Luminescence Properties of Co-Doped Eu3+, Bi3+ Lu2O3/Polyvinylpyrrolidone Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results
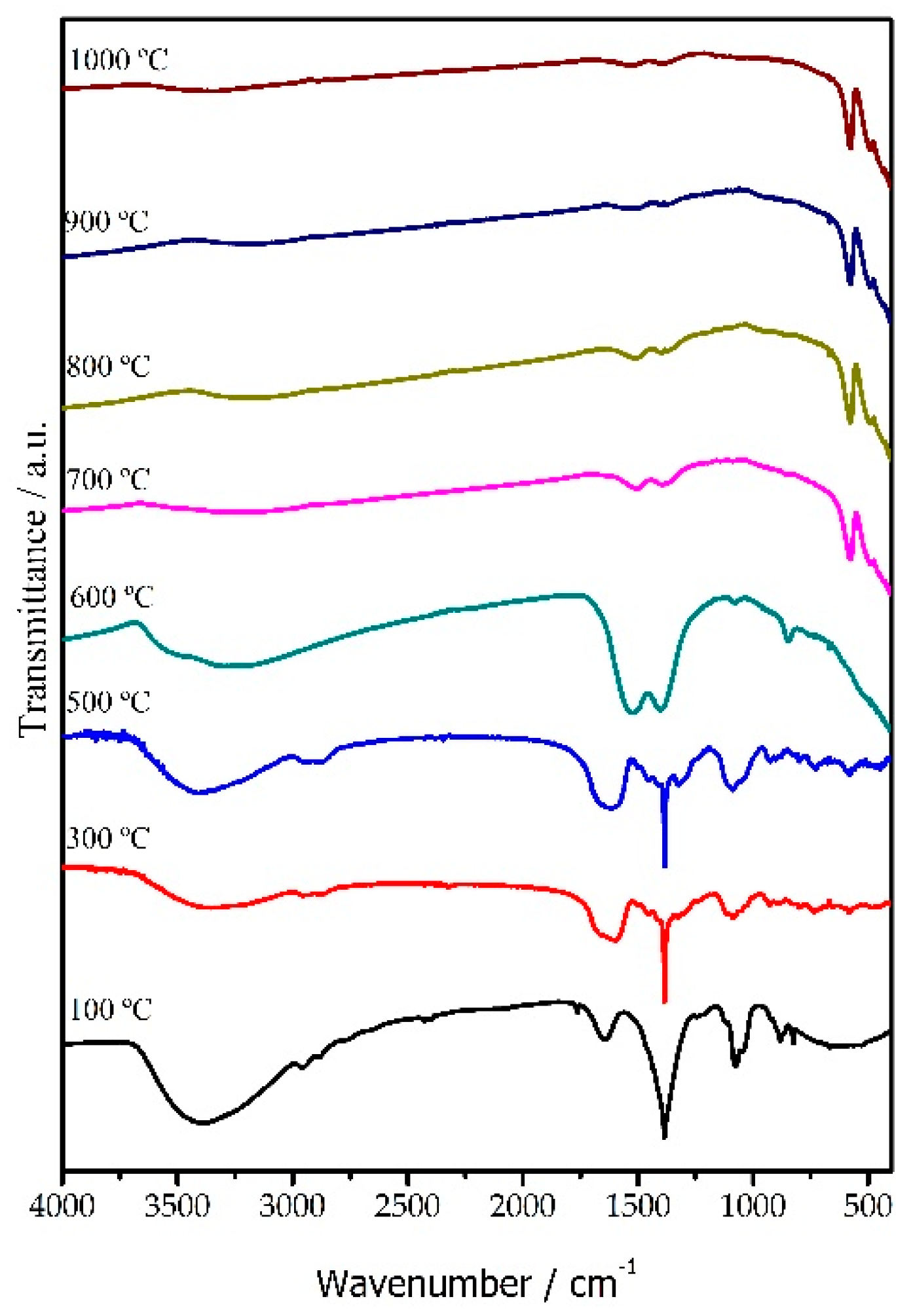
3.1. Structural Studies
3.2. Morphology of Thin Films
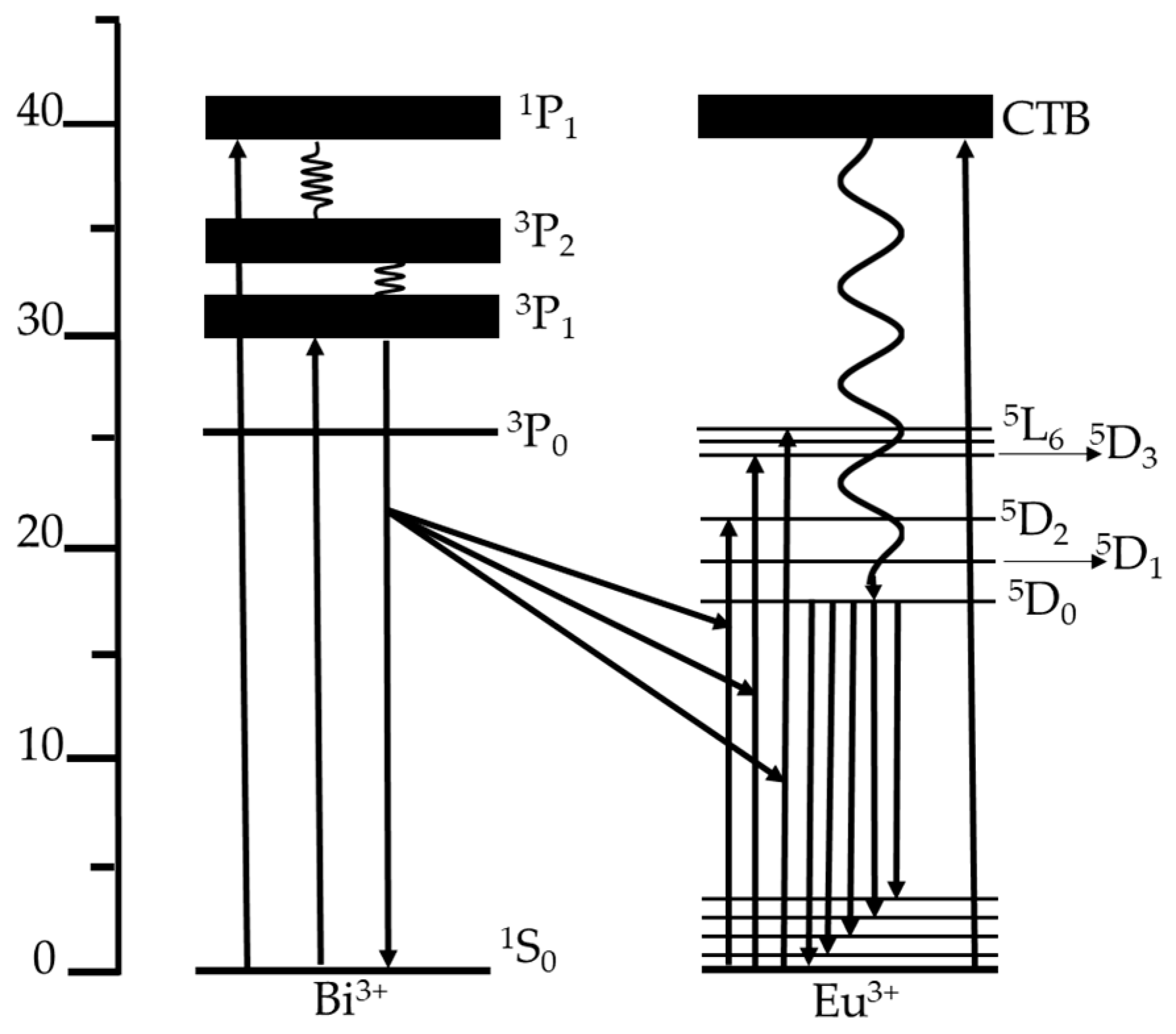
3.3. Luminescence Results and Comission Internationale de l’Éclairage (CIE) Chromaticity
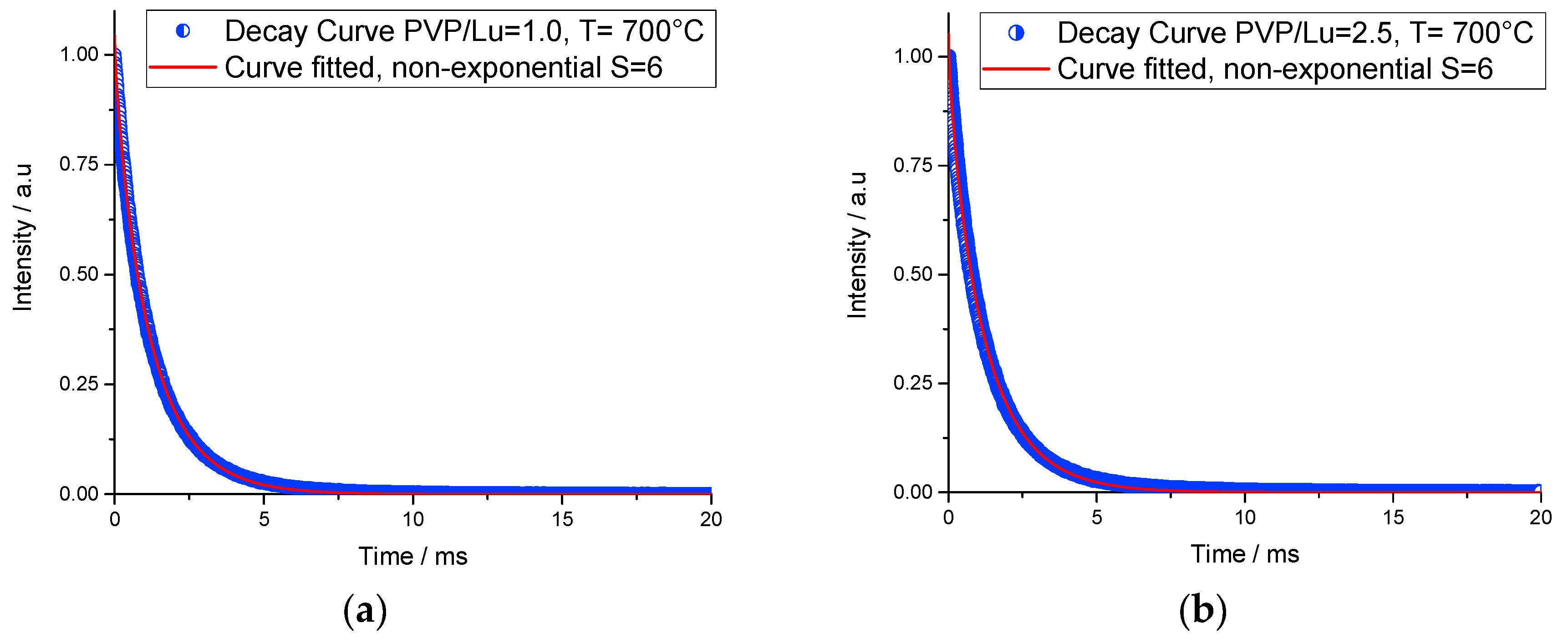
3.4. Lifetime Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Somalu, M.R.; Samat, A.A.; Muchtar, A.; Osman, N. Polymer-based approach in ceramic materials processing for energy device applications. Acad. J. Polym. Sci. 2018, 1, 555571. [Google Scholar]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Organic-inorganic hybrid functional materials: An integrated platform for applied technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Portier, J.; Choy, J.H.; Subramanian, M.A. Inoganic-organic-hybrids as precursors to functional materials. Int. J. Inorg. Mater. 2001, 3, 581–592. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Egger, P.; Sorarù, G.D.; Diré, S. Sol-gel synthesis of polymer-YSZ hybrid materials for SOFC technology. J. Eur. Ceram. Soc. 2004, 24, 1371–1374. [Google Scholar] [CrossRef]
- MacEdo, D.A.; Cesário, M.R.; Cela, B.; Melo, D.M.A.; Paskocimas, C.A.; Martinelli, A.E.; Nascimento, R.M. Influence of polymerizing agent on structure and spectroscopic properties of nano-crystalline La0.8Sr0.2MnO3 powders. Cryst. Res. Technol. 2010, 45, 1166–1170. [Google Scholar] [CrossRef]
- Farhadian Azizi, K.; Bagheri-Mohagheghi, M.M. Transition from anatase to rutile phase in titanium dioxide (TiO2) nanoparticles synthesized by complexing sol-gel process: Effect of kind of complexing agent and calcinating temperature. J. Sol-Gel Sci. Technol. 2013, 65, 329–335. [Google Scholar] [CrossRef]
- Kozuka, H.; Takenaka, S.; Tokita, H.; Hirano, T.; Higashi, Y.; Hamatani, T. Stress and cracks in gel-derived ceramic coatings. J. Sol-Gel Sci. Technol. 2003, 26, 681–686. [Google Scholar] [CrossRef]
- Park, W.-K.; Kim, J.-Y.; Kim, S.-R.; Kim, T.; Iwasaki, M. Fabrication of a PVP (polyvinylpyrrolidone)-assisted TiO2 film using a high-concentrated TiO2 nano sol and its optical properties. J. Ceram. Soc. Jpn. 2011, 119, 745–751. [Google Scholar] [CrossRef]
- Tamrakar, R.K.; Bisen, D.P.; Robinson, C.S.; Sahu, I.P.; Brahme, N. Ytterbium doped gadolinium oxide (Gd2O3:Yb3+) phosphor: Topology, morphology, and luminescence behaviour. Indian J. Mater. Sci. 2014, 2014, 396147. [Google Scholar] [CrossRef]
- Rath, M.K.; Acharya, S.K.; Kim, B.H.; Lee, K.T.; Ahn, B.G. Photoluminescence properties of sesquioxide doped ceria synthesized by modified sol-gel route. Mater. Lett. 2011, 65, 955–958. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Guo, H. Electrospinning synthesis and luminescent properties of Lu2O3:Eu3+ nanofibers. J. Rare Earths 2010, 28, 232–235. [Google Scholar] [CrossRef]
- Li, R.; Gai, S.; Wang, L.; Wang, J.; Yang, P. Facile synthesis and multicolor luminescent properties of uniform Lu2O3:Ln (Ln = Eu3+, Tb3+, Yb3+/Er3+, Yb3+/Tm3+, and Yb3+/Ho3+) nanospheres. J. Colloid Interface Sci. 2012, 368, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zych, E.; Trojan-Piegza, J.; Kępiński, L. Homogeneously precipitated Lu2O3:Eu nanocrystalline phosphor for X-ray detection. Sens. Actuators B Chem. 2005, 109, 112–118. [Google Scholar] [CrossRef]
- Zych, E.; Trojan-Piegza, J.; Dorenbos, P. Radioluminescence of Lu2O3:Eu nanocrystalline powder and vacuum-sintered ceramic. Radiat. Meas. 2004, 38, 471–474. [Google Scholar] [CrossRef]
- Jia, G.A.; You, H.P.; Zheng, Y.H.; Liu, K.; Guo, N.; Zhang, H.J. Synthesis and characterization of highly uniform Lu2O3:Ln3+ (Ln = Eu, Er, Yb) luminescent hollow microspheres. CrystEngComm 2010, 12, 2943–2948. [Google Scholar] [CrossRef]
- Jung, K.Y.; Lee, H.W.; Jung, H.K. Luminescent properties of (Sr, Zn)Al2O4:Eu2+, Bi3+ particles as a potential green phosphor for UV LEDs. Chem. Mater. 2006, 18, 2249–2255. [Google Scholar] [CrossRef]
- Wu, X.; Liang, Y.; Chen, R.; Liu, M.; Li, Y. Preparation and photoluminescence properties of Y2O3: Eu, Bi phosphors by molten salt synthesis for white light-emitting diodes. J. Mater. Sci. 2011, 46, 5581–5586. [Google Scholar] [CrossRef]
- Kang, F.; Hu, Y.; Wu, H.; Ju, G.; Mu, Z.; Li, N. Luminescence investigation of Eu3+–Bi3+ co-doped CaMoO4 phosphor. J. Rare Earths 2011, 29, 837–842. [Google Scholar] [CrossRef]
- Takeshita, S.; Isobe, T.; Sawayama, T.; Niikura, S. Low-temperature wet chemical precipitation of YVO4:Bi3+,Eu3+ nanophosphors via citrate precursors. Prog. Cryst. Growth Charact. Mater. 2011, 57, 127–136. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Deng, K.; Wei, X.; Chen, Y.; Yin, M. Luminescent properties of LuVO4: Bi3+, Eu3+ red phosphor for light-emitting diodes. J. Nanosci. Nanotechnol. 2014, 14, 3631–3634. [Google Scholar] [CrossRef] [PubMed]
- Neeraj, S.; Kijima, N.; Cheetham, A.K. Novel red phosphors for solid state lighting; The system BixLn1−xVO4; Eu3+/Sm3+(Ln = Y, Gd). Solid State Commun. 2004, 131, 65–69. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Z.; Teng, L.; Wei, R.; Hu, F.; Guo, H. Self-calibrated optical thermometer based on luminescence from SrLu2O4:Bi3+,Eu3+ phosphors. RSC Adv. 2018, 8, 35422–35428. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Z.; He, X.; Wei, Y.; Zou, Z.; Zhang, J.; Wang, Z.; Zhang, Z.; Wang, Y. How to design ultraviolet emitting persistent materials for potential multifunctional applications: A living example of a NaLuGeO4:Bi3+, Eu3+ phosphor. J. Mater. Chem. C 2017, 5, 4310–4318. [Google Scholar] [CrossRef]
- Kennedy, I.M.; Koivunen, M.M.; Gee, S.M.; Cummins, C.M.; Perron, R.M.; Dosev, D.M.; Hammock, B.D. Nanoscale fluoro-immuno assays with lanthanide oxide nanoparticles. Proc. SPIE 2004, 5593, 329–339. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Lenczewska, K.; Stefanski, M.; Hreniak, D. Synthesis, structure and NIR luminescence properties of Yb3+ and Bi3+-activated vanadate GdVO4. J. Rare Earths 2016, 34, 837–842. [Google Scholar] [CrossRef]
- Shang, Y.; Hao, S.; Yang, C.; Chen, G. Enhancing solar cell efficiency using photon upconversion materials. Nanomaterials 2015, 5, 1782–1809. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.D.; Bescher, E.P. Physical properties of sol-gel coatings. J. Sol-Gel Sci. Technol. 2000, 19, 23–29. [Google Scholar] [CrossRef]
- Jeon, H.J.; Yi, S.C.; Oh, S.G. Preparation and antibacterial effects of Ag–SiO2 thin films by sol-gel method. Biomaterials 2003, 24, 4921–4928. [Google Scholar] [CrossRef]
- Guo, H.; Yin, M.; Dong, N.; Xu, M.; Lou, L.; Zhang, W. Effect of heat-treatment temperature on the luminescent properties of Lu2O3:Eu film prepared by Pechini sol-gel method. Appl. Surf. Sci. 2005, 243, 245–250. [Google Scholar] [CrossRef]
- Xie, J.; Deng, L.; Shi, Y.; Xie, J.; Qiu, H.; Song, G. The influence of Pr3+ co-doping on the luminescent properties of Lu2O3:5 mol % Eu films. J. Lumin. 2011, 131, 970–974. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, K.I.; Sakka, S. Study on the properties of coating films prepared from metal alkoxides. J. Ceram. Soc. Jpn. 1982, 90, 328–333. [Google Scholar]
- Kozuka, H. Stress evolution on gel-to-ceramic thin film conversion. J. Sol-Gel Sci. Technol. 2006, 40, 287–297. [Google Scholar] [CrossRef]
- Liu, X.; Han, K.; Gu, M.; Huang, S.; Liu, B.; Ni, C. Optical properties of GdTaO4:Eu3+ thick films prepared from a PVP-containing solution. Appl. Surf. Sci. 2009, 255, 4680–4683. [Google Scholar] [CrossRef]
- Rho, Y.H.; Dokko, K.; Kanamura, K. Li+ ion diffusion in LiMn2O4 thin film prepared by PVP sol-gel method. J. Power Sources 2006, 157, 471–476. [Google Scholar] [CrossRef]
- Morales Ramírez, A.; García Hernández, M.; Yepez Ávila, J.; García Murillo, A.; Carrillo Romo, F.; De La Rosa, E.; Garibay Febles, V.; Reyes Miranda, J. Eu3+, Bi3+ codoped Lu2O3 nanopowders: Synthesis and luminescent properties. J. Mater. Res. 2013, 28, 1365–1371. [Google Scholar] [CrossRef]
- Chi, Y.; Chuang, S.S.C. Infrared and TPD Studies of nitrate adsorbed on Tb4O7, La2O3, BaO, and MgO/γ-Al2O3. J. Phys. Chem. B 2000, 104, 4673–4683. [Google Scholar] [CrossRef]
- Tu, W.; Zuo, X.; Liu, H. Study on the Interaction between polyvinylpyrrolidone and platinum metals during the formation of the colloidal metal nanoparticles. Chin. J. Polym. Sci. 2008, 26, 23–29. [Google Scholar] [CrossRef]
- Mcdevitt, N.T.; Baun, W.L. Infrared absorption study of metal oxides in the low frequency region (700–240 cm−1). Spectrochim. Acta 1964, 20, 799–808. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandhasamy, S.; Pandey, A.; Minakshi, M. Polyvinylpyrrolidone assisted sol-gel route LiCo1/3Mn1/3Ni1/3PO4 composite cathode for aqueous rechargeable battery. Electrochim. Acta 2012, 60, 170–176. [Google Scholar] [CrossRef]
- Fukada, H.; Konagai, M.; Ueda, K.; Miyata, T.; Minami, T. Photoluminescent and electroluminescent characteristics of various Bi-activated niobate-based oxide phosphor thin films. Thin Solid Films 2009, 517, 6054–6057. [Google Scholar] [CrossRef]
- Liu, H.Q.; Wang, L.L.; Chen, S.Q.; Zou, B. Effect of concentration on the luminescence of Eu3+ ions in nanocrystalline La2O3. J. Lumin. 2007, 126, 459–463. [Google Scholar] [CrossRef]
- Medina, D.Y.; Orozco, S.; Hernandez, I.; Hernandez, R.T.; Falcony, C. Characterization of europium doped lanthanum oxide films prepared by spray pyrolysis. J. Non-Cryst. Solids 2011, 357, 3740–3743. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Q.L.; Shao, S.F.; Liu, W.P.; Wan, S.M.; Yin, S.T. Phase transition, structure and luminescence of Eu:YAG nanophosphors by co-precipitation method. J. Alloy Compd. 2009, 470, 306–310. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium (III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-L.; Wang, Q.-L.; Xu, X.-Y.; Li, J.-Z.; Gao, L.-B.; Kang, W.-K.; Shi, J.-S.; Wang, J. Energy transfer from Bi3+ to Eu3+ triggers exceptional long-wavelength excitation band in ZnWO4:Bi3+, Eu3+ phosphors. J. Mater. Chem. C 2013, 1, 8033–8040. [Google Scholar] [CrossRef]
- Li, K.; Lian, H.; Shang, M.; Lin, J. A novel greenish yellow-orange red Ba3Y4O9:Bi3+, Eu3+ phosphor with efficient energy transfer for UV-LEDs. Dalton Trans. 2014, 44, 20542–20550. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Lahlouh, B.; Fiory, A.T. Analytical study of thermal annealing behaviour of erbium emission in Er2O3-sol–gel silica films. J. Phys. D Appl. Phys. 2011, 44, 315401. [Google Scholar] [CrossRef]
- Sahu, B.S.; Delachat, F.; Slaoui, A.; Carrada, M.; Ferblantier, G.; Muller, D. Effect of annealing treatments on photoluminescence and charge storage mechanism in silicon-rich SiNx:H films. Nanoscale Res. Lett. 2011, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Guild, J. The C.I.E. colorimetric standards and their use. Trans. Opt. Soc. 1931, 33, 73. [Google Scholar] [CrossRef]
- Lou, Z.; Hao, J. Cathodoluminescence of rare-earth-doped zinc aluminate films. Thin Solid Films 2004, 450, 334–340. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels of the trivalent lanthanide aquo ions. III. Tb3+. J. Chem. Phys. 1968, 49, 4447–4449. [Google Scholar] [CrossRef]
- Chang, Y.S.; Lin, H.J.; Chai, Y.L.; Li, Y.C. Preparation and luminescent properties of europium-activated YInGe2O7 phosphors. J. Alloy. Compd. 2008, 460, 421–425. [Google Scholar] [CrossRef]
- Inokuti, M.; Hirayama, F. Influence of energy transfer by the exchange mechanism on donor luminescence. J. Chem. Phys. 1965, 43, 1978–1989. [Google Scholar] [CrossRef]




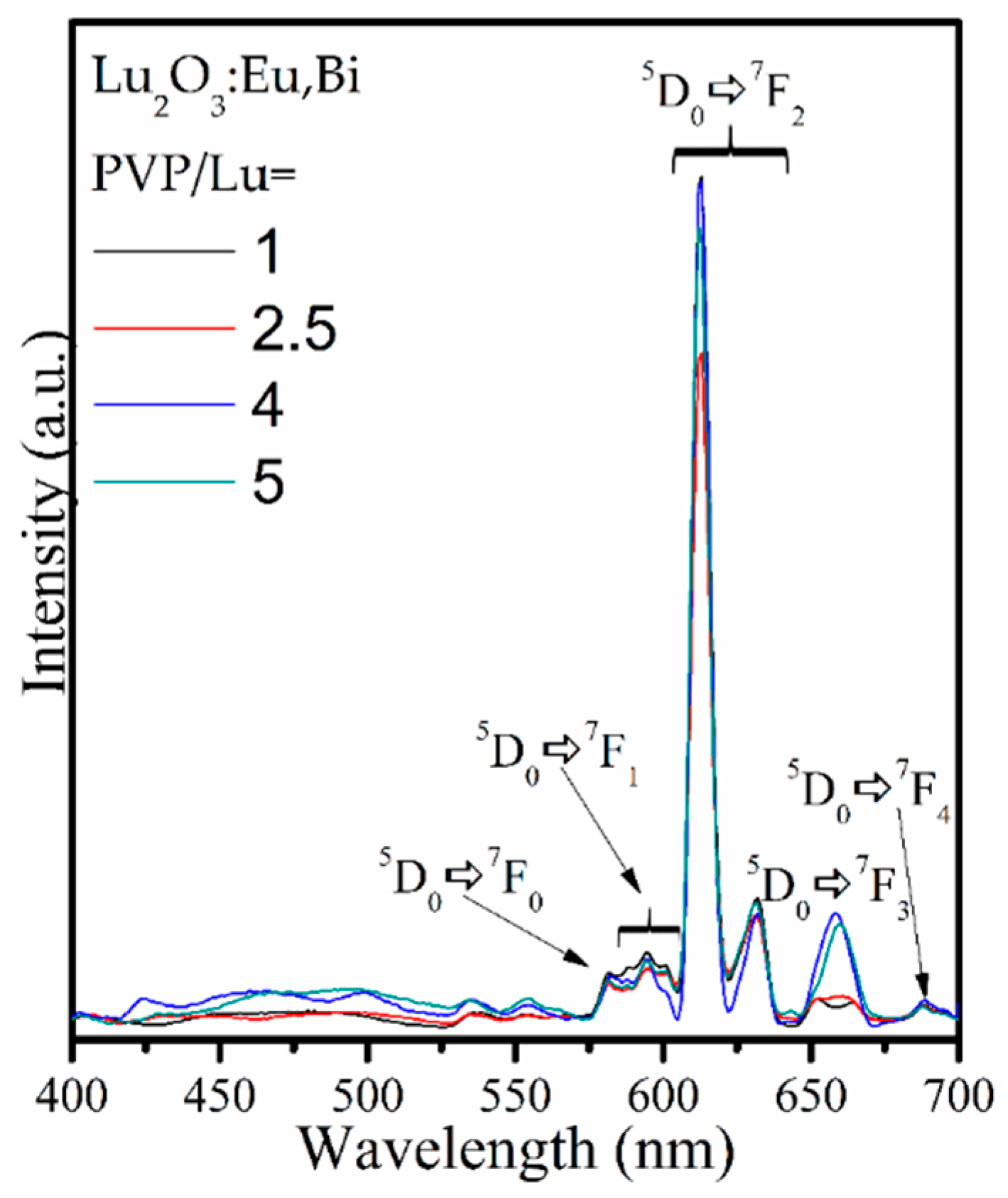

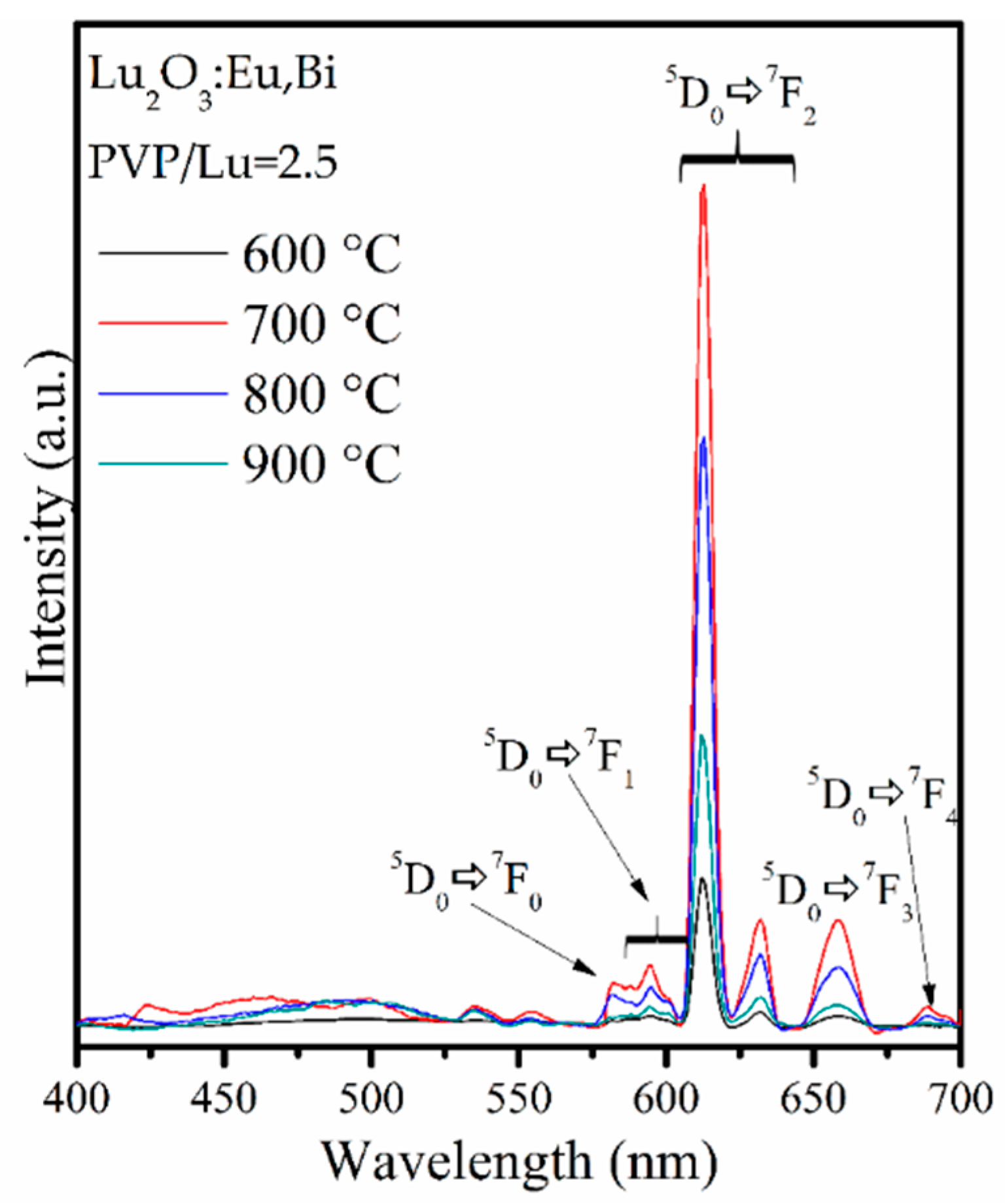

| System | PVP/Lu | R |
|---|---|---|
| A | 1.0 | 10.04 |
| B | 2.5 | 13.94 |
| C | 4.0 | 14.58 |
| D | 5.0 | 12.80 |
| System | PVP/Lu | x | y | Color Temperature | CP |
|---|---|---|---|---|---|
| a | 1.0 | 0.63 | 0.362 | 1895 K | 0.95 |
| b | 2.5 | 0.626 | 0.365 | 1829 K | 0.96 |
| c | 4.0 | 0.618 | 0.374 | 1717 K | 0.96 |
| d | 5.0 | 0.642 | 0.35 | 2185 K | 0.97 |
| PVP/Lu Ratio | 5D0 → 7F0 | 5D0 → 7F1 | 5D0 → 7F2 | 5D0 → 7F3 | 5D0 → 7F4 |
|---|---|---|---|---|---|
| 1.0 | 2.29 | 11.23 | 80.36 | 4.63 | 1.48 |
| 2.5 | 2.30 | 10.45 | 80.81 | 4.89 | 1.55 |
| 4.0 | 2.13 | 7.40 | 73.26 | 15.02 | 2.19 |
| 5.0 | 2.22 | 9.30 | 75.35 | 11.72 | 1.41 |
| PVP/Lu Ratio | τ (ms) | Parameters of Non-exponential Decay | ||
|---|---|---|---|---|
| R2 | S | γ | ||
| 1.0 | 1.52 | 0.99931 | 6 | 0.32 |
| 2.5 | 1.58 | 0.99927 | 6 | 0.36 |
| 4.0 | 1.64 | 0.99934 | 6 | 0.32 |
| 5.0 | 2.05 | 0.99555 | Simple Exponential | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Ramírez, Á.d.J.; García-Hernández, M.; Medina-Velázquez, D.Y.; Ruiz-Guerrero, M.d.R.; Juárez-López, F.; Reyes-Miranda, J. Luminescence Properties of Co-Doped Eu3+, Bi3+ Lu2O3/Polyvinylpyrrolidone Films. Coatings 2018, 8, 434. https://doi.org/10.3390/coatings8120434
Morales-Ramírez ÁdJ, García-Hernández M, Medina-Velázquez DY, Ruiz-Guerrero MdR, Juárez-López F, Reyes-Miranda J. Luminescence Properties of Co-Doped Eu3+, Bi3+ Lu2O3/Polyvinylpyrrolidone Films. Coatings. 2018; 8(12):434. https://doi.org/10.3390/coatings8120434
Chicago/Turabian StyleMorales-Ramírez, Ángel de Jesús, Margarita García-Hernández, Dulce Yolotzin Medina-Velázquez, María del Rosario Ruiz-Guerrero, Fernando Juárez-López, and Joan Reyes-Miranda. 2018. "Luminescence Properties of Co-Doped Eu3+, Bi3+ Lu2O3/Polyvinylpyrrolidone Films" Coatings 8, no. 12: 434. https://doi.org/10.3390/coatings8120434





