Application of Transglutaminase Crosslinked Whey Protein–Pectin Coating Improves Egg Quality and Minimizes the Breakage and Porosity of Eggshells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Film Forming Solution (FFS)
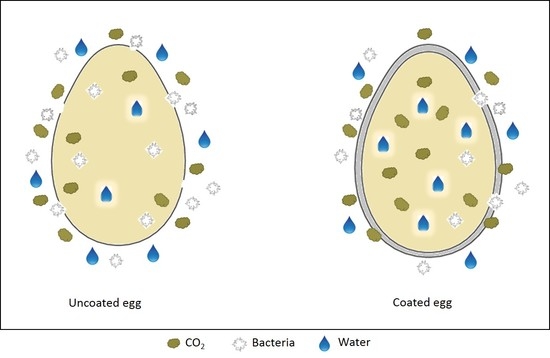
2.2.2. Egg Coating
2.2.3. Eggs Quality Parameters
Weight Loss (%)
Egg Yolk Index (YI)
pH Measurement
Albumen CO2 Content
2.2.4. Eggshell Thickness
2.2.5. Eggshell Strength
2.2.6. Eggshell Permeability to Blue Lake Dye
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. CO2 Loss and pH Value
3.3. Yolk Index
3.4. Egg Shell Parameters and Bacterial Penetration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- 4To Informe De Labores 2015–2016; Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA): Ciudad de México, Mexico, 2016; Available online: https://www.gob.mx/cms/uploads/attachment/file/254118/CuartoInformeDeLabores_SAGARPA.pdf (accessed on 28 November 2018). (In Mexican)
- Leading egg producing countries worldwide in 2016 (in number of eggs in billions). Available online: https://www.statista.com/statistics/263971/top-10-countries-worldwide-in-egg-production (accessed on 27 November 2018).
- Egg Marketing: A Guide for the Production and Sale of Eggs; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2003.
- Stadelman, W.J.; Cotterill, O.J. The preservation of quality in shell eggs. In Egg Science and Technology, 3rd ed.; AVI Publishing Company: Westport, CT, USA, 1986; pp. 499–521. [Google Scholar]
- Stadelman, W.J.; Cotterill, O.J. Quality identification of shell eggs. In Egg Science and Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 55–82. [Google Scholar]
- Nongtaodum, S.; Jangchud, A.; Jangchud, K.; Dhamvithee, P.; No, H.K.; Prinyawiwatkul, W. Oil coating affects internal quality and sensory acceptance of selected attributes of raw eggs during storage. J. Food Sci. 2013, 78, S329–S335. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.V.; Raj, K.R.; Nidheesh, T.; Pal, G.K.; Sakhare, P.Z. Application of chitosan for improvement of quality and shelf life of table eggs under tropical room conditions. J. Food Sci. Technol. 2015, 52, 6345–6354. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Lv, X.; Chi, Y.; Wu, Y.; Shao, H. Internal quality of coated eggs with soy protein isolate and montmorillonite: effects of storage conditions. Int. J. Food Prop. 2017, 20, 1921–1934. [Google Scholar] [CrossRef]
- Xie, L.; Hettiarachchy, N.S.; Ju, Z.Y.; Meullenet, J.; Wang, H.; Slavik, M.F.; Janes, M.E. Edible film coating to minimize eggshell breakage and reduce post-wash bacterial contamination measured by dye penetration in eggs. J. Food Sci. 2002, 67, 280–284. [Google Scholar] [CrossRef]
- Caner, C.; Cansiz, Ö. Chitosan coating minimises eggshell breakage and improves egg quality. J. Sci. Food Agric. 2008, 88, 56–61. [Google Scholar] [CrossRef]
- Yuceer, M.; Caner, C. Antimicrobial lysozyme–chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 2014, 94, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Alleoni, A.C.C.; Antunes, A.J. Internal quality of eggs coated with whey protein concentrate. Sci. Agric. 2004, 61, 276–280. [Google Scholar] [CrossRef]
- Edirisinghe, E.D.M.T.; Jayasinghe, J.M.P.; Himali, S.M.C.; Abeyrathne, E.D.N.S. Effect of Beeswax and Gammalu (Pterocarpus marsupium) Latex Coating on Internal and Sensory Attributes of Chicken Eggs Stored at Room Temperature. Int. J. Res. Agric. Sci. 2017, 4, 76–81. [Google Scholar]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the Art in the Development and Properties of Protein-Based Films and Coatings and Their Applicability to Cellulose Based Products: An Extensive Review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Caner, C. Whey protein isolate coating and concentration effects on egg shelf life. J. Sci. Food Agric. 2005, 85, 2143–2148. [Google Scholar] [CrossRef]
- Biladeau, A.M.; Keener, K.M. The effects of edible coatings on chicken egg quality under refrigerated storage. Poult. Sci. 2009, 88, 1266–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhim, J.-W.; Weller, C.L.; Gennadios, A. Effects of soy protein coating on shell strength and quality of shell eggs. Food Sci. Biotechnol. 2004, 13, 455–459. [Google Scholar]
- Wardy, W.; Torrico, D.D.; Herrera Corredor, J.A.; No, H.K.; Zhang, X.; Xu, Z.; Prinyawiwatkul, W. Soybean oil-chitosan emulsion affects internal quality and shelf-life of eggs stored at 25 and 4 °C. Int. J. Food Sci. Technol. 2013, 48, 1148–1156. [Google Scholar] [CrossRef]
- Bhale, S.; No, H.K.; Prinyawiwatkul, W.; Farr, A.J.; Nadarajah, K.; Meyers, S.P. Chitosan coating improves shelf life of eggs. J. Food Sci. 2003, 68, 2378–2383. [Google Scholar] [CrossRef]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Mariniello, L.; Damiao, A.E.; Masi, P.; Porta, R. Chitosan−whey protein edible films produced in the absence or presence of transglutaminase: Analysis of their mechanical and barrier properties. Biomacromolecules 2006, 7, 744–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Mariniello, L.; Masi, P.; Porta, R. Transglutaminase-catalyzed preparation of chitosan–ovalbumin films. Enzyme Microb. Technol. 2007, 40, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Porta, R.; Mariniello, L.; Di Pierro, P.; Sorrentino, A.; Giosafatto, C.V.L. Transglutaminase crosslinked pectin-and chitosan-based edible films: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, P.; Rossi Marquez, G.; Mariniello, L.; Sorrentino, A.; Villalonga, R.; Porta, R. Effect of transglutaminase on the mechanical and barrier properties of whey protein/pectin films prepared at complexation pH. J. Agric. Food Chem. 2013, 61, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Marquez, G.; Di Pierro, P.; Esposito, M.; Mariniello, L.; Porta, R. Application of Transglutaminase-Crosslinked Whey Protein/Pectin Films as Water Barrier Coatings in Fried and Baked Foods. Food Bioprocess Technol. 2014, 7, 447–455. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. Technol. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT Food Sci. Technol. 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Sabbah, M.; Giosafatto, C.V.L.; Esposito, M.; Di Pierro, P.; Mariniello, L.; Porta, R. Transglutaminase Cross-Linked Edible Films and Coatings for Food Applications. In Enzymes in Food Biotechnology; Academic Press: New York, NY, USA, 2019; pp. 369–388. [Google Scholar]
- DSM Egg Quality Manual; DSM Nutritional Products Ltd.: Basel, Switzerland. Available online: https://www.dsm.com/content/dam/dsm/anh/en_US/documents/DSM-egg-quality-manual.PDF (accessed on 28 November 2018).
- Keener, K.M.; LaCrosse, J.D.; Babson, J.K. Chemical method for determination of carbon dioxide content in egg yolk and egg albumen. Poult. Sci. 2001, 80, 983–987. [Google Scholar] [CrossRef] [PubMed]
- De Zuniga, A.G.; Anderson, M.E.; Marshall, R.T.; Iannotti, E.L. A model system for studying the penetration of microorganisms into meat. J. Food Prot. 1991, 54, 256–258. [Google Scholar] [CrossRef]
- Kim, J.W.; Slavik, M.F. Changes in eggshell surface microstructure after washing with cetylpyridinium chloride or trisodium phosphate. J. Food Prot. 1996, 59, 859–863. [Google Scholar] [CrossRef]
- Lucisano, M.; Hidalgo, A.; Comelli, E.M.; Rossi, M. Evolution of chemical and physical albumen characteristics during the storage of shell eggs. J. Agric. Food Chem. 1996, 44, 1235–1240. [Google Scholar] [CrossRef]
- Marketing quality eggs. In Egg Marketing: A Guide for the Production and Sales of Eggs; Agricultural Services Bulletin n.150; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2003; pp. 29–41.
- Xu, D.; Wang, J.; Ren, D.; Wu, X. Effects of Chitosan Coating Structure and Changes during Storage on Their Egg Preservation Performance. Coatings 2018, 8, 317. [Google Scholar] [CrossRef]
- Stadelman, W.J. The preservation of quality in shell eggs. In Egg Science and Technology, 4th ed.; CRC Press: New York, NY, USA, 1995; pp. 67–79. [Google Scholar]
- Scott, T.A.; Silversides, F.G. The effect of storage and strain of hen on egg quality. Poult. Sci. 2000, 79, 1725–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silversides, F.G.; Scott, T.A. Effect of storage and layer age on quality of eggs from two lines of hens. Poult. Sci. 2001, 80, 1240–1245. [Google Scholar] [CrossRef] [PubMed]



| Sample | Day 0 | Day 7 | Day 15 | |
|---|---|---|---|---|
| CO2 (mg/g albumen) | Uncoated | 1.79 ± 0.08 | 1.67 ± 0.06 | 1.55 ± 0.02 |
| Coated | 1.98 ± 0.06 | 1.92 ± 0.05 | 1.89 ± 0.08 | |
| Albumen pH | Uncoated | 8.34 ± 0.13 | 9.01 ± 0.3 | 9.67 ± 0.2 |
| Coated | 8.29 ± 0.39 | 8.41 ± 0.13 | 8.53 ± 0.8 | |
| Yolk pH | Uncoated | 6.11 ± 0.12 | 6.43 ± 0.21 | 6.93 ± 0.61 |
| Coated | 6.10 ± 0.09 | 6.06 ± 0.12 | 6.21 ± 0.31 | |
| Sample | Eggshell Thickness (µm) | Shell Strength (N) | Dye Dots/Egg (Number) |
|---|---|---|---|
| Uncoated | 422 ± 32 | 40 ± 7.3 | 90 ± 4.2 |
| Coated | 483 ± 45 | 57 ± 6.2 | 6.4 ± 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávalos-Saucedo, C.A.; Rossi-Márquez, G.; Regalado-González, C.; Alonzo-Macías, M.; Di Pierro, P. Application of Transglutaminase Crosslinked Whey Protein–Pectin Coating Improves Egg Quality and Minimizes the Breakage and Porosity of Eggshells. Coatings 2018, 8, 438. https://doi.org/10.3390/coatings8120438
Dávalos-Saucedo CA, Rossi-Márquez G, Regalado-González C, Alonzo-Macías M, Di Pierro P. Application of Transglutaminase Crosslinked Whey Protein–Pectin Coating Improves Egg Quality and Minimizes the Breakage and Porosity of Eggshells. Coatings. 2018; 8(12):438. https://doi.org/10.3390/coatings8120438
Chicago/Turabian StyleDávalos-Saucedo, Cristian Aarón, Giovanna Rossi-Márquez, Carlos Regalado-González, Maritza Alonzo-Macías, and Prospero Di Pierro. 2018. "Application of Transglutaminase Crosslinked Whey Protein–Pectin Coating Improves Egg Quality and Minimizes the Breakage and Porosity of Eggshells" Coatings 8, no. 12: 438. https://doi.org/10.3390/coatings8120438