Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grass Pea Flour Characterization
2.2.1. Protein Content
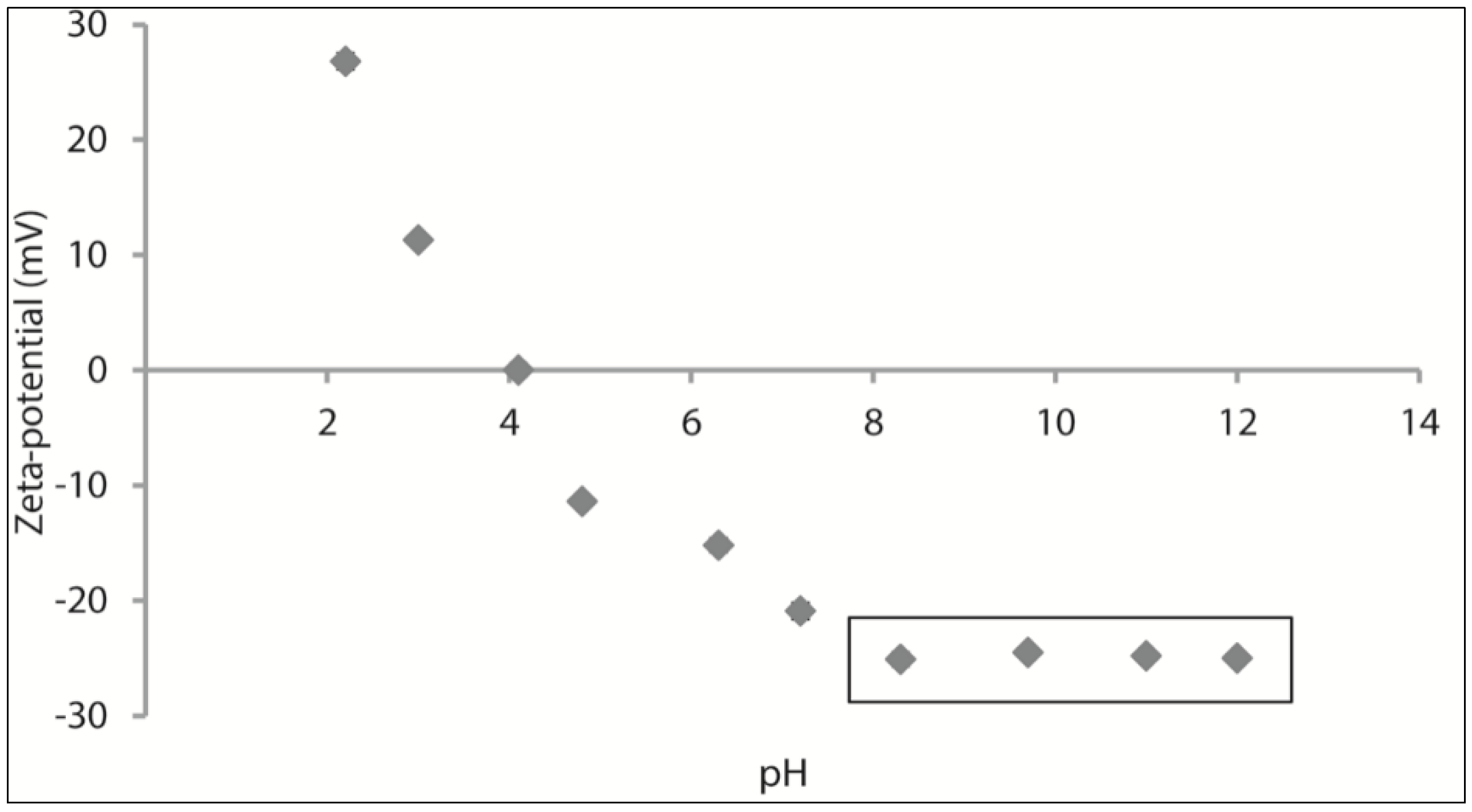
2.2.2. Zeta-Potential and Particle Size of Grass Pea Flour Suspension
2.3. Film Forming Solutions Preparation and Characterization
2.3.1. mTGase Preparation
2.3.2. Film Forming Solution (FFS) Preparation
2.3.3. Zeta-Potential and Particle Average Size
2.3.4. Viscosity
2.4. Film Preparation and Characterization
2.4.1. Film Casting
2.4.2. Thickness
2.4.3. Opacity
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. Mechanical Properties
2.4.6. In Vitro Film Digestion
2.4.7. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4.8. Densitometry Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Stability of Grass Pea Flour Suspension and FFSs
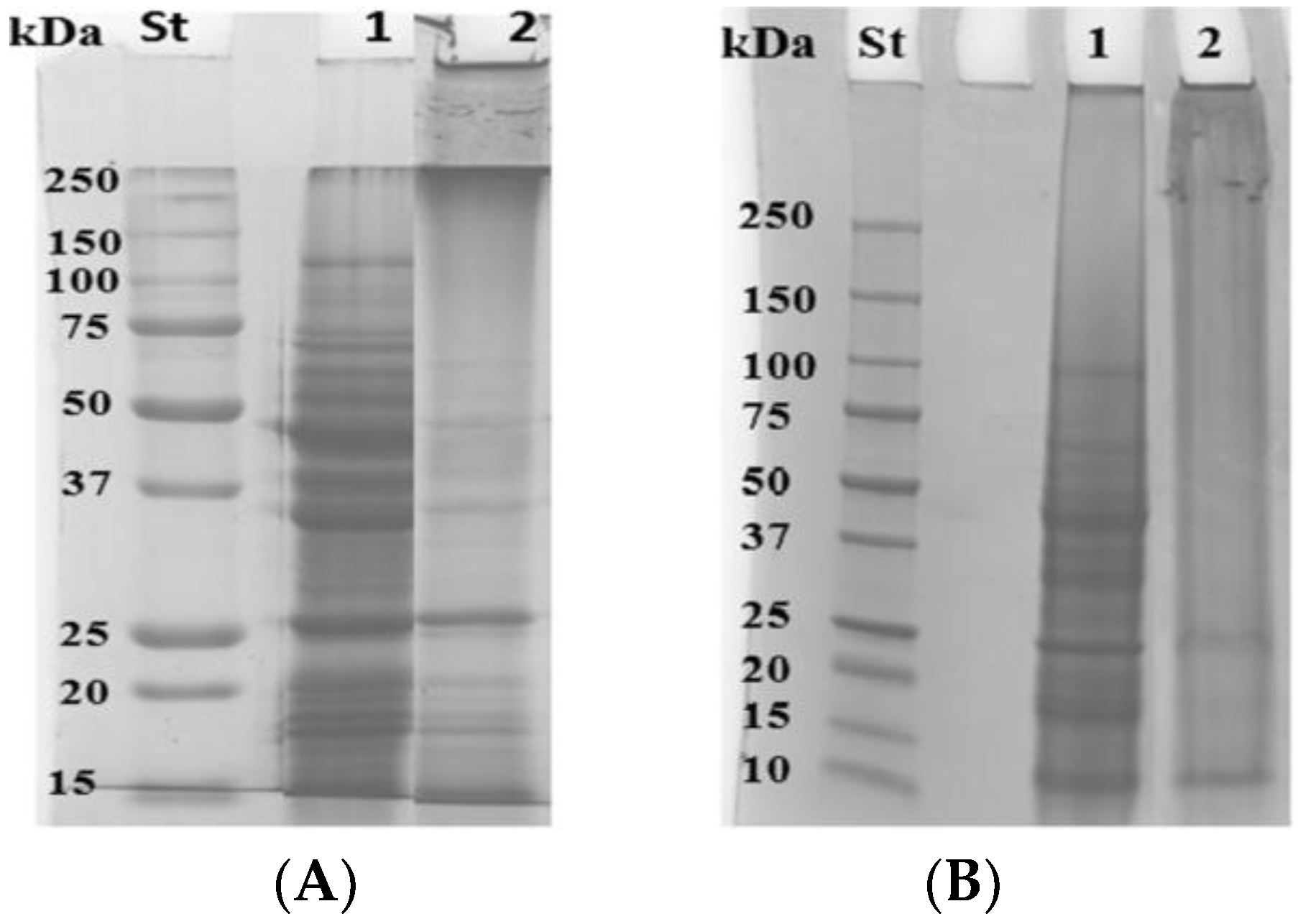
3.2. Modification of Grass Pea Flour Proteins by Means of mTGase
3.3. Opacity
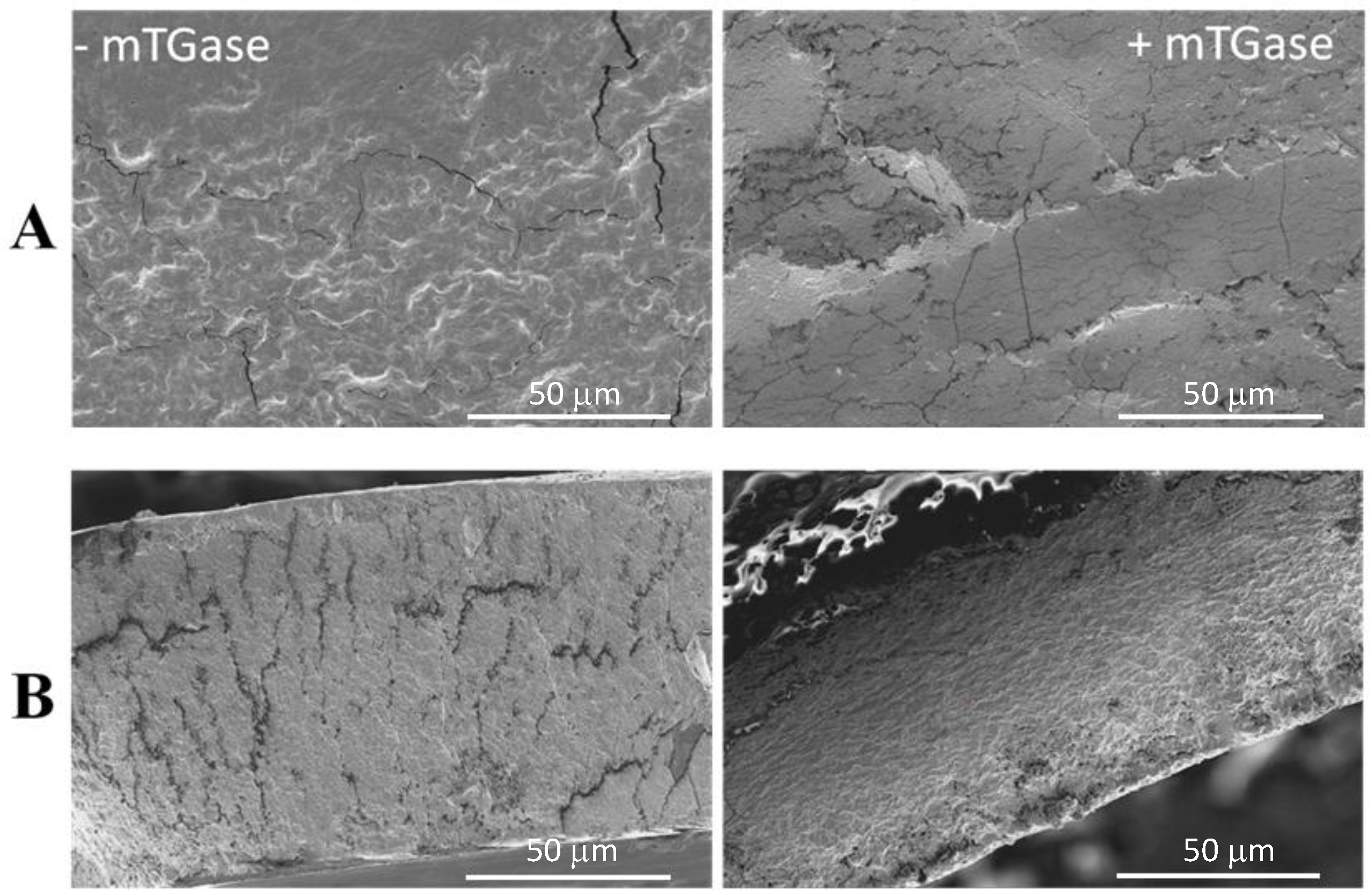
3.4. Scanning Electron Microscopy (SEM)
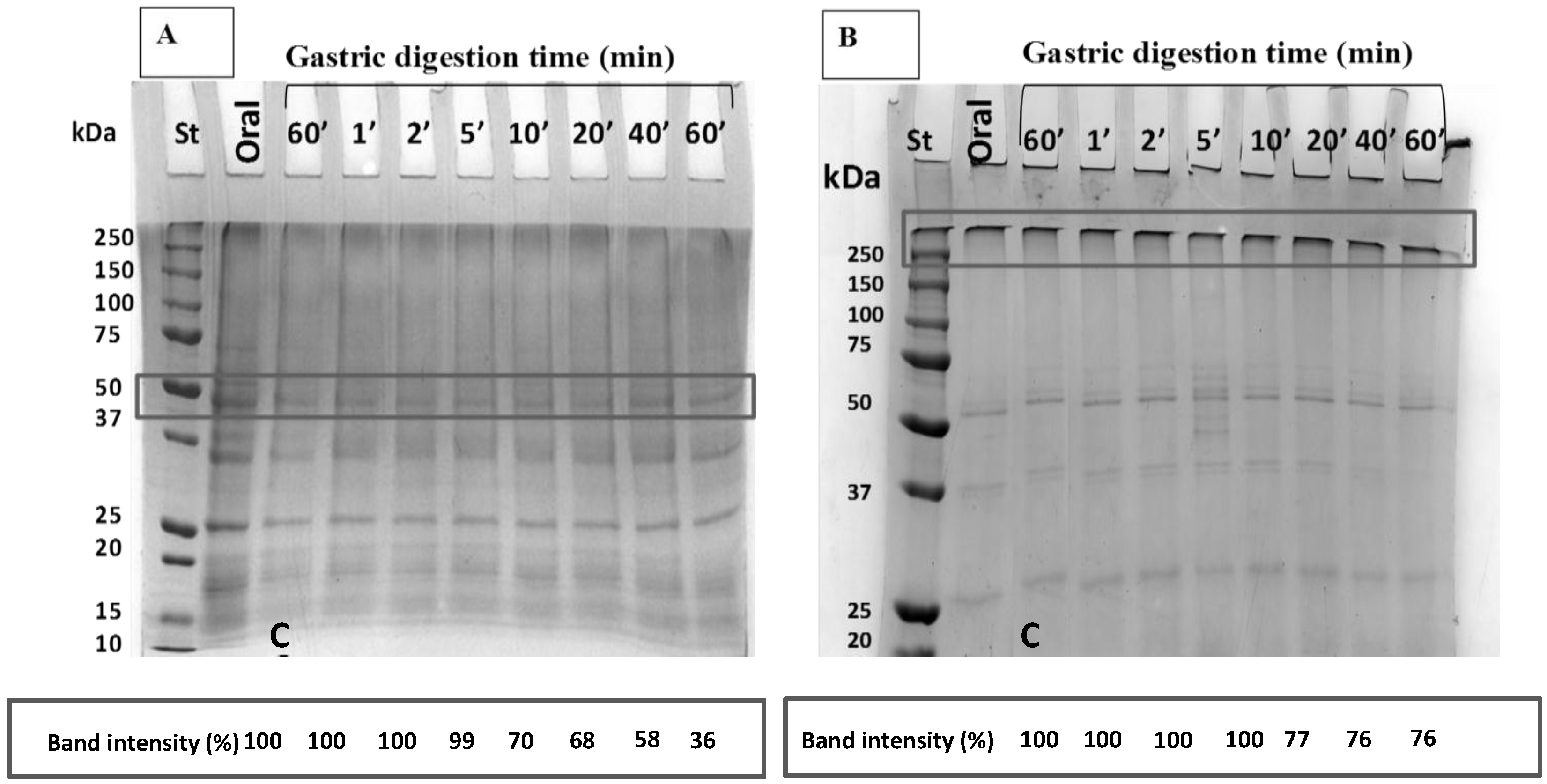
3.5. Oral, Gastric and Duodenal in Vitro Digestion of Grass Pea Flour-Based Edible Films
3.6. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, M.; Di Pierro, P.; Cammarota, M.; Dell’Olmo, E.; Arciello, A.; Porta, R. Development and properties of new chitosan-based films plasticized with spermidine and/or glycerol. Food Hydrocoll. 2019, 87, 245–252. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, A.P.; Mackie, A.; Porta, R.; Mariniello, L. Trehalose containing hydrocolloid edible films prepared in the presence of transglutaminase. Biopolymers 2014, 101, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Materials European Bioplastics V. Available online: https://www.european-bioplastics.org/bioplastics/materials/ (accessed on 1 October 2018).
- Porta, R.; Di Pierro, P.; Rossi-Marquez, G.; Mariniello, L.; Kadivar, M.; Arabestani, A. Microstructure and properties of bitter vetch (Vicia ervilia) protein films reinforced by microbial transglutaminase. Food Hydrocoll. 2015, 50, 102–107. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. Technol. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT Food Sci. Technol. 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Campbell, C.G. Grass Pea, Lathyrus Sativus L.; Promoting the conservation and use of underutilized and neglected crops; IPGRI: Rome, Italy, 1997; Volume 18, pp. 1–91. [Google Scholar]
- Al-Asmar, A.; Naviglio, D.; Giosafatto, C.V.L.; Mariniello, L. Hydrocolloid-based coatings are effective at reducing acrylamide and oil content of french fries. Coatings 2018, 8, 147. [Google Scholar] [CrossRef]
- Kieliszek, M.; Misiewicz, A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol. 2014, 59, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, A.; Giosafatto, C.V.L.; Sirangelo, I.; De Simone, C.; Di Pierro, P.; Porta, R.; Mariniello, L. Higher susceptibility to amyloid fibril formation of the recombinant ovine prion protein modified by transglutaminase. Biochim. Biophys. Acta. 2012, 1822, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Giosafatto, C.V.L.; Di Pierro, P.; Sorrentino, A.; Mariniello, L. Transglutaminase-mediated modification of ovomucoid. Effects on its trypsin inhibitory activity and antigenic properties. Amino Acids. 2013, 44, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kjeldahl, J. Neuemethodezurbestimmung des stickstoffs in organischenkörpern. Zeitschrift für Analytische Chemie 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N. Relationship between protein characteristics and film-forming properties of kidney bean, field pea and amaranth protein isolates. Int. J. Food Sci. Technol. 2015, 50, 1033–1043. [Google Scholar] [CrossRef]
- ASTM D882-97 Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM: Philadelphia, PA, USA, 1997.
- Giosafatto, C.V.L.; Rigby, N.M.; Wellner, N.; Ridout, M.; Husband, F.; Mackie, A.R. Microbial transglutaminase-mediated modification of ovalbumin. Food Hydrocoll. 2012, 26, 261–267. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Bourlieu, C.; Ménard, O.; Bouzerzour, K.; Mandalari, G.; Macierzanka, A.; Mackie, A.R.; Dupont, D. Specificity of Infant Digestive Conditions: Some Clues for Developing Relevant In Vitro Models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature 1970, 227, 680–985. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and Zeta potential—What they are and what they are not? J. Controlled Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Giosafatto, C.V.L.; Al-Asmar, A.; Masi, P.; Aponte, M.; Mariniello, L. Grass pea (Lathyrus sativus) flour: Microstructure, physico-chemical properties and in vitro digestion. Eur. Food Res. Technol. 2018, 1–8. [Google Scholar] [CrossRef]
- Nio, N.; Motoki, M.; Takinami, K. Gelation of Casein and Soybean Globulins by Transglutaminase. Agric. Biol. Chem. 1985, 49, 2283–2286. [Google Scholar]
- Temiz, H.; Dağyıldız, K. Effects of Microbial Transglutaminase on Physicochemical, Microbial and Sensorial Properties of Kefir Produced by Using Mixture Cow’s and Soymilk. Korean J. Food Sci. Anim. Resour. 2017, 37, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, L.; Giosafatto, C.V.L.; Di Pierro, P.; Sorrentino, A.; Porta, R. Synthesis and resistance to in vitro proteolysis of transglutaminase cross-linked phaseolin, the major storage protein from Phaseolus vulgaris. J. Agric. Food Chem. 2007, 55, 4717–4721. [Google Scholar] [CrossRef] [PubMed]
- Monogioudi, E.; Faccio, G.; Lille, M.; Poutanen, K.; Mattinen, M. Effect of enzymatic crosslinking of β-casein on proteolysis by pepsin. Food Hydrocoll. 2011, 25, 71–81. [Google Scholar] [CrossRef]
- Motoki, M.; Aso, H.; Seguro, K.; Nio, N. αs1-Caseinfilm prepared using transglutaminase. Agric. Biol. Chem. 1987, 51, 993–996. [Google Scholar]
- Mahmoud, R.; Savello, P.A. Solubility and hydrolyzability of films produced by transglutaminase catalytic cross-linking of whey protein. J. Dairy Sci. 1993, 76, 29–35. [Google Scholar] [CrossRef]
- Yildirim, M.; Hettiarachchy, N.S. Biopolymers Produced by Cross-linking Soybean 11S Globulin with Whey Proteins using Transglutaminase. J. Food Sci. 1997, 62, 270–275. [Google Scholar] [CrossRef]
- Larré, C.; Desserme, C.; Barbot, J.; Gueguen, J. Properties of deamidated gluten films enzymatically cross-linked. J. Agric. Food Chem. 2000, 48, 5444–5449. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, L.; Di Pierro, P.; Esposito, C.; Sorrentino, A.; Masi, P.; Porta, R. Preparation and mechanical properties of edible pectin-soy flour films obtained in the absence or presence of transglutaminase. J. Biotechnol. 2003, 102, 191–198. [Google Scholar] [CrossRef]
- Tang, C.H.; Jiang, Y.; Wen, Q.B.; Yang, X.Q. Effect of transglutaminase treatment on the properties of cast films of soy protein isolates. J. Biotechnol. 2005, 120, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Di Pierro, P.; Roviello, V.; Sabbah, M. Tuning the functional properties of bitter vetch (Vicia ervilia) protein films grafted with spermidine. Int. J. Mol. Sci. 2017, 18, 2658. [Google Scholar] [CrossRef] [PubMed]
- Products and markets—Viscofan. Available online: http://www.viscofan.com/products-and-markets (accessed on 1 October 2018).
- Uso delle risorse—Materbi. Available online: http://materbi.com/uso-delle-risorse/ (accessed on 1 October 2018).

| Sample pH 9 | Average Size (d/nm) | Polydispersity Index | Zeta-Potential (mV) |
|---|---|---|---|
| FFS | 139.40 ± 1.06 a | 0.53 ± 0.01 a | −27.10 ± 1.90 a |
| FFS + mTGase | 127.30 ± 2.50 a | 0.57 ± 0.02 a | −28.00 ± 1.63 a |
| Film Features | Thickness (mm) | Opacity (mm−1) |
|---|---|---|
| Grass Pea-Based Films | 0.084 ± 0.005 b | 7.74 ± 0.26 b |
| Grass Pea-Based Films + mTGase | 0.12 ± 0.02 a | 4.04 ± 0.06 c |
| Kidney Bean-Based Films * | 0.064 ± 0.002 | 8.9 ± 0.3 |
| Field Pea-Based Film * | 0.064 ± 0.002 | 7.3 ± 0.3 |
| CTA | 0.131 ± 0.001 a | 0.54 ± 0.09 d |
| PP5 | 0.054 ± 0.003 c | 32.02 ± 3.35 a |
| Film Type | TS (MPa) Resistance | EB (%) Extensibility | YM (MPa) Stiffness |
|---|---|---|---|
| Films | 0.70 ± 0.03 b | 32.2 ± 4.4 b | 26.2 ± 0.7 a |
| Films + mTGase | 1.04 ± 0.10 a | 59.1 ± 6.1 a | 17.1 ± 2.8 b |
| * BVPC | 1.59 ± 0.18 | 32.08 ± 2.52 | 78.14 ± 3.04 |
| * BVPC + mTGase | 2.14 ± 0.47 | 21.04 ± 1.29 | 65.13 ± 2.10 |
| ** Viscofan NDX® | 36.6 ± 8.1 | 13.1 ± 2.9 | 356 ± 29 |
| ** Mater Bi (S-301)® | 18.4 ± 2.7 | 317.9 ± 35.9 | 75.2 ± 2.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase. Coatings 2018, 8, 435. https://doi.org/10.3390/coatings8120435
Giosafatto CVL, Al-Asmar A, D’Angelo A, Roviello V, Esposito M, Mariniello L. Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase. Coatings. 2018; 8(12):435. https://doi.org/10.3390/coatings8120435
Chicago/Turabian StyleGiosafatto, C. Valeria L., Asmaa Al-Asmar, Antonio D’Angelo, Valentina Roviello, Marilena Esposito, and Loredana Mariniello. 2018. "Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase" Coatings 8, no. 12: 435. https://doi.org/10.3390/coatings8120435
APA StyleGiosafatto, C. V. L., Al-Asmar, A., D’Angelo, A., Roviello, V., Esposito, M., & Mariniello, L. (2018). Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase. Coatings, 8(12), 435. https://doi.org/10.3390/coatings8120435








