3. Results and Discussion
Fe-Ni alloys with different Ni contents ranging from 35 to 43 wt % were electroformed under the bath conditions and process parameters given in
Table 1.
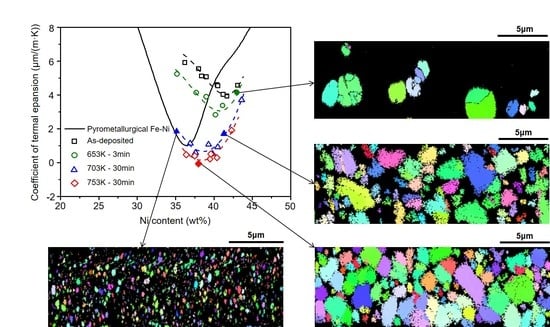
Figure 1 shows the experimentally measured CTEs of the electroformed Fe-Ni alloys and the CTEs of Fe-Ni alloys found in the literature [
1] for comparison. The CTEs as a function of the alloy composition of the electroformed Fe-Ni alloys differ substantially from the CTE curve of the Fe-Ni alloy produced using a pyrometallurgical method. The CTEs of the as-deposited Fe-Ni alloys are much higher than that of Invar, and the minimum CTE of approximately 4 μm/(m·K) occurs at a Ni composition of approximately 41 wt %. However, the CTEs of the electroformed Fe-Ni alloys were drastically reduced after heat treatment.
As presented in
Figure 1, the electroformed Fe-Ni alloys with Ni contents ranging from 36 to 41 wt % after annealing above approximately 753 K (480 °C) for 30 min exhibited CTEs lower than that of conventional Invar [
1] and similar to that of the commercial Invar applied currently for the OLED FMM. Therefore,
Figure 1 raises several questions. First, why is the CTE in the as-deposited state of the electroformed Invar considerably higher than that of the pyrometallurgically produced Invar? Second, why do the CTEs of the electroformed Fe-Ni alloys decrease with heat treatment? Third, why does the composition of the alloy that exhibits the minimum CTE differ between the electroformed and pyrometallurgically produced Fe-Ni alloys? These phenomena are clarified based on the experimental results as follows.
A sample of the Fe-Ni alloy sheet was intentionally fabricated to exhibit a compositional gradient along the thickness direction. To achieve this, the electroforming conditions were modified based on the conditions given in
Table 1 as follows: 97 g/L of FeSO
4·7H
2O, 85 g/L of NiSO
4·6H
2O, pH = 2.5, current density rate of 0.2 mA/cm
2∙min from 10 to 20 mA/cm
2, and the other conditions were fixed.
Figure 2a presents the compositional gradient from 40 wt % Ni at the surface (hereafter, the D-surface) that initially deposited onto the cathode to 31 wt % Ni at the other surface (hereafter, the F-surface) of a sample with a thickness of 10 µm. This compositional gradient was maintained within the measurement error after the samples were annealed at temperatures up to 753 K (480 °C).
Figure 2b,c depict the X-ray diffraction (XRD) data collected for the D-surface and the F-surface, respectively. Because the thickness of the samples, 10 μm, could be penetrated by X-rays, the peaks in the XRD patterns of the D-surfaces reflect the structures of the sides of the F-surfaces and vice versa, although the XRD intensities decrease with increasing distance from the measurement position.
In the Fe-Ni alloy system, true thermodynamic equilibrium is never reached at temperatures below 653 K (380 °C) because of the slow inter-diffusion of Fe and Ni atoms. Consequently, the stable phases with body-centered cubic (BCC) structures contain as much as approximately 4 wt % Ni, and metastable BCC phases can contain up to approximately 25 wt % Ni [
3]. However, in the as-deposited state of the electroformed Fe-Ni alloys fabricated in the current work, the BCC phase, hereafter denoted by α, and the face-centered cubic (FCC) phase, hereafter denoted by γ′, coexist in the composition range between 36 and 41 wt % Ni. The single α phase exists in the compositions with less than 36 wt % Ni, and the single γ′ phase exists in the compositions of more than 41 wt % Ni. The authors of a previous study [
4] reported similar results concerning the relation between the phases and the alloy compositions in electrodeposits fabricated using bath conditions and process parameters that differ from those used in the present work. The broadening of the peaks observed in the XRD patterns of the as-deposited state are attributed to the electroformed Fe-Ni alloy that consists of nanometer-sized crystallites. The mean grain size calculated using Scherrer’s formula [
5], i.e.,
t = 0.9λ/
Bcosθ
B, is approximately 7 nm. A previous study [
6] reported that the morphology of nanometer-sized grains observed using a transmission electron microscope is equiaxed.
The EBSD orientation maps observed in the cross-sections of the annealed samples are given in
Figure 2d–f. When the electroformed Fe-Ni alloy with the compositional gradient shown in
Figure 2a was annealed at 653 K (380 °C) for 3 min, abnormal grain growth (AGG) of the nanometer-sized crystallites occurred only in the section of the sample with a Ni content > 36 wt %, i.e., to a distance approximately 4 μm from the D-surface, see
Figure 2d. Because the resolution limit of the EBSD measurements is 20 nm, the nanocrystalline section of the sample appears dark in the EBSD map. As presented in
Figure A1, AGG occurs during annealing above 623 K (350 °C) in the electroformed nanocrystalline Fe-Ni alloys with the FCC structure [
7]. Therefore, as evident in
Figure 2d, AGG occurred only in the γ′ phase; the α phase maintained the nanocrystalline state. Hereafter, the FCC phase formed by AGG during annealing is denoted by γ″ to distinguish it from γ′, which is the as-deposited FCC phase.
In
Figure 2e, which shows the EBSD map for the cross-section of the sample annealed at 703 K (430 °C) for 30 min, grain growth is also observed in a part of the nanocrystalline α phase, but far from AGG in terms of the scale change in grain size. Importantly, it was analyzed using EBSD that the grown grains were transformed into the FCC structure, i.e., the α → γ″ phase transition occurred. Notably, grain growth of the α phase was not observed. The dark area in the EBSD map represents the α phase that has maintained its nanocrystalline state, which is confirmed by broadening of the α{110}-peak in the XRD pattern of the F-surface of the sample as depicted in
Figure 2c. When the sample was annealed at 753 K (480 °C) for 30 min, as observed in
Figure 2f, a small portion of the α phase still maintained the nanocrystalline state among far more developed grains. Despite the microstructure changes, such as the α → γ″ phase transition and grain growth of the transformed γ″ phase, the through-thickness compositional gradient is maintained within the measurement error, as shown in
Figure 2a. This result indicates that the microstructure changes occurred without a compositional change.
To examine the stability of the α phase in the as-deposited state and the phase transition that occurs during annealing, the Fe-35 wt % Ni alloy sheet consisting of the single α phase was electroformed. The process conditions given in
Table 1 were controlled to make the α phase possess two different morphologies of grains, i.e., one is nanocrystalline and the other is columnar.
Figure 3a shows the EBSD map of the cross-section of the sample and the XRD data corresponding to the D-surface. In the entire sample thickness of approximately 30 μm, the initially deposited section of the sample up to approximately 5 μm from the D-surface consisted of the nanocrystalline α phase (dark area in the EBSD map) and a columnar type of large-sized grains, represented by red color, developed in the other section.
When this sample was annealed at 703 K (430 °C) for 30 min and then subsequently water-quenched, as shown in
Figure 3b, the spherical γ″ grains were grown. These γ″ grains were formed by the transformation of the nanocrystalline α phase; additionally, the columnar grains of the α phase transformed into the γ″ phase without a morphological change (the γ″ phase is represented by the green color in
Figure 3b). The α → γ″ phase transition occurring in the nanocrystalline region is more homogeneous than that of the columnar grains. This effect will be discussed later. The amount of the remaining α phase that did not transform into the γ″ phase can be predicted in the Fe-Ni equilibrium phase diagram [
8].
Notably, in
Figure 3, the α → γ″ phase transition can neither occur through atomic diffusion [
9] nor a massive transformation [
10] reported in the Fe-Ni alloy system. The annealing temperature of 703 K (430 °C) is too low for Fe and Ni atoms to move extensively. Another important point concerning
Figure 3 is that the α phase is metastable with respect to maintaining the nanostructure in the as-deposited state but is too unstable for grain growth to occur during annealing. When the grains of the α phase are thermally activated, the transformation of the α phase into the stable γ″ phase may occur faster prior to the occurrence of grain growth in the α phase.
The electroformed Fe-36 wt % Ni alloy consists of a mixture of the α and γ′ phases in the as-deposited state and of the single γ″ phase when fully annealed.
Figure 4 gives an example of the atom configurations for the Fe-36 wt % Ni (Fe-35 at.% Ni) composition, where Fe and Ni atoms are represented by red and green colors, respectively. The FCC structure, as drawn by thin lines, is equivalent to the body-centered tetragonal (BCT) structure. The BCT structure may transform into the BCC structure by altering the interatomic distances without extensive atomic movement [
11]. The lattice parameters of the α and γ′ phases measured using XRD were 0.286 nm (2.86 Å) and 0.360 nm (3.60 Å), respectively (see
Figure A2). Thus, the spatial volumes occupied by the same numbers of atoms in occupy each phase are almost equal, i.e., 2 × 2.86
3/3.60
3 ≒ 1. This structural accommodation enables the unstable α phase to co-deposit with the relatively stable γ′ phase during electroforming for Ni-rich compositions ranging from 36 to 41 wt %.
The difference of which phase, α or γ′, is electrodeposited for the Fe-36 wt % Ni composition modifies not only the interatomic distances to fit the corresponding phase but also neighboring circumstances of the Fe atoms. Consider the Fe atoms indicated by the arrows seen in
Figure 4. The number of the nearest-neighbor Fe atoms with respect to each Fe atom is either 5 for the BCC structure or 8 for the FCC structure. Therefore, the α → γ″ phase transition, as shown in
Figure 3, corresponds to the change from 5 to 8 in the number of the nearest-neighbor Fe atoms. When the Fe-Ni alloy assumes the FCC structure, the maximum number of the nearest-neighbor Fe atoms is 8 at compositions near 36 wt % Ni and decreases with increasing Ni content. In the case in which the number of nearest-neighbor Fe atoms for the BCC structure is 8 at maximum, it is pure Fe. Chikazumi [
12] demonstrated that the Invar anomalies are associated with the energy state of each Fe atom, which strongly depends on the number of nearest-neighbor Fe atoms.
As shown in
Figure 1, for the Fe-36 wt % Ni composition, the CTE of the as-deposited state of the electroformed Invar, which is overwhelmingly dominated by the α phase, is approximately 6 μm/(m·K), whereas it approaches zero in the sample consisting of the single γ″ phase after annealing at 753 K (480 °C) for 30 min. This phenomenon where the CTE decreases because of the α → γ″ phase transition is readily explained by the increase in the number of the nearest-neighbor Fe atoms from 5 to 8. CTEs lower than that of conventional Invar are observed for the samples consisting of the single γ″ phase with Ni contents ranging from 36 to 41 wt %, thus exhibiting a decrease in the number of the nearest-neighbor Fe atoms from 8 to 7 after annealing at 753 K (480 °C) for 30 min. However, even in the samples consisting of the single γ″ phase, when the Ni content is increased to more than 41 wt %, the CTE drastically increases because of the decrease in the number of the nearest-neighbor Fe atoms.
The fact that the Fe-36–41 wt % Ni alloys annealed at 703 K (430 °C) for 30 min exhibit CTEs greater than those of the alloys of the single γ″ phase is attributable to the co-existence of the α phase with the reduced number of nearest-neighbor Fe atoms. Thus, the presence of a small amount of the γ″ phase is responsible for the CTEs of the samples annealed at 653 K (380 °C) for 3 min decreasing only slightly compared with the CTE of the as-deposited material. Nagayama et al. [
13] also reported a substantial decrease of the CTEs due to the BCC → FCC phase transition in electroformed Fe-Ni alloys that exhibited CTEs greater than that of conventional Invar. It is noted that the anomalous co-deposition behavior of Fe and Ni ions during the electrodeposition of Fe-Ni alloys appears significant with increasing Fe contents. As a result, the α phase would grow into a columnar type of large-sized grains rather than nanocrystals as observed in the materials of Nagayama et al. [
13], see also
Figure 3. The development of the columnar structure in the electroformed Fe-Ni alloys results in through-thickness inhomogeneity of the alloy composition. In this case, the gain of low thermal expansivity is difficult in the final products even after sophisticated heat treatment. The homogeneous α → γ″ phase transition that takes place in nanostructured Fe-Ni alloys might lead to their low thermal expansivity. For this, the process conditions given in
Table 1 should be accurately applied for electroforming alloys with Fe-rich compositions.
The previous discussion clarifies the first issue of why the CTE in the as-deposited state of the electroformed Invar is considerably higher than that of pyrometallurgicallay produced Invar and the second issue of why the CTEs of the electroformed Fe-Ni alloys decrease via heat treatment that were brought up by
Figure 1. The third issue of why the alloy composition for the minimum CTE differs for the electroformed and pyrometallurgically produced Fe-Ni alloys can also be clarified based on the number of nearest-neighbor Fe atoms. The minimum Ni content at which the single γ′ phase can exist in the as-deposited state is approximately 41 wt %; thus, the maximum number of nearest-neighbor Fe atoms is 7. It follows that the minimum CTE in the as-deposited state of the electroformed Invar is observed for the Fe-41 wt % Ni composition.
However, to explain significant difference of the CTE vs. composition curve between the electroformed Invar and the conventional Invar, other factors such as grain boundary phenomena and alloying elements should be considered together with the effect of the phase structure as mentioned above. The as-deposited Invar consisting of nanometer-sized crystallites possesses much larger amount of grain boundaries than the coarse-grained conventional Invar. It has recently been reported that the grain boundary segregation and films of grain boundary phases can allow to “hide” a lot of atoms in grain boundaries [
14] and thus change the concentration in the matrix [
15]. These grain boundary phenomena can make the CTE curve shift towards the Ni richer composition as well as the higher CTE value in the as-deposited Invar in comparison to the conventional Invar as observed in
Figure 1. On the other hand, there is a fundamental difference between the electroformed and pyrometallurgically produced Fe-Ni alloys in terms of alloying elements. The Fe-Ni alloys fabricated in the current work are a nearly perfect binary Fe-Ni alloy system while most of pyrometallurgically produced Invar alloys contain various kinds of alloying elements [
16]. It is well known that different commercial Invar alloys reveal different relationships of CTE vs. composition [
1]. Effects of alloying elements might be related to the phenomenon that the Invar effect drastically disappears except for around 36 wt % Ni composition in the pyrometallurgically produced Invar while the electroformed Fe-Ni binary alloys after annealing perform low thermal expansivity persistently from 36 up to 41 wt % Ni contents. This is a striking result for practical applications because the anomalous co-deposition behavior occurring during the deposition process is relatively moderated with increasing Ni content.
Finally, the decrease of the CTEs due to the γ′ → γ″ state transition in the alloys consisting of a single FCC phase, i.e., those with Ni contents greater than 41 wt %, should be clarified. In fact, the α → γ″ phase transition that occurs during the annealing of the electroformed Fe-Ni alloys may result from the α → γ′ phase transition followed by the γ′ → γ″ state transition.
Figure A2 reveals that the lattice parameter of the FCC phase decreases from 0.360 to 0.358 nm (3.60–3.58 Å) because of the γ′ → γ″ state transition. The anomalously low thermal expansivity of Invar originates from instability of ferromagnetism around the Invar compositions [
12]. This is strongly related to the electron spin state of Fe atoms. The Weiss model [
17], which serves as the basis of the prevailing models of the Invar anomalies, explains that Fe atoms of the FCC structure of an Fe-Ni alloy can assume two different electron configurations, i.e., the low-spin state and the high-spin state, and that the “Invar effect” occurs because of the thermal activation of the low-spin state, which possesses a smaller atomic volume. In terms of the lattice parameter, the γ′ and γ″ phases observed in the electroformed Fe-Ni alloys may correspond to the high-spin state and the low-spin state, respectively, in the electron configurations [
18]. The lattice parameter of the commercial Invar applied currently for the OLED FMM is approximately 0.358 nm (3.58 Å) at room temperature as measured using XRD under the same conditions used in the present study.