Spectroscopic and Structural Analyses of Opuntia Robusta Mucilage and Its Potential as an Edible Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vegetative Material
2.2. Obtention of Mucilage
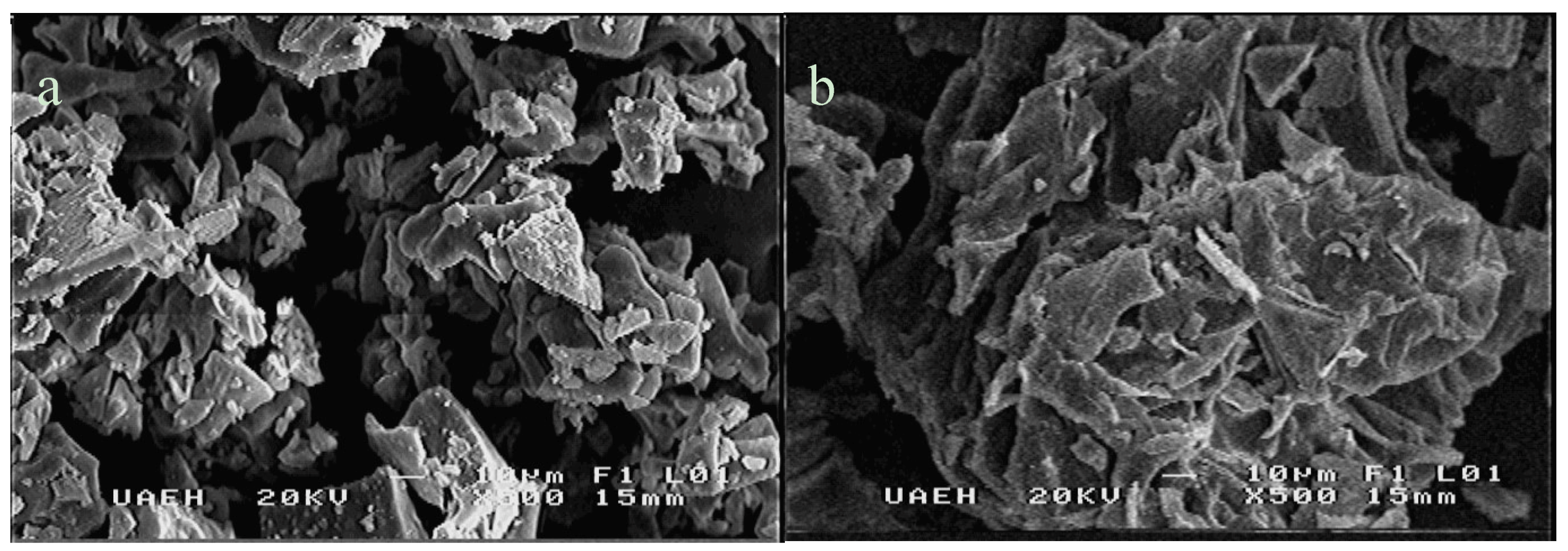
2.3. Scanning Electron Microscopy
2.4. FT-IR Spectroscopy
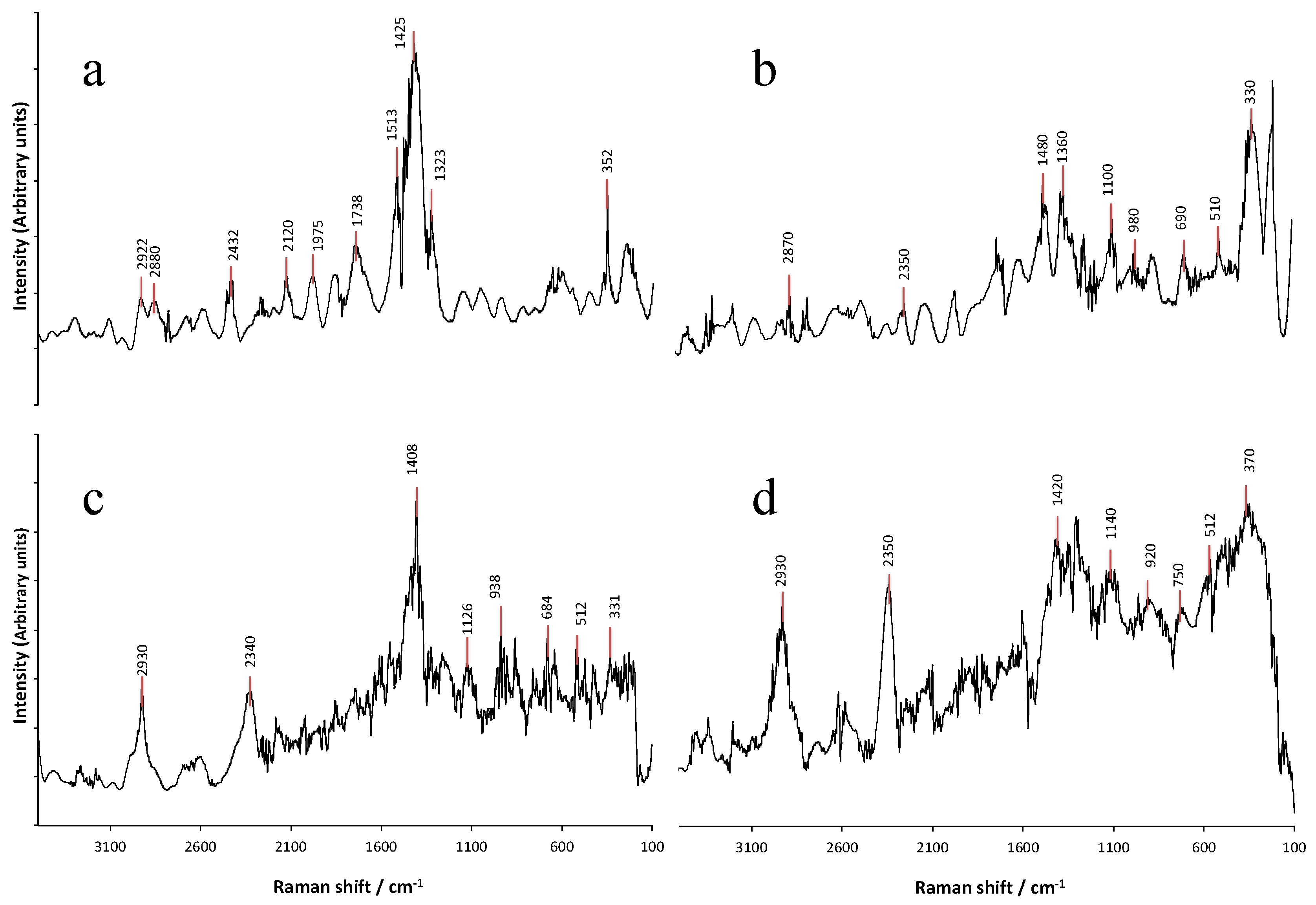
2.5. Raman Spectroscopy
2.6. Edible Coating Preparation
Edible Coating Application
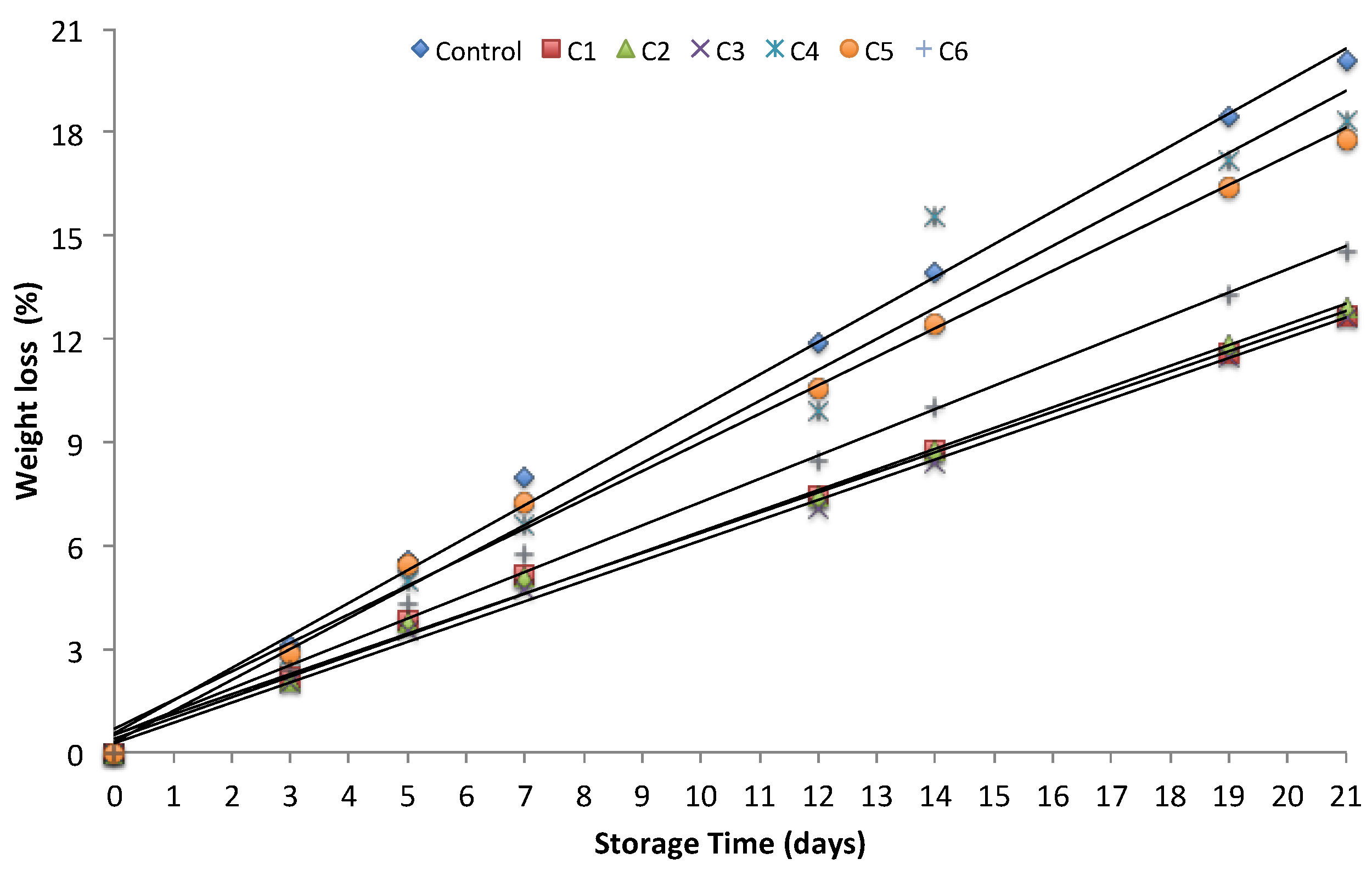
2.7. Weight Loss During Postharvest Storage
2.8. Texture Measurement
2.9. Determination of Lycopene
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Mucilage
3.1.1. Morphology of Opuntia Mucilage
3.1.2. FT-IR Analysis of Structural Changes in Mucilage Due to the Extraction Process and the Source
3.1.3. Raman Spectroscopy Analysis of Structural Changes in the Mucilage Due to the Extraction Process and the Source
3.2. Mucilage Used as Edible Coating
3.2.1. Weight Loss of Tomatoes
3.2.2. Effect on the Firmness of Tomatoes
3.2.3. Effect on the Lycopene Content of Tomatoes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2010, 58, 42–47. [Google Scholar] [CrossRef]
- Rodríguez-Garcia, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Moctezuma, E.; Gutierrez-Pacheco, M.M.; Tapia-Rodríguz, M.R.; Ortega-Ramírez, L.A.; Ayala-Zavala, J.F. Oregano (Lippia graveolens) essential oil added within pectin edible coatings prevents fungal decay and increases the antioxidant capacity of treated tomatoes. J. Sci. Food Agric. 2016, 96, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.F.; Islam, M.Z.; Sarker, M.S.H.; Hasan, S.K.; Mizan, R. Development and performance study of controlled atmosphere for fresh tomato. World J. Eng. Tech. 2016, 4, 168–175. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Pop, O.L.; Dulf, F.V.; Socaciu, C. Antimicrobial efficiency of edible films in food industry. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2015, 43, 302–312. [Google Scholar] [CrossRef]
- Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Serrano, M. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J. Sci. Food Agric. 2008, 88, 1287–1293. [Google Scholar] [CrossRef]
- Limchoowong, N.; Sricharoen, P.; Techawongstien, S.; Chanthai, S. An iodine supplementation of tomato fruits coated with an edible film of the iodide-doped chitosan. Food Chem. 2016, 200, 223–229. [Google Scholar] [CrossRef]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Reyes-González, L.R.; Regalado, C. Characterization and antimicrobial effect of starch-based edible coating suspensions. Food Hydrocoll. 2016, 52, 906–913. [Google Scholar] [CrossRef]
- Trachtenberg, S.; Mayer, A.M. Composition and properties of Opuntia ficus-indica mucilage. Phytochemistry 1981, 20, 2665–2668. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica cladodes as rich source of bioactive compounds with health-promoting properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Bernardino-Nicanor, A.; Hinojosa-Hernández, E.N.; Juárez-Goiz, J.M.S.; Montañez-Soto, J.L.; Ramírez-Ortiz, M.E.; González-Cruz, L. Quality of Opuntia robusta and its use in development of mayonnaise-like product. J. Food Sci. Technol. 2015, 52, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and characterization of edible films based on mucilage of Opuntia ficus-Indica (L.). J. Food Sci. 2010, 75, E347–E352. [Google Scholar] [CrossRef] [PubMed]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- de la Paz Salgado-Cruz, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.; Farrera-Rebollo, R.R.; Méndez-Méndez, J.V.; Díaz-Ramírez, M. Chia (Salvia hispanica L.) seed mucilage release characterisation. A microstructural and image analysis study. Ind. Crop. Prod. 2013, 51, 453–462. [Google Scholar] [CrossRef]
- du Toit, A.; de Wit, M.; Hugo, A. Cultivar and harvest month influence the nutrient content of Opuntia spp. cactus pear cladode mucilage extracts. Molecules 2018, 23, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Ishurd, O.; Zgheel, F.; Elghazoun, M.; Elmabruk, M.; Kermagi, A.; Kennedy, J.F.; Knill, C.J. A novel (1→ 4)-α-d-glucan isolated from the fruits of Opuntia ficus indica (L.) Miller. Carbohydr. Polym. 2010, 82, 848–853. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Rodríguez-García, M.E.; Gutiérrez-Cortez, E.; del Carmen Valderrama-Bravo, M.; Rojas-Molina, J.I.; Rivera-Muñoz, E.M. Physicochemical and rheological characterization of Opuntia ficus mucilage at three different maturity stages of cladode. Eur. Polym. J. 2016, 78, 226–234. [Google Scholar] [CrossRef]
- Lian, X.; Wang, C.; Zhang, K.; Li, L. The retrogradation properties of glutinous rice and buckwheat starches as observed with FT-IR, 13C NMR and DSC. Int. J. Biol. Macromol. 2014, 64, 288–293. [Google Scholar] [CrossRef]
- Díaz, K.M.; Reyes, T.F.; Cabrera, L.P.; Sánchez, M.D.; García, M.A.; de Posada Piñán, E. Characterization of laser-treated Opuntia using FT-IR spectroscopy and thermal analysis. Appl. Phys. A 2013, 112, 221–224. [Google Scholar] [CrossRef]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, M.V.; Mahajan, Y.S. Assessment of feasibility of natural coagulants in turbidity removal and modeling of coagulation process. Desalin. Water Treat. 2014, 52, 5812–5821. [Google Scholar] [CrossRef]
- Kalegowda, P.; Chauhan, A.S.; Urs, S.M.N. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ruelas-Chacon, X.; Contreras-Esquivel, J.C.; Montañez, J.; Aguilera-Carbo, A.F.; Reyes-Vega, M.L.; Peralta-Rodriguez, R.D.; Sanchéz-Brambila, G. Guar gum as an edible coating for enhancing shelf-life and improving postharvest quality of roma tomato (Solanum lycopersicum L.). J. Food Qual. 2017, 2017, 8608304. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Kupski, L.; Rocha, M.D.; Oliveira, M.D.S.; Buffon, J.G.; Furlong, E.B. Application of protein-phenolic based coating on tomatoes (Lycopersicum esculentum). Food Sci. Technol. 2012, 32, 594–598. [Google Scholar] [CrossRef]
- Clifford, S.C.; Arndt, S.K.; Popp, M.; Jones, H.G. Mucilages and polysaccharides in Ziziphus species (Rhamnaceae): Localization, composition and physiological roles during drought-stress. J. Exp. Bot. 2002, 53, 131–138. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid. Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Irfan, P.K.; Vanjakshi, V.; Prakash, M.K.; Ravi, R.; Kudachikar, V.B. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 2013, 82, 70–75. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vasilakakis, M.; Diamantidis, G.; Mignani, I. The effect of postharvest calcium application on tissue calcium concentration, quality attributes, incidence of flesh browning and cell wall physicochemical aspects of peach fruits. Food Chem. 2007, 100, 1385–1392. [Google Scholar] [CrossRef]
- García, J.M.; Herrera, S.; Morilla, A. Effects of postharvest dips in calcium chloride on strawberry. J. Agric. Food. Chem. 1996, 44, 30–33. [Google Scholar] [CrossRef]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino Vesuviano” tomatoes. J. Food Compos. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Montesano, D.; Fallarino, F.; Cossignani, L.; Bosi, A.; Simonetti, M.S.; Puccetti, P.; Damiani, P. Innovative extraction procedure for obtaining high pure lycopene from tomato. Eur. Food Res. Technol. 2008, 226, 327. [Google Scholar] [CrossRef]
- Abebe, Z.; Tola, Y.B.; Mohammed, A. Effects of edible coating materials and stages of maturity at harvest on storage life and quality of tomato (Lycopersicon Esculentum Mill.) fruits. Afr. J. Agric. Res. 2017, 12, 550–565. [Google Scholar] [CrossRef]
- Javanmardi, J.; Kubota, C. Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Biol. Technol. 2006, 41, 151–155. [Google Scholar] [CrossRef]


| Edible Coating Sample | % Soluble Solids | Solvent | Cladode Tissue |
|---|---|---|---|
| C1 | 12 | Water | parenchyma |
| C2 | 12 | Ethanol | parenchyma |
| C3 | 12 | Mix * | parenchyma |
| C4 | 12 | Water | chlorenchyma |
| C5 | 12 | Ethanol | chlorenchyma |
| C6 | 12 | Mix * | chlorenchyma |
| Sample | Lycopene Content (mg·100 g) | |
|---|---|---|
| Fresh Weight Basis | Dry Weight Basis | |
| Control | 168 ± 12 | 3685 ± 250 |
| C1 | 132 ± 9.0 | 2714 ± 184 |
| C2 | 137 ± 11 | 2854 ± 218 |
| C3 | 143 ± 13 | 3069 ± 284 |
| C4 | 163 ± 14 | 3456 ± 303 |
| C5 | 160 ± 18 | 3385 ± 299 |
| C6 | 152 ± 12 | 3096 ± 263 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardino-Nicanor, A.; Montañez-Soto, J.L.; Conde-Barajas, E.; Negrete-Rodríguez, M.d.l.L.X.; Teniente-Martínez, G.; Vargas-León, E.A.; Juárez-Goiz, J.M.S.; Acosta-García, G.; González-Cruz, L. Spectroscopic and Structural Analyses of Opuntia Robusta Mucilage and Its Potential as an Edible Coating. Coatings 2018, 8, 466. https://doi.org/10.3390/coatings8120466
Bernardino-Nicanor A, Montañez-Soto JL, Conde-Barajas E, Negrete-Rodríguez MdlLX, Teniente-Martínez G, Vargas-León EA, Juárez-Goiz JMS, Acosta-García G, González-Cruz L. Spectroscopic and Structural Analyses of Opuntia Robusta Mucilage and Its Potential as an Edible Coating. Coatings. 2018; 8(12):466. https://doi.org/10.3390/coatings8120466
Chicago/Turabian StyleBernardino-Nicanor, Aurea, José Luis Montañez-Soto, Eloy Conde-Barajas, María de la Luz Xochilt Negrete-Rodríguez, Gerardo Teniente-Martínez, Enaim Aída Vargas-León, José Mayolo Simitrio Juárez-Goiz, Gerardo Acosta-García, and Leopoldo González-Cruz. 2018. "Spectroscopic and Structural Analyses of Opuntia Robusta Mucilage and Its Potential as an Edible Coating" Coatings 8, no. 12: 466. https://doi.org/10.3390/coatings8120466






