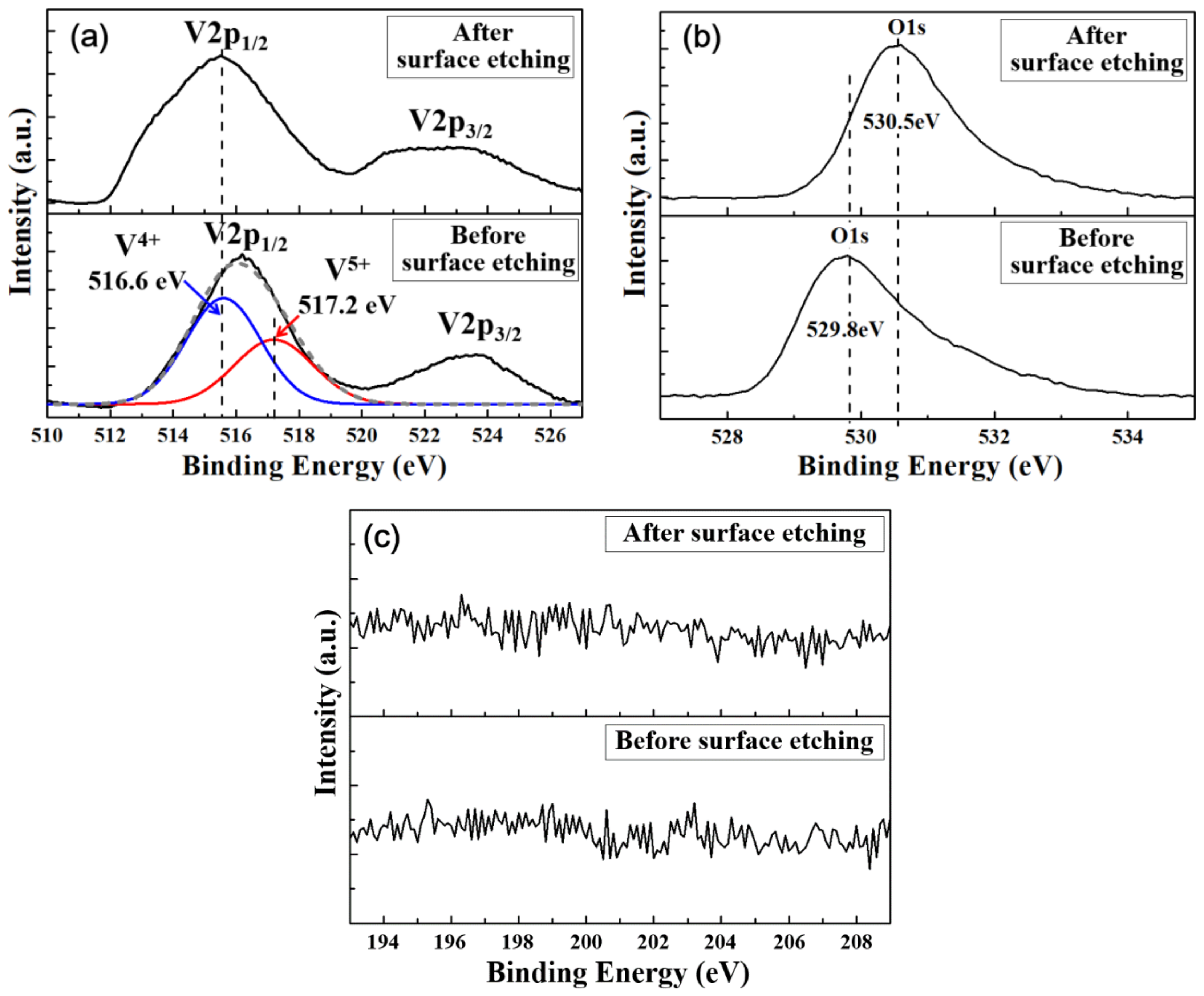
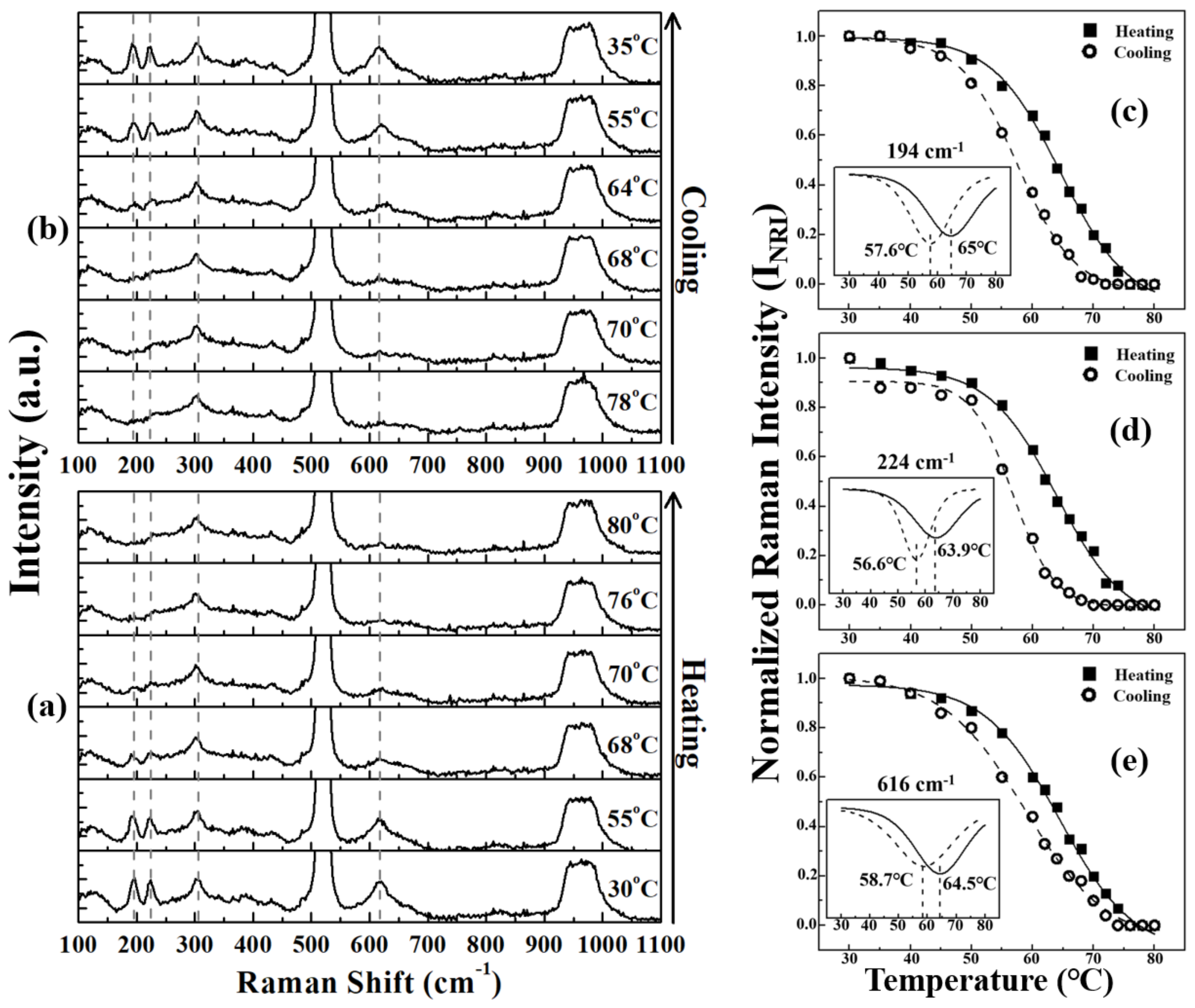
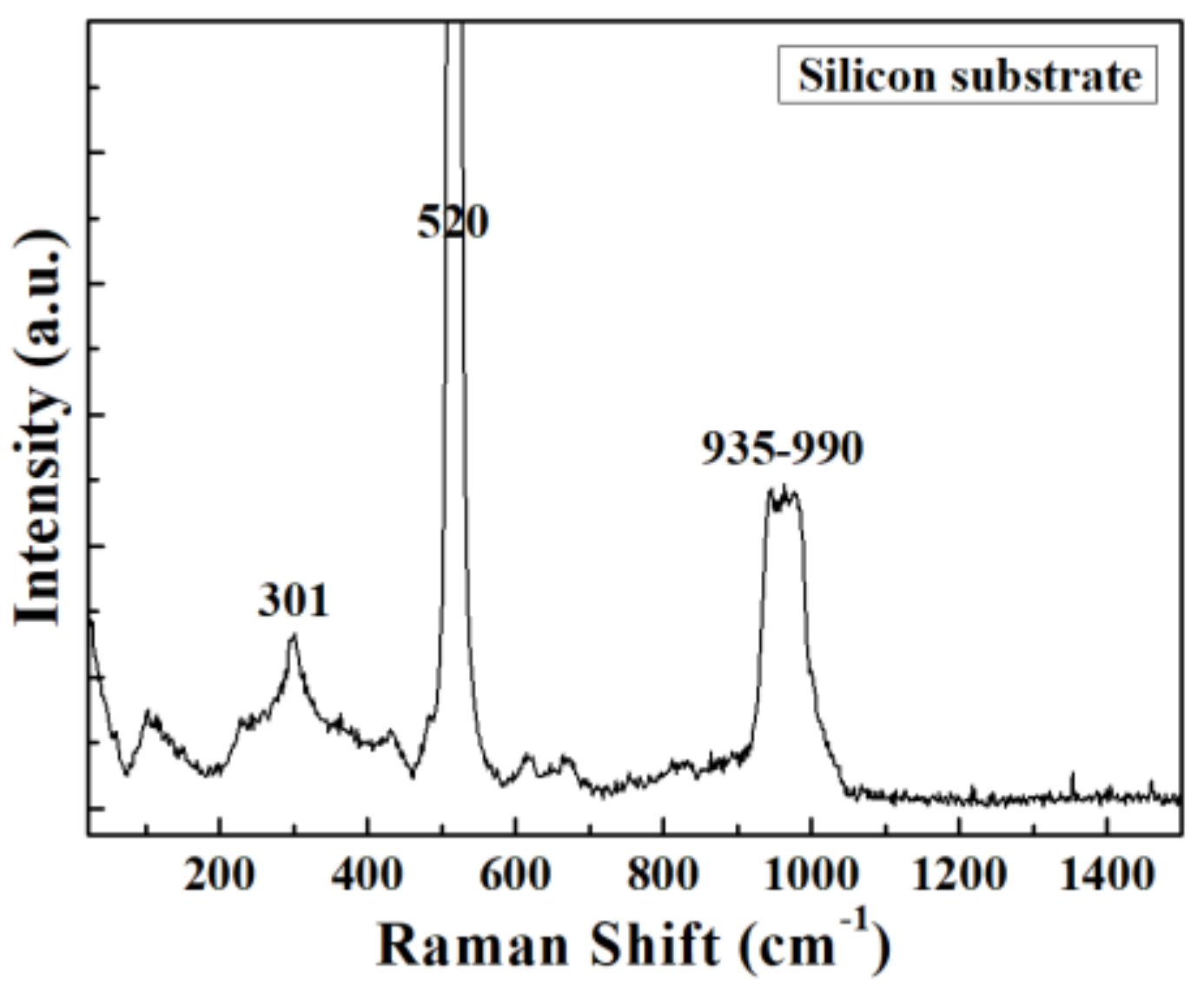
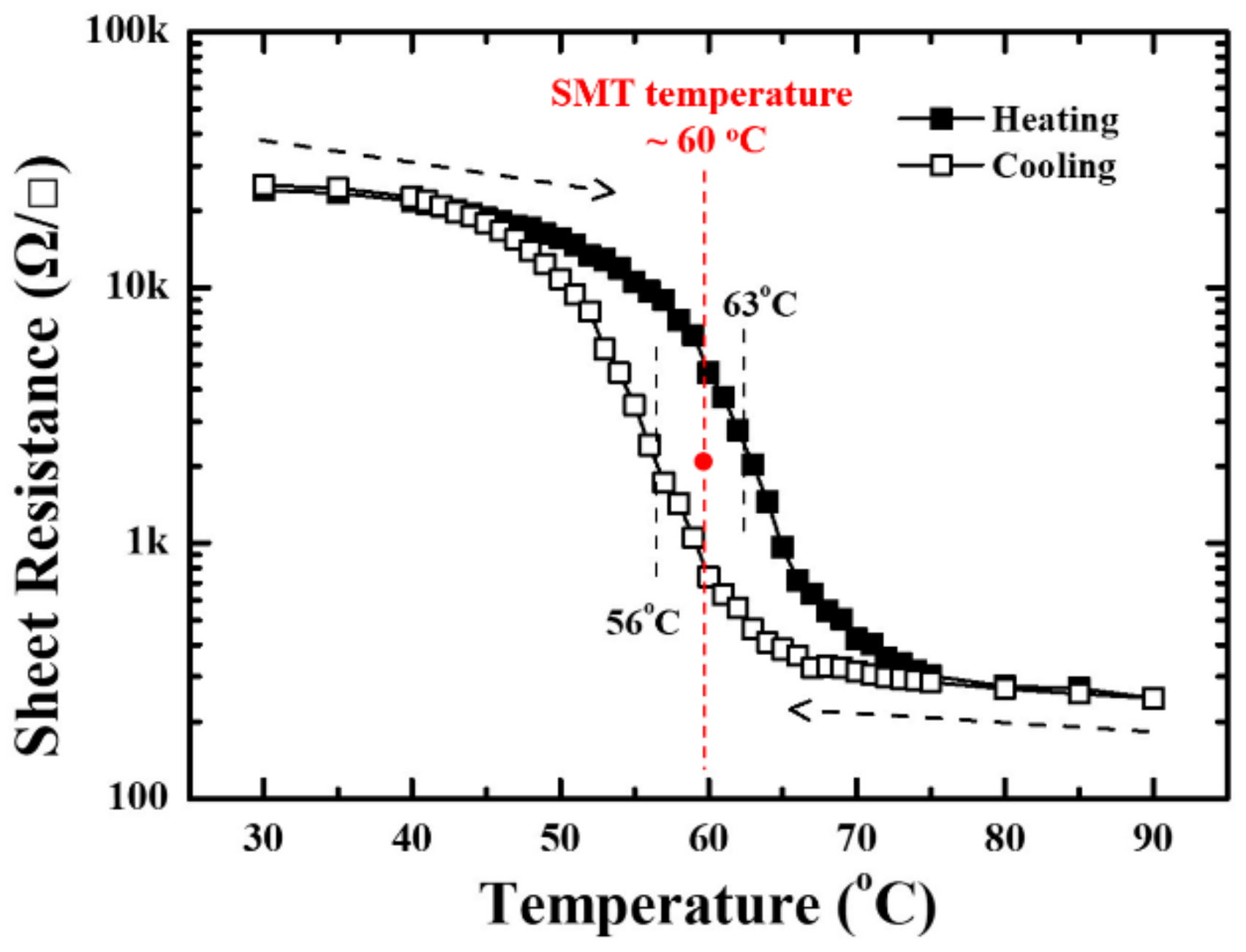
| Elemental Content | Before Surface Etching | After Surface Etching |
|---|---|---|
| V (at.%) | 25.7 | 33.1 |
| O (at.%) | 74.3 | 66.9 |
| Cl (at.%) | <0.1 | <0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-J.; Chang, Y.-H. Growth without Postannealing of Monoclinic VO2 Thin Film by Atomic Layer Deposition Using VCl4 as Precursor. Coatings 2018, 8, 431. https://doi.org/10.3390/coatings8120431
Lee W-J, Chang Y-H. Growth without Postannealing of Monoclinic VO2 Thin Film by Atomic Layer Deposition Using VCl4 as Precursor. Coatings. 2018; 8(12):431. https://doi.org/10.3390/coatings8120431
Chicago/Turabian StyleLee, Wen-Jen, and Yong-Han Chang. 2018. "Growth without Postannealing of Monoclinic VO2 Thin Film by Atomic Layer Deposition Using VCl4 as Precursor" Coatings 8, no. 12: 431. https://doi.org/10.3390/coatings8120431





