Chitosan Based Regenerated Cellulose Fibers Functionalized with Plasma and Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasound Treatment
2.3. Plasma Treatment
2.4. Molecular and Supramolecular Properties
2.5. Moisture and Water Sorption Properties
2.6. Tensile Properties
2.7. Thermal Stability
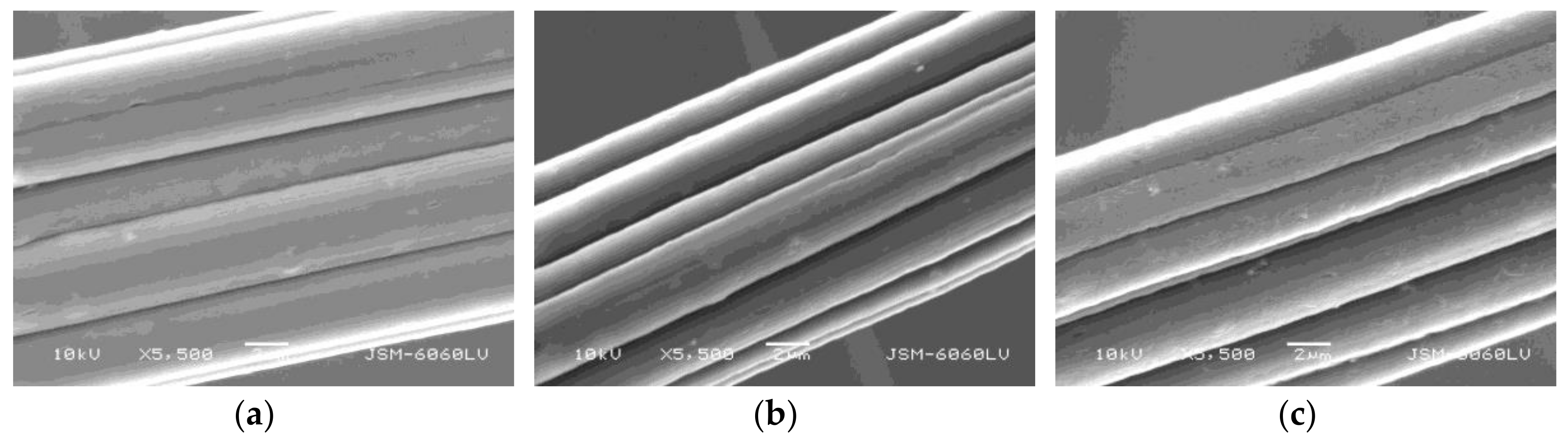
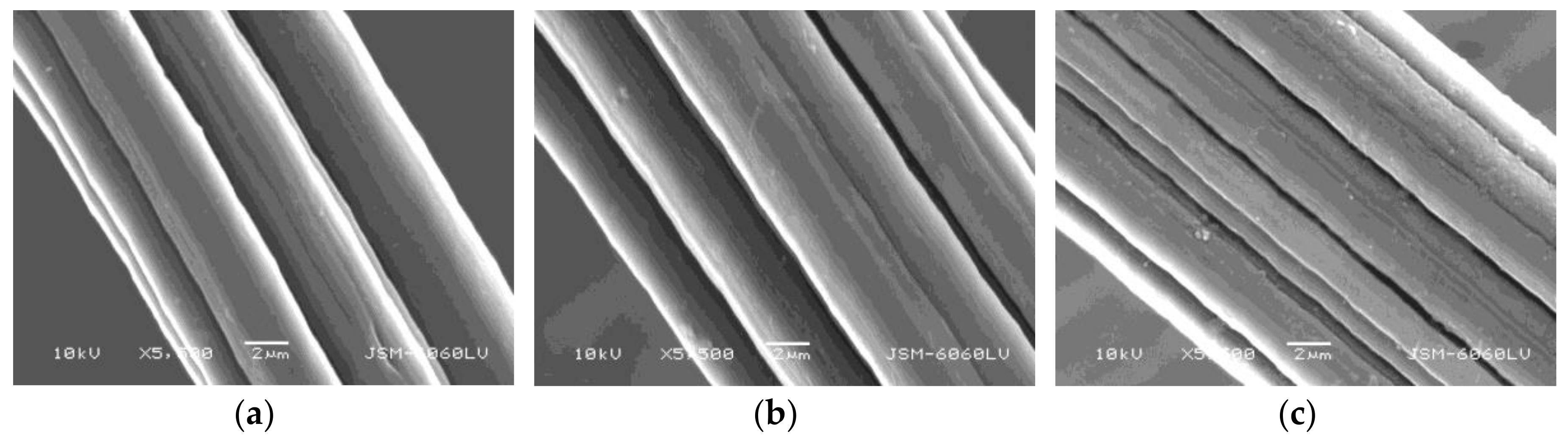
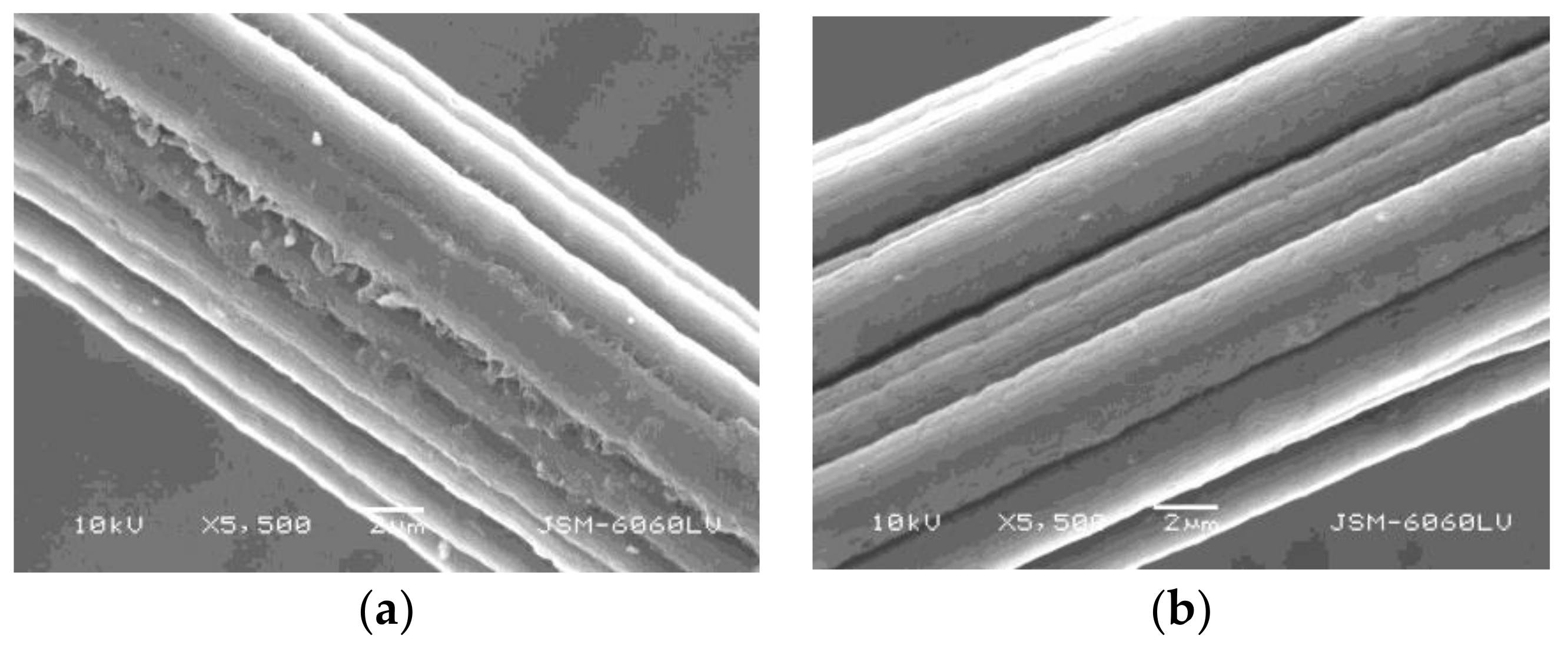
2.8. Surface Morphology
3. Results and Discussion
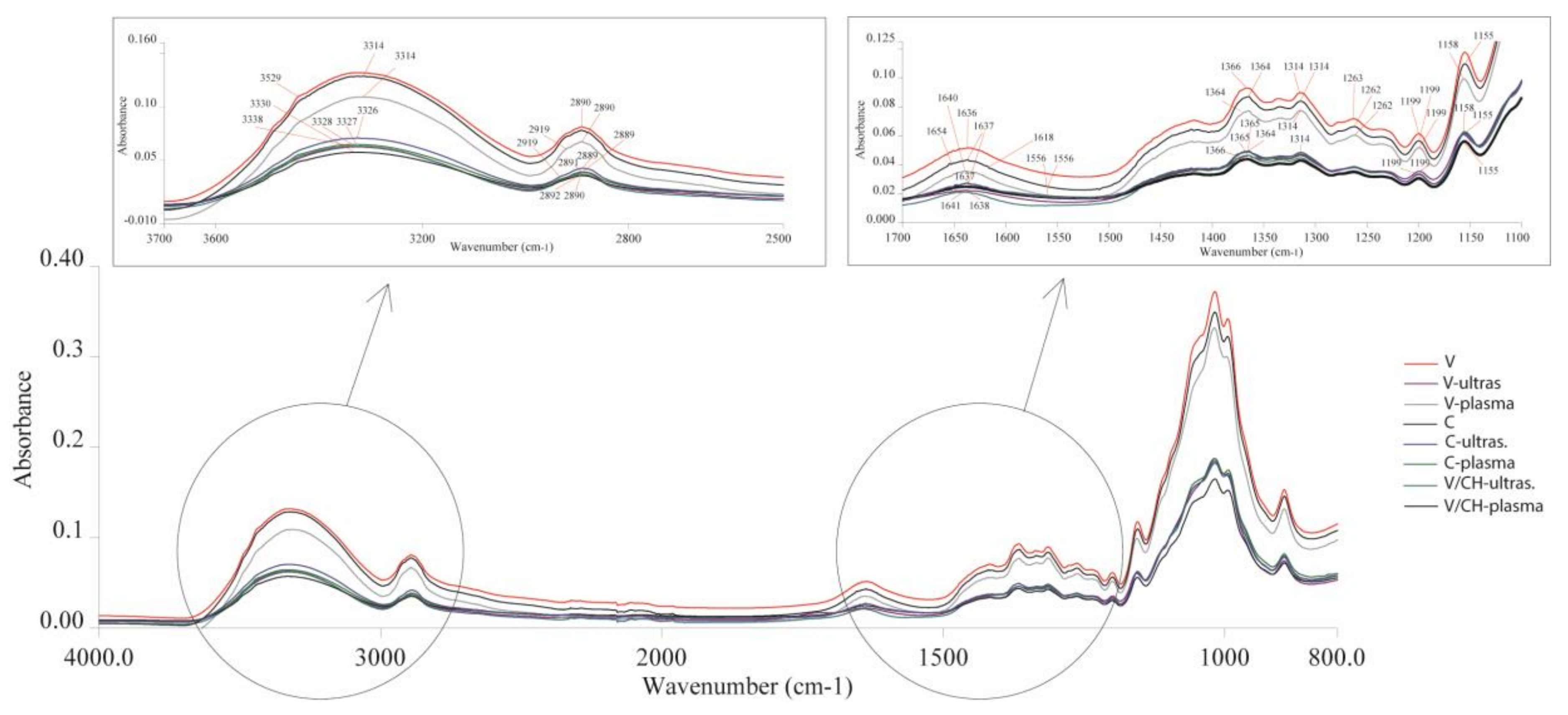
3.1. Structural Characteristics
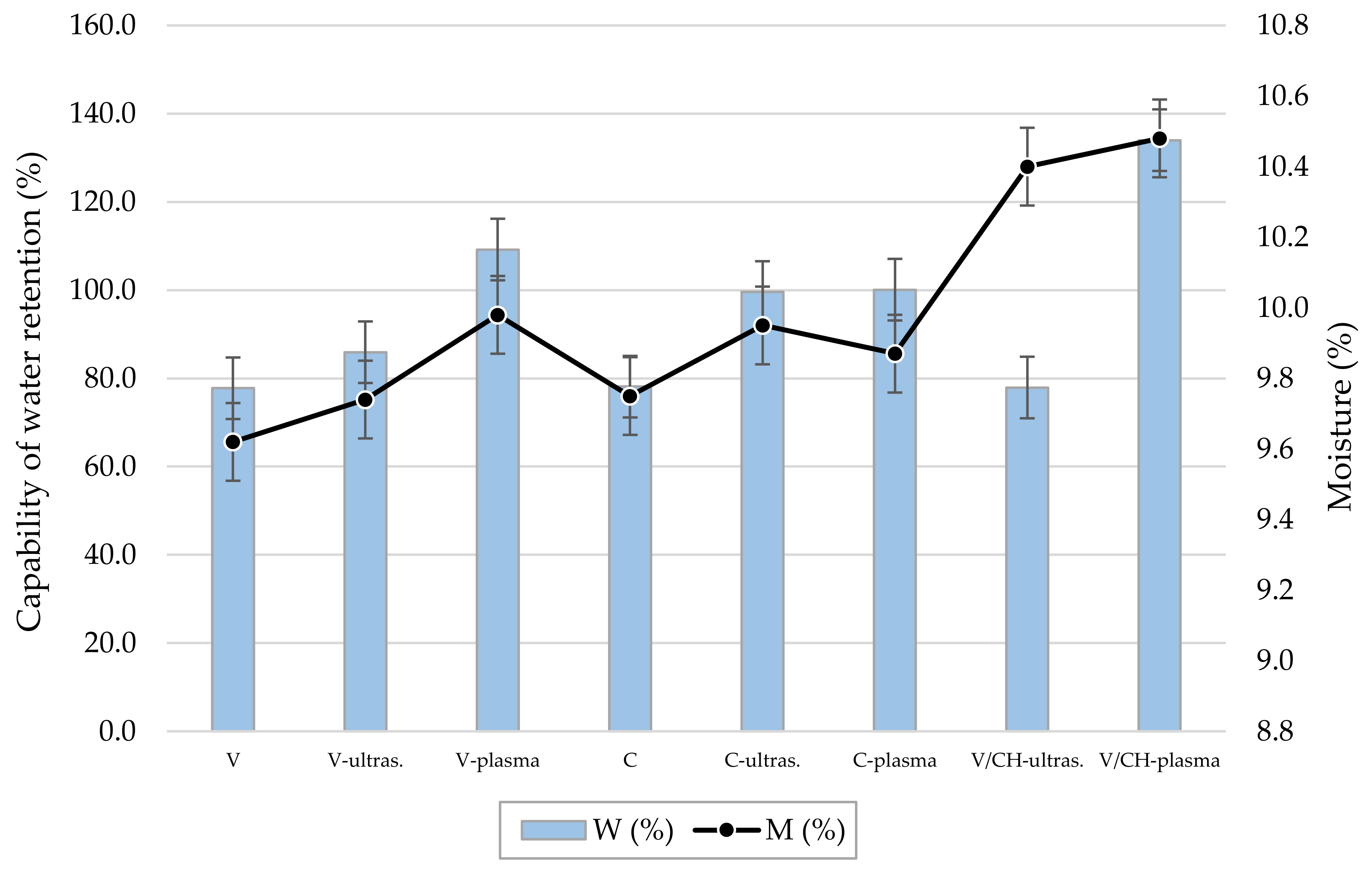
3.2. Sorption Properties
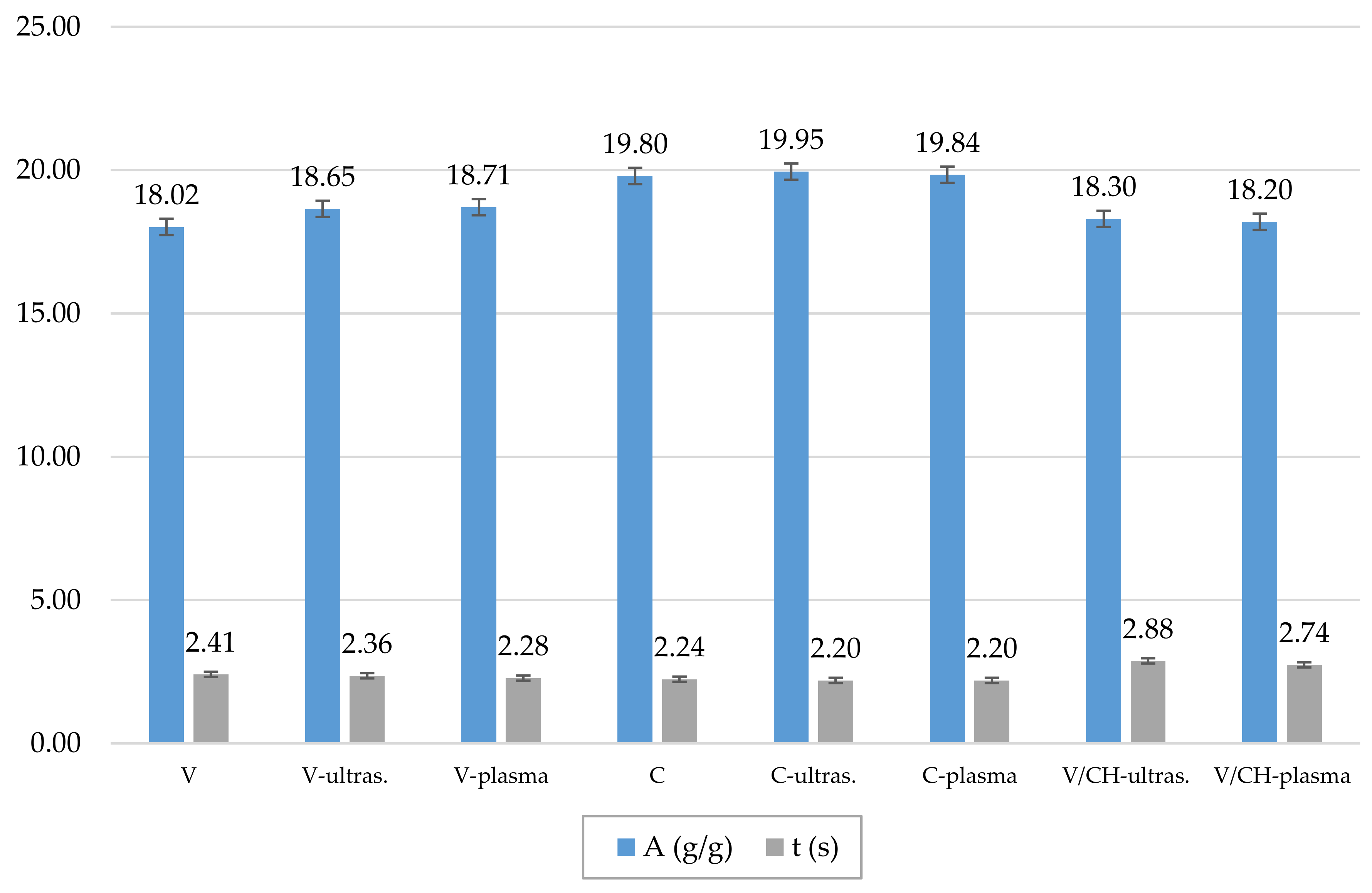
3.3. Tensile Properties
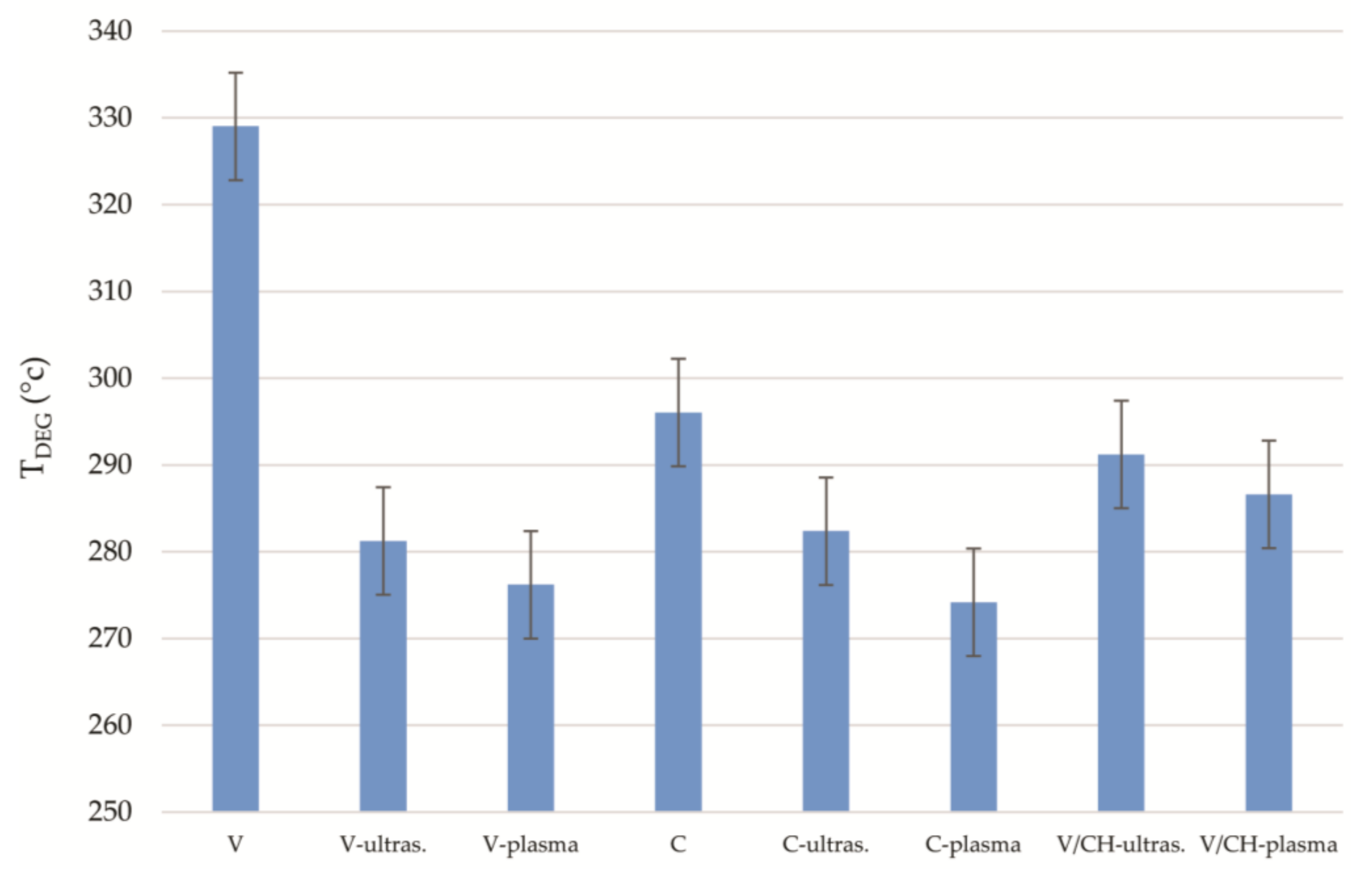
3.4. Thermal Stability
3.5. Surface Morphology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Božič, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohyd. Polym. 2012, 87, 2388–2398. [Google Scholar] [CrossRef]
- Pedersen, G.L.; Screws, G.A.; Cedroni, D.M. Biopolishing of cellulosic fabrics. Can. Text. J. 1992, 109, 31. [Google Scholar]
- Anand, S.C.; Kennedy, J.F.; Miraftab, M.; Rajendran, S. Medical Textiles and Biomaterials for Healthcare; Woodhead Publishing Limited: Cambridge, UK, 2005; pp. 90–98. [Google Scholar]
- Vrabič Brodnjak, U.; Gregor-Svetec, D. Preparation and characterization of chitosan coatings onto regular cellulose fibers with ultrasound technique. J. Coat. Technol. Res. 2013, 10, 247–254. [Google Scholar] [CrossRef]
- Bhuiyan, M.R.; Hossain, M.A.; Zakaria, M.; Islam, M.N.; Uddin, M.Z. Chitosan coated cotton fiber: Physical and antimicrobial properties for apparel use. J. Polym. Environ. 2017, 25, 334–342. [Google Scholar] [CrossRef]
- Ahamed, M.N.; Sankar, S.; Kashif, P.M.; Basha, S.H.; Sastry, T.P. Evaluation of biomaterial containing regenerated cellulose and chitosan incorporated with silver nanoparticles. Int. J. Biol. Macromol. 2015, 72, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Qin, A.; Li, X.; He, C. High tenacity regenerated chitosan fibers prepared by using the binary ionic liquid solvent (Gly·HCl)-[Bmim] Cl. Carbohyd. Polym. 2013, 97, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polm. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Liu, X.D.; Nishi, N.; Tokura, S.; Sakairi, N. Chitosan coated cotton fiber: preparation and physical properties. Carbohyd. Polym. 2001, 44, 233–238. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, Z.; Luo, X.; Zhang, H.; Liu, Y.; Cai, N.; Xue, Y.; Yu, F. Citrate-modified maghemite enhanced binding of chitosan coating on cellulose porous membranes for potential application as wound dressing. Carbohyd. Polym. 2017, 166, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Fras, L.; Ristić, T.; Tkavc, T. Adsorption and Antibacterial Activity of Soluble and Precipitated Chitosan on Cellulose Viscose Fibers. J. Eng. Fiber. Fabr. 2012, 7, 50–57. [Google Scholar]
- Vrabič Brodnjak, U.; Gregor-Svetec, D.; Klančnik, M. Influence of enzymatic treatment on the structural, sorption and dyeing properties of viscose and chitosan/cellulose fibers. Text. Res. J. 2016, 86, 990–1005. [Google Scholar] [CrossRef]
- Kitkulnumchai, Y.; Ajavakom, A.; Sukwattanasinitt, M. Treatment of oxidized cellulose fabric with chitosan and its surface activity towards anionic reactive dyes. Cellulose 2008, 15, 599–608. [Google Scholar] [CrossRef]
- Shen, S.S.; Yang, J.J.; Liu, C.X.; Bai, R.B. Immobilization of copper ions on chitosan/cellulose acetate blend hollow fiber membrane for protein adsorption. RSC Adv. 2017, 7, 10424–10431. [Google Scholar] [CrossRef]
- Shimizu, Y.; Dohmyou, M.; Yoshikawa, M.; Takagishi, T. Dyeing chitin/cellulose composite fibers with reactive dyes. Text. Res. J. 2004, 74, 34–38. [Google Scholar] [CrossRef]
- HPS, A.K.; Saurabh, C.K.; Adnan, A.S.; Fazita, M.N.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohyd. Polym. 2016, 150, 216–226. [Google Scholar]
- Ahmad, M.; Ahmed, S.; Swami, B.L.; Ikram, S. Adsorption of heavy metal ions: Role of chitosan and cellulose for water treatment. Langmuir 2015, 79, 109–155. [Google Scholar]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Wu, T.; Farnood, R. A preparation method of cellulose fiber networks reinforced by glutaraldehyde-treated chitosan. Cellulose 2015, 22, 1955–1961. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Effect of plasma superficial treatments on antibacterial functionalization and coloration of cellulosic fabrics. Appl. Surf. Sci. 2017, 392, 1126–1133. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Y. The application of He/O2 atmospheric pressure plasma jet and ultrasound in desizing of blended size on cotton fabrics. Appl. Surf. Sci. 2012, 258, 7787–7793. [Google Scholar] [CrossRef]
- Erra, P.; Molina, R.; Jocic, D.; Julia, M.R.; Cuesta, A.; Tascon, J.M.D. Shrinkage properties of wool treated with low temperature plasma and chitosan biopolymer. Text. Res. J. 1999, 69, 811–815. [Google Scholar] [CrossRef]
- Perincek, S.; Uzgur, A.E.; Duran, K.; Dogan, A.; Korlu, A.E.; Bahtiyari, İ.M. Design parameter investigation of industrial size ultrasound textile treatment bath. Ultrason. Sonochem. 2009, 16, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Ghoranneviss, M.; Sharifi, S.D. Effect of atmospheric pressure plasma treatment/followed by chitosan grafting on antifelting and dyeability of wool fabric. J. Fusion Energy 2014, 33, 177–183. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Wang, Q.; Sun, Q.; Lucia, L.A.; Chen, J. Ultrasound-assisted xylanase treatment of chemi-mechanical poplar pulp. BioResources 2016, 11, 4104–4112. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Abdel-Halim, E.S.; Al-Othman, Z.A. Low-Temperature Bleaching of Cotton Cellulose using an Ultrasound-assisted Tetraacetylethylenediamine/Hydrogen Peroxide/Triethanolamine System. BioResources 2016, 11, 2784–2796. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.; Zhao, Z.; Yi, S.; Mu, J.; Wang, X. Influence of ultrasound pretreatment on wood physiochemical structure. Ultrason. Sonochem. 2017, 34, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Tissera, N.D.; Wijesena, R.N.; De Silva, K.N. Ultrasound energy to accelerate dye uptake and dye–fiber interaction of reactive dye on knitted cotton fabric at low temperatures. Ultrason. Sonochem. 2016, 29, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.Q.; Sitaru, R.; Denes, F.; Young, R.A. Mechanisms of oxygen-and argon-RF-plasma-induced surface chemistry of cellulose. Plasmas Polym. 1997, 2, 199–224. [Google Scholar] [CrossRef]
- Prabhu, S.; Vaideki, K.; Anitha, S. Effect of microwave argon plasma on the glycosidic and hydrogen bonding system of cotton cellulose. Carbohyd. Polym. 2017, 156, 34–44. [Google Scholar] [CrossRef] [PubMed]
| Sample | Fibers | Type of Treatment |
|---|---|---|
| V | Regenerated cellulose fibers | Untreated |
| V-ultras. | Regenerated cellulose fibers | Ultrasound |
| V-plasma | Regenerated cellulose fibers | Plasma |
| C | Biocomposite regenerated cellulose/chitosan | Untreated |
| C-ultras. | Biocomposite regenerated cellulose/chitosan | Ultrasound |
| C-plasma | Biocomposite regenerated cellulose/chitosan | Plasma |
| V/CH-ultras. | Regenerated cellulose fibers | Chitosan+ultrasound |
| V/CH-plasma | Regenerated cellulose fibers | Chitosan+plasma |
| Sample | DP | s | Xc (%) | s | for | s |
|---|---|---|---|---|---|---|
| V | 510.58 | 0.25 | 75.50 | 0.27 | 0.4457 | 0.0388 |
| V-ultras. | 485.63 | 0.69 | 75.17 | 0.56 | 0.3584 | 0.0254 |
| V-plasma | 408.65 | 1.00 | 74.41 | 0.71 | 0.3321 | 0.0287 |
| C | 458.40 | 0.88 | 74.07 | 0.48 | 0.4603 | 0.1446 |
| C-ultras. | 441.27 | 0.75 | 69.75 | 0.34 | 0.3359 | 0.1147 |
| C-plasma | 410.36 | 0.87 | 70.82 | 0.93 | 0.3778 | 0.0416 |
| V/CH-ultras. | 439.76 | 0.36 | 62.59 | 0.28 | 0.3639 | 0.0585 |
| V/CH-plasma | 437.88 | 0.47 | 67.58 | 0.88 | 0.3427 | 0.0769 |
| Sample | σ (cN/dtex) | s | E (%) | s | σy (cN/dtex) | s | E0 (GPa) | s |
|---|---|---|---|---|---|---|---|---|
| V | 2.51 | 0.60 | 33.20 | 2.00 | 0.80 | 0.02 | 4.59 | 0.21 |
| V-ultras. | 2.48 | 0.19 | 35.82 | 1.07 | 0.72 | 0.04 | 4.25 | 0.45 |
| V-plasma | 2.28 | 0.81 | 37.64 | 0.87 | 0.73 | 0.10 | 3.89 | 0.36 |
| C | 2.15 | 0.77 | 36.71 | 1.05 | 1.30 | 0.04 | 6.52 | 0.32 |
| C-ultras. | 2.85 | 0.65 | 39.23 | 0.97 | 1.24 | 0.08 | 3.55 | 0.74 |
| C-plasma | 2.86 | 0.54 | 41.76 | 0.89 | 1.38 | 0.09 | 3.84 | 0.65 |
| V/CH-ultras. | 2.61 | 0.40 | 36.20 | 1.16 | 0.77 | 0.08 | 2.34 | 0.28 |
| V/CH-plasma | 2.46 | 0.69 | 46.00 | 1.04 | 0.85 | 0.14 | 2.39 | 0.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrabič Brodnjak, U.; Jesih, A.; Gregor-Svetec, D. Chitosan Based Regenerated Cellulose Fibers Functionalized with Plasma and Ultrasound. Coatings 2018, 8, 133. https://doi.org/10.3390/coatings8040133
Vrabič Brodnjak U, Jesih A, Gregor-Svetec D. Chitosan Based Regenerated Cellulose Fibers Functionalized with Plasma and Ultrasound. Coatings. 2018; 8(4):133. https://doi.org/10.3390/coatings8040133
Chicago/Turabian StyleVrabič Brodnjak, Urška, Adolf Jesih, and Diana Gregor-Svetec. 2018. "Chitosan Based Regenerated Cellulose Fibers Functionalized with Plasma and Ultrasound" Coatings 8, no. 4: 133. https://doi.org/10.3390/coatings8040133






