Fungal Growth on Coated Wood Exposed Outdoors: Influence of Coating Pigmentation, Cardinal Direction, and Inclination of Wood Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coatings
2.2. Wood Samples
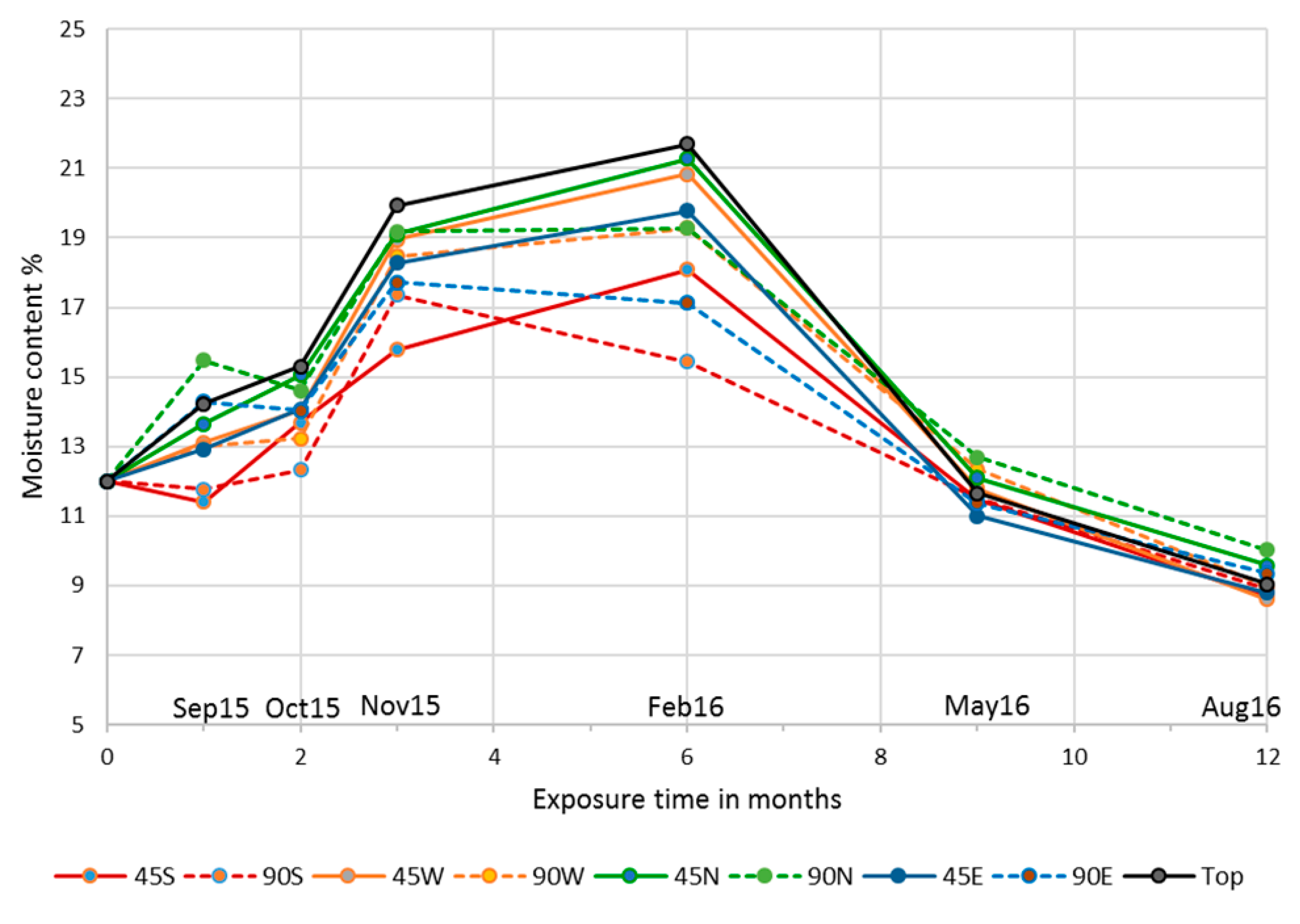
2.3. Natural Weathering Test
2.4. Visual Assessment of Fungal Growth
2.5. Fungal Enumeration on Wood Panels
- a surface analysis
- an in-depth analysis
2.6. Fungal Identification
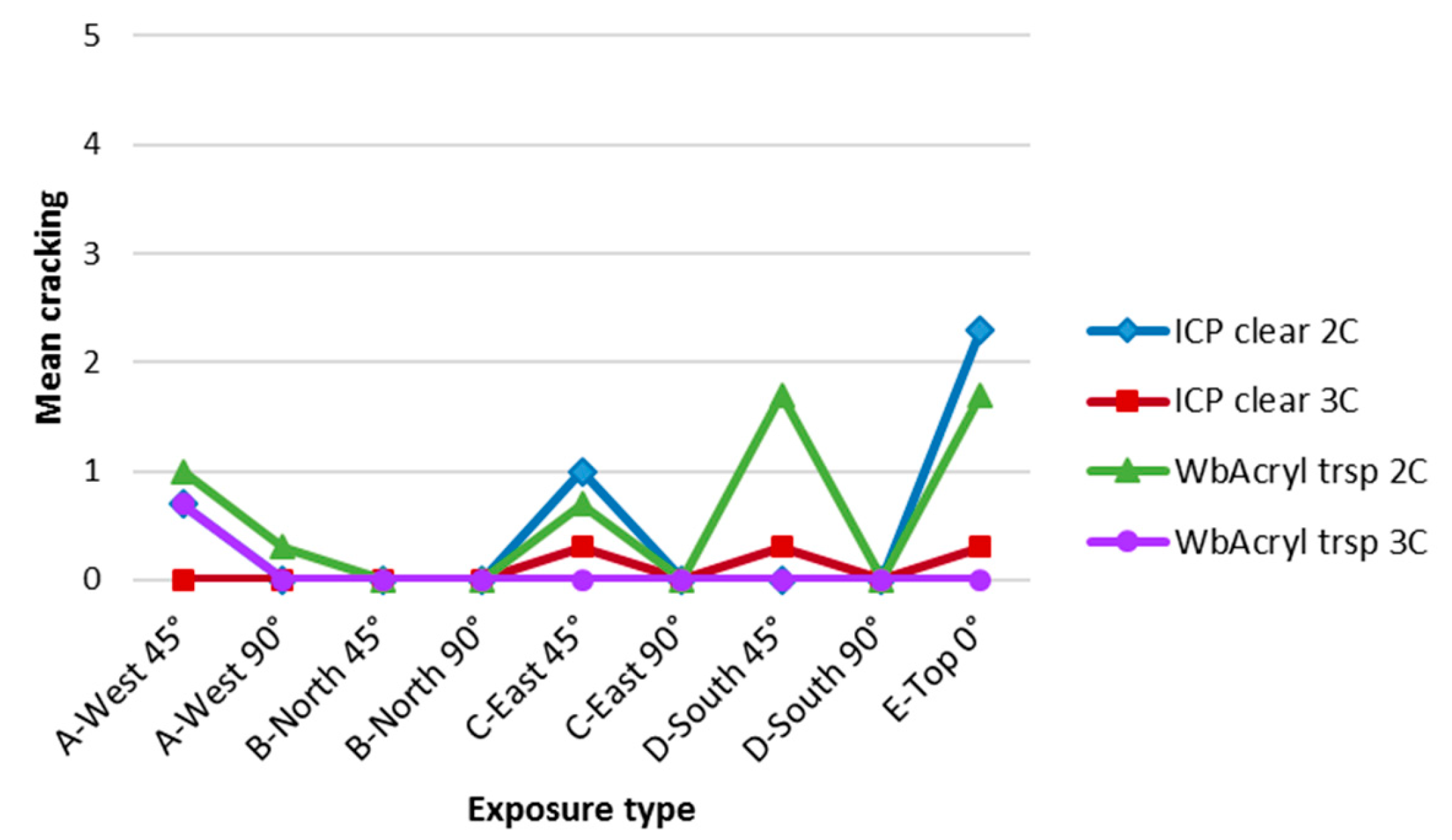
2.7. Cracking
3. Results
3.1. Fungal Growth
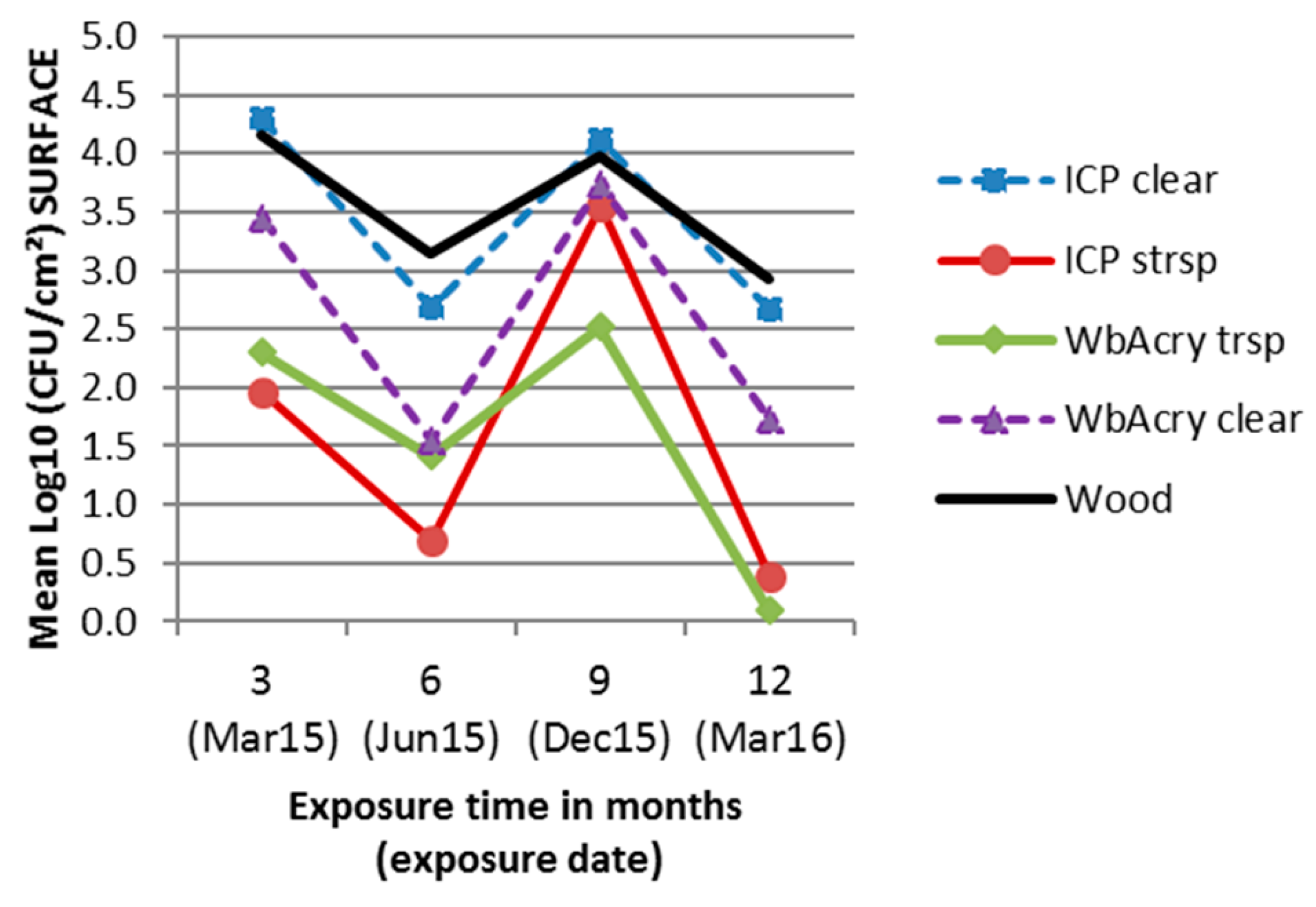
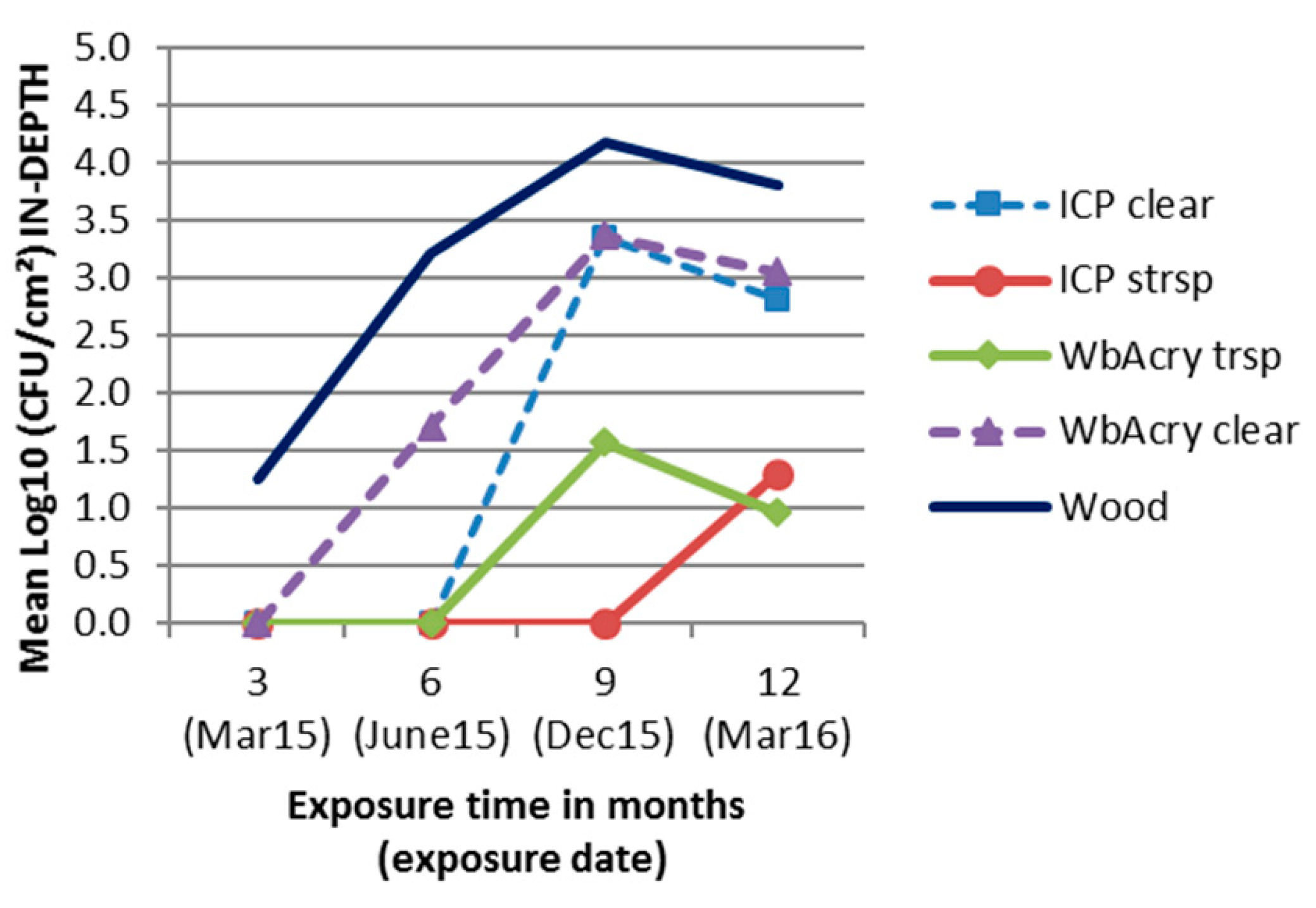
3.2. Fungal Enumeration
3.3. Fungal Identification
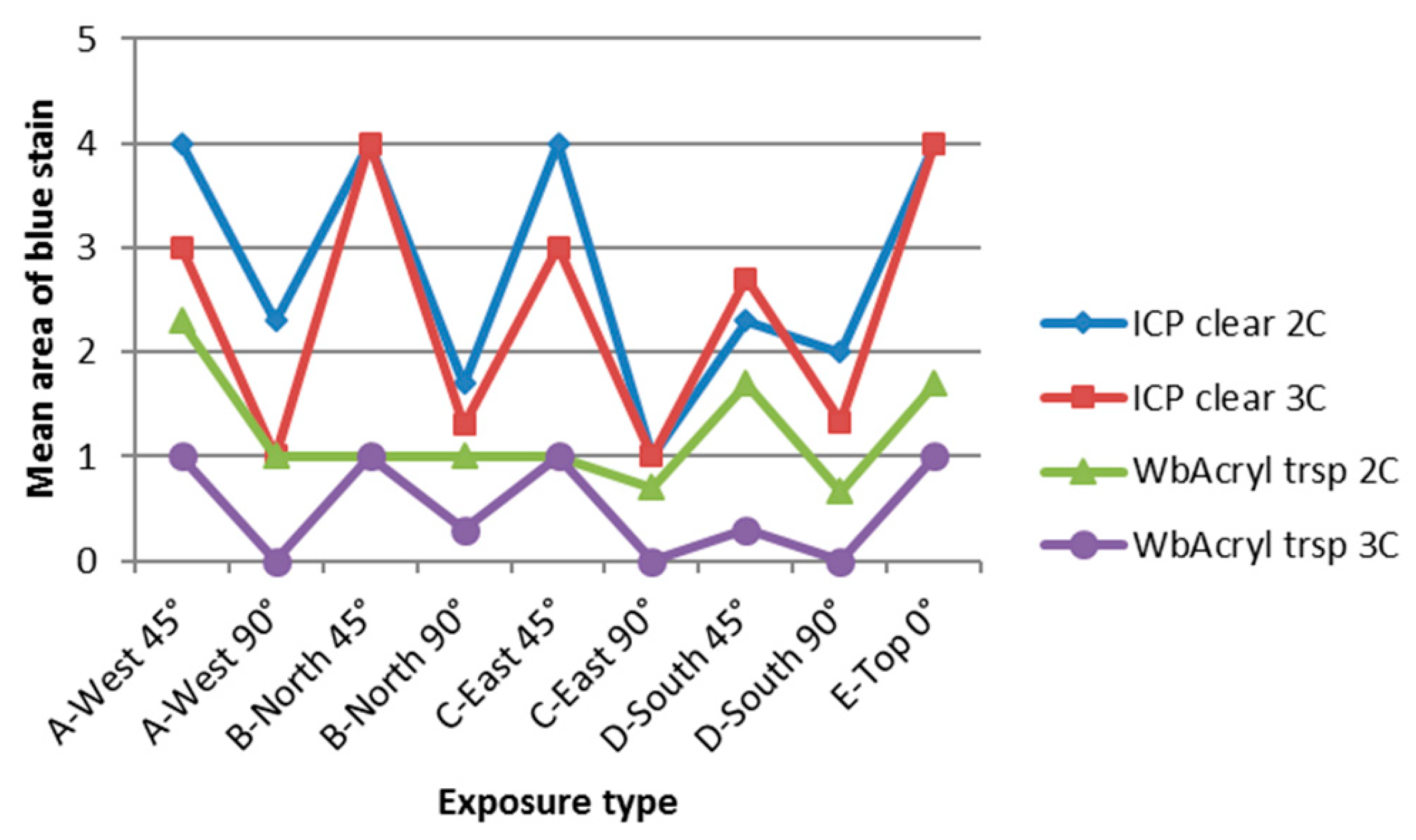
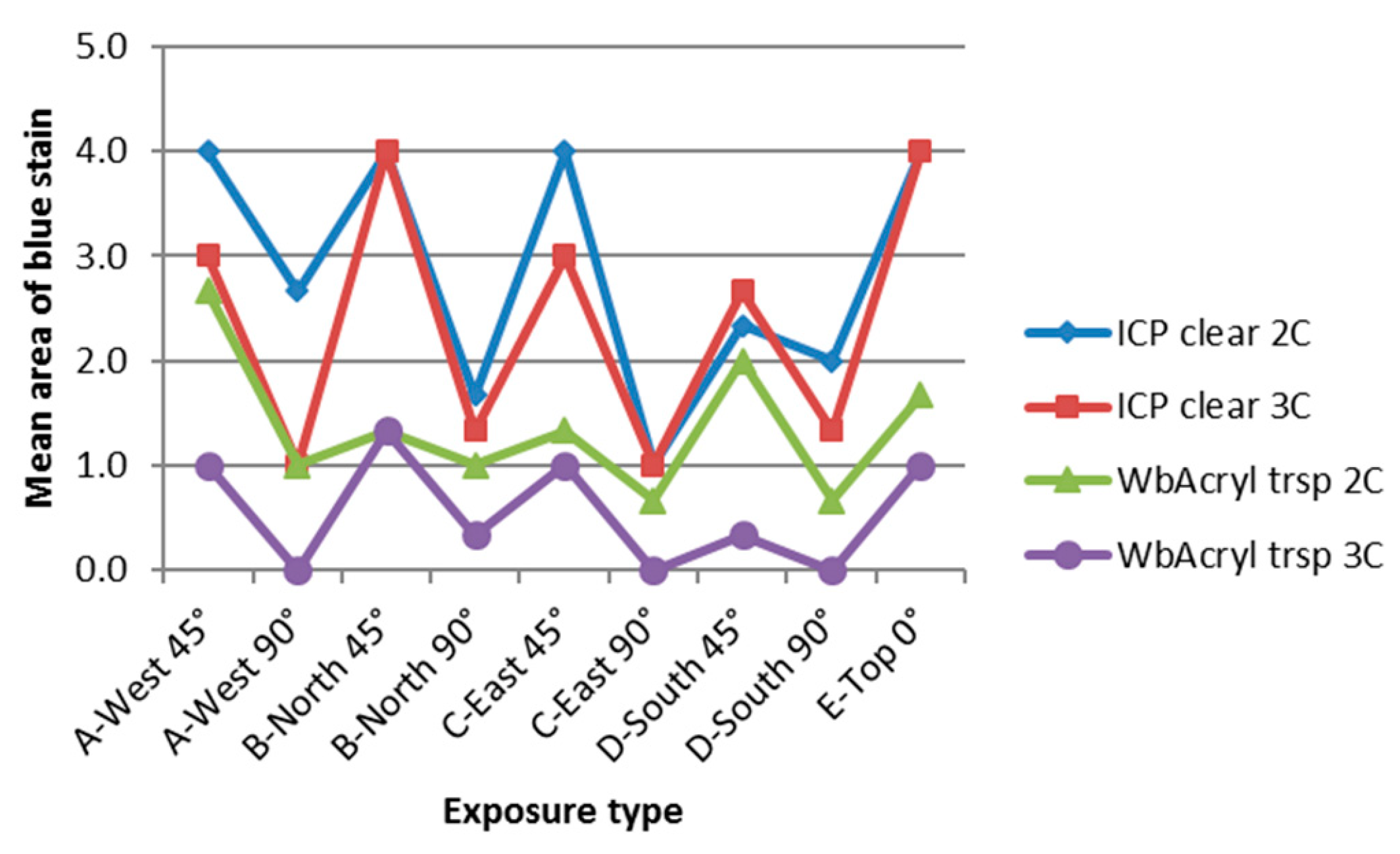
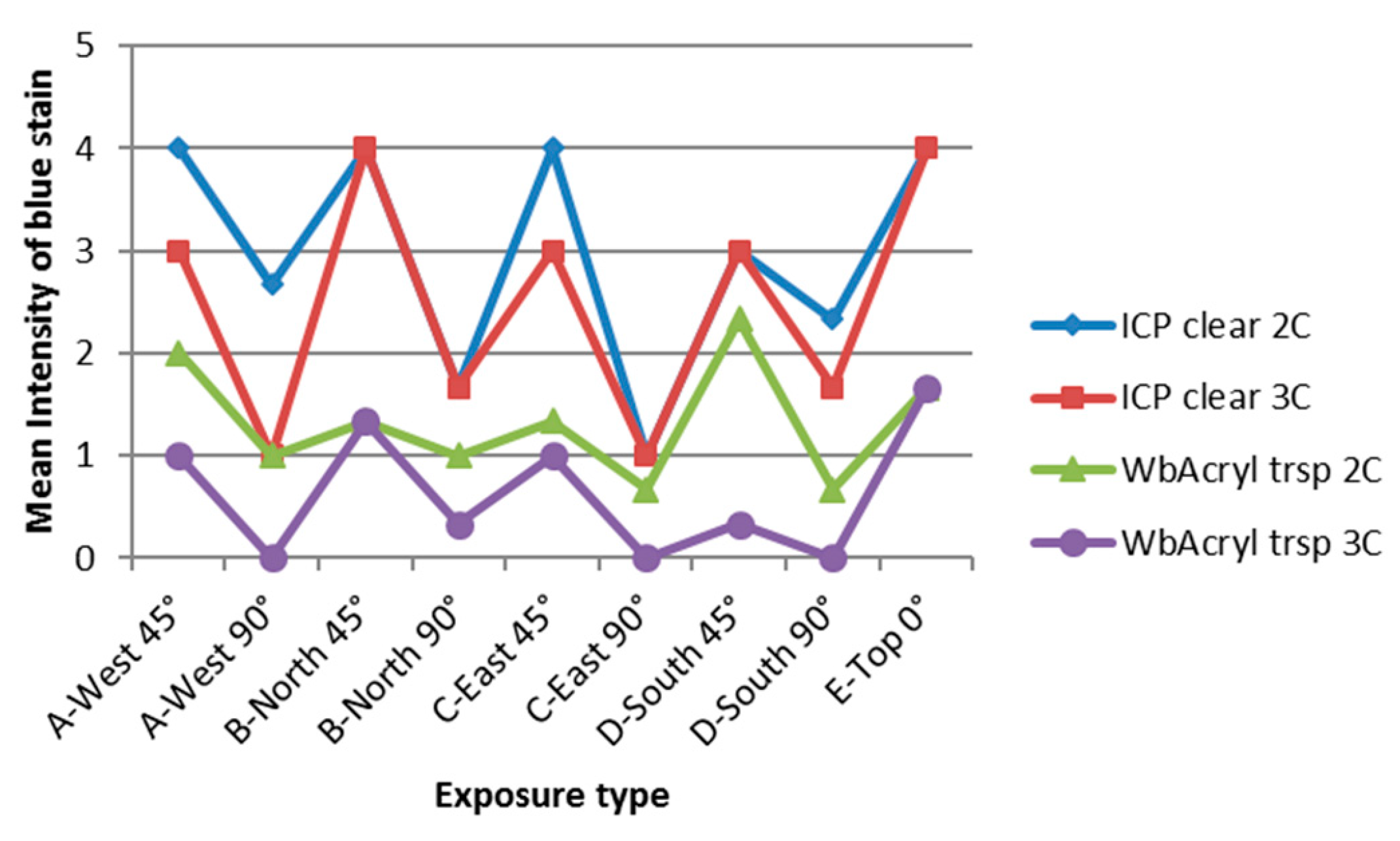
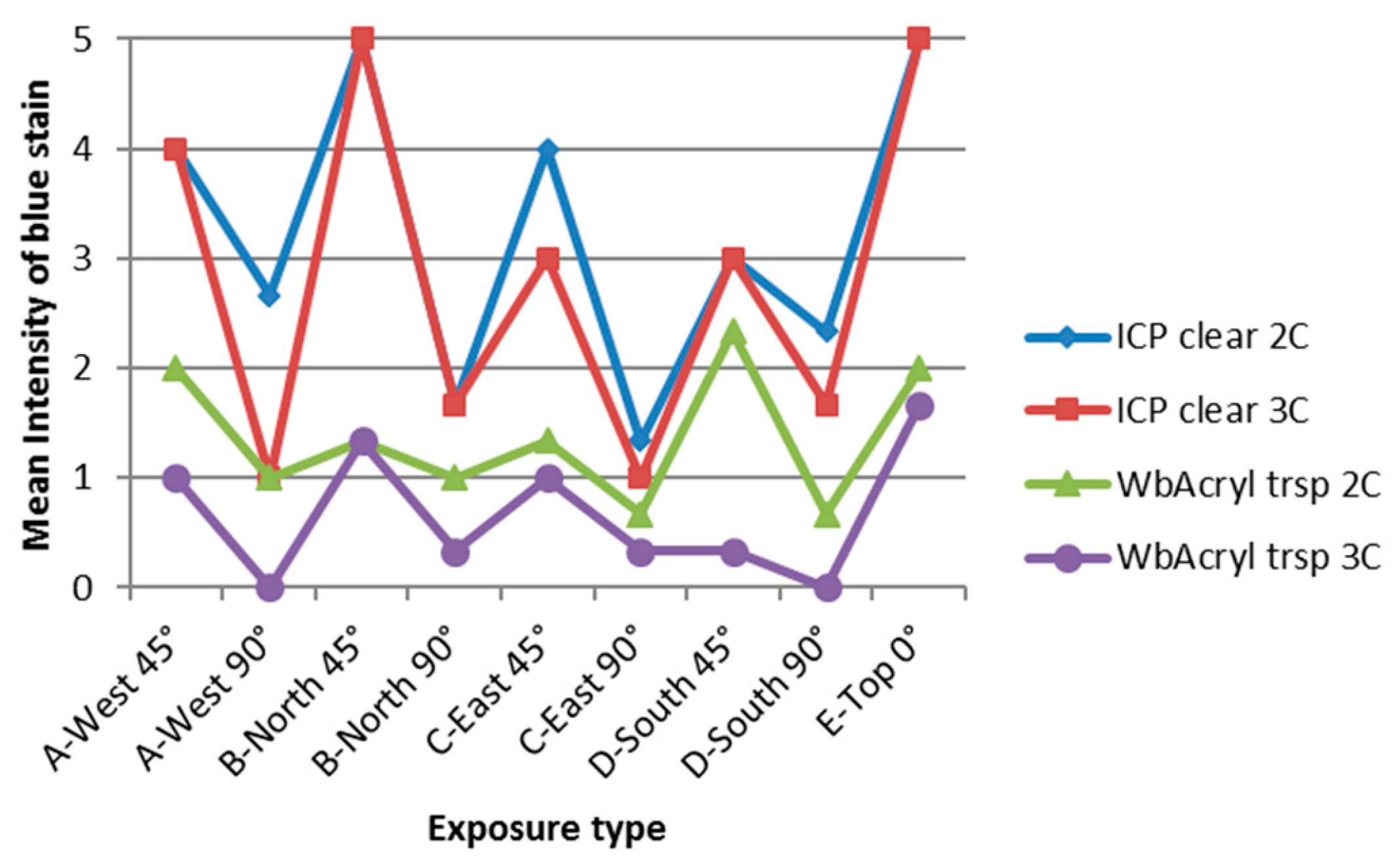
3.4. Influence of Cardinal Direction and Surfaces Inclination on Fungal Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, P.D.; Haase, J.G.; Shakri, A.; Seman, B.M.; Kiguchi, M. The search for durable exterior clear coatings for wood. Coatings 2015, 5, 830–864. [Google Scholar] [CrossRef]
- Jellison, J.; Goodell, B.; Daniel, G. The biology and microscopy of building molds: Medical and molecular aspects. In Development of Commercial Wood Preservatives: Environmental, and Health Issues; Schultz, T.P., Militz, H., Freeman, M.H., Goodell, B., Nicholas, D.D., Eds.; American Chemical Society: Washington, DC, USA, 2008. [Google Scholar]
- Cogulet, A.; Blanchet, P.; Landry, V.; Morris, P. Weathering of wood coated with semi-clear coating: Study of interactions between photo and biodegradation. Int. Biodeterior. Biodegrad. 2018, 129, 33–41. [Google Scholar] [CrossRef]
- De Meijer, M. Review on the durability of exterior wood coatings with reduced VOC-content. Prog. Org. Coat. 2001, 43, 217–225. [Google Scholar] [CrossRef]
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. Off. J. Eur. Union 2012, L167, 1–123.
- Jensen, H.; Sandve, M.; Lystvet, S.M. Exterior paint for the future—Will there be any dry-film preservatives left? In Proceedings of the International Research Group on Wood Protection, 48th Annual Meeting, Ghent, Belgium, 4–8 June 2017. [Google Scholar]
- Gaylarde, C.G.; Morton, L.H.G.; Loh, K.; Shirakawa, M.A. Biodeterioration of external architectural paint films—A review. Int. Biodeterior. Biodegrad. 2011, 65, 1189–1198. [Google Scholar] [CrossRef]
- Gobakken, L.R.; Vestøl, G.I. Mould growth on spruce claddings and the effect of selected influencing factors after 4 years of outdoor testing. In Proceedings of the International Research Group on Wood Protection, 43rd Annual Meeting, Kuala Lumpur, Malaysia, 6–10 May 2015. [Google Scholar]
- Gobakken, L.R.; Westin, M. Surface mould growth on five modified wood substrates coated with three different coating systems when exposed outdoors. Int. Biodeterior. Biodegrad. 2008, 62, 397–402. [Google Scholar] [CrossRef]
- Van Acker, J.; Stevens, M.; Brauwers, C.; Rijckaert, V.; Mol, E. Blue stain resistance of exterior wood coatings as a function of their typology. In Proceedings of the International Research Group on Wood Protection, 29th Annual Meeting, Maastricht, The Netherlands, 14–19 June 1998. [Google Scholar]
- EN 927–2 Paint and Varnishes—Coating Materials and Coating Systems for Exterior Wood—Part 2: Performance Specification; European Committee for Standardization: Brussels, Belgium, 2014.
- EN 927–3 Paint and Varnishes—Coating Materials and Coating Systems for Exterior Wood—Part 3: Natural Weathering Test; European Committee for Standardization: Brussels, Belgium, 2012.
- EN 16 492 Paint and Varnishes—Evaluation of the Surface Disfigurement Caused by Fungi and Algae on Coatings; European Committee for Standardization: Brussels, Belgium, 2014.
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 4628-4 Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 4: Assessment of Degree of Cracking; European Committee for Standardization: Brussels, Belgium, 2003.
- Hansen, K. Molds and moldicide formulations for exterior paints and coatings. In Development of Commercial Wood Preservatives: Environmental, and Health Issues; Schultz, T.P., Militz, H., Freeman, M.H., Goodell, B., Nicholas, D.D., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008. [Google Scholar]
- Urbanczyk, M.M.; Bester, K.; Borho, N.; Schoknecht, U.; Bollmann, U.E. Influence of pigments on phototransformation of biocides in paints. J. Hazard. Mater. 2019, 364, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Anahid, S.; Yaghmaei, S.; Ghobadinejad, Z. Heavy metal tolerance of fungi. Sci. Iran. 2011, 18, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Wiśnicka, R.; Krzepiłko, A.; Wawryn, J.; Biliński, T. Iron toxicity in yeast. Acta Microbiol. Pol. 1997, 46, 339–347. [Google Scholar] [PubMed]
- Kržišnik, D.; Boštjan, L.; Thaler, N.; Humar, M. Influence of natural and artificial weathering on the colour change of different wood and wood-based materials. Forests 2018, 9, 488. [Google Scholar] [CrossRef]
- Ballantyne, E.R. The effect of orientation and latitude on the solar radiation received by test panels and fences during weathering studies. Build. Sci. 1974, 9, 191–196. [Google Scholar] [CrossRef]
- Gobakken, L.R.; Vestøl, G.I. Effects of microclimate, wood temperature and surface colour on fungal disfigurement on wooden claddings. In Proceedings of the International Research Group on Wood Protection, 43rd Annual Meeting, Kuala Lumpur, Malaysia, 6–10 May 2012. [Google Scholar]
- Forsthuber, B.; Grüll, G.; Arnold, M.; Podgorski, L.; Bulian, F. Service life prediction of exterior wood coatings. In Proceedings of the 5th International Conference on Processing Technologies for the Forest and Bio-based Products Industries, Freising, Germany, 20–21 September 2018. [Google Scholar]
- Grüll, G.; Truskaller, M.; Podgorski, L.; Bollmus, S.; Tscherne, F. Maintenance procedures and definition of limit states for exterior wood coatings. Eur. J. Wood Wood Prod. 2011, 69, 443–450. [Google Scholar] [CrossRef]
- Grüll, G.; Truskaller, M.; Podgorski, L.; Bollmus, S.; De Windt, I.; Suttie, E. Moisture conditions in coated wood panels during 24 months natural weathering at five sites in Europe. Wood Mater. Sci. Eng. 2013, 8, 95–110. [Google Scholar] [CrossRef] [Green Version]






| Coating Reference | Type of Coating | Pigmentation |
|---|---|---|
| ICP strsp | Solventborne ICP | Semi-transparent |
| ICP clear | Solventborne ICP | Clear |
| WbAcry trsp | Waterborne acrylic | Semi-transparent |
| WbAcry clear | Waterborne acrylic | Clear |
| Components | ICP Strsp (wt %) | ICP Clear (wt %) |
|---|---|---|
| Synolac 6005 WD 65 | 52.82 | 52.93 |
| Sicoflush red L2817 | 4.63 | – |
| Sicoflush yellow L1916 | 2.30 | – |
| Bentone 34 | 0.60 | 0.65 |
| Octa-Solingen Calcium 10 | 2.77 | 2.99 |
| Octa-Solingen Cobalt 10 | 0.37 | 0.40 |
| Octa-Solingen Zirconium 18 | 0.30 | 0.32 |
| Omacide IPBC | 0.72 | 1.00 |
| Tinuvin 292 | 0.45 | 0.49 |
| Troysan Anti-skin B | 0.20 | 0.22 |
| Shellsol D40 | 34.84 | 41.00 |
| Total | 100.00 | 100.00 |
| Components | WbAcry Trsp (wt %) | WbAcry Clear (wt %) |
|---|---|---|
| DSM Neocryl XK188 | 86.0 | 89.7 |
| Water | 2.0 | 2.0 |
| BASF Luconyl 1916 yellow | 2.4 | – |
| BASF Luconyl 2817 red | 1.2 | – |
| BASF Luconyl 0060 black | 0.1 | – |
| Ammonia 25% | 0.1 | 0.1 |
| Ethyldiglycol | 5.0 | 5.0 |
| BASF Lusolvan FBH | 1.0 | 1.0 |
| BASF Dehydran 1293 | 0.5 | 0.5 |
| Dow Rocima 250 | 1.25 | 1.25 |
| Munzing Tafigel PUR 45 | 0.45 | 0.45 |
| Total | 100.00 | 100.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgorski, L.; Reynaud, C.; Montibus, M. Fungal Growth on Coated Wood Exposed Outdoors: Influence of Coating Pigmentation, Cardinal Direction, and Inclination of Wood Surfaces. Coatings 2019, 9, 27. https://doi.org/10.3390/coatings9010027
Podgorski L, Reynaud C, Montibus M. Fungal Growth on Coated Wood Exposed Outdoors: Influence of Coating Pigmentation, Cardinal Direction, and Inclination of Wood Surfaces. Coatings. 2019; 9(1):27. https://doi.org/10.3390/coatings9010027
Chicago/Turabian StylePodgorski, Laurence, Céline Reynaud, and Mathilde Montibus. 2019. "Fungal Growth on Coated Wood Exposed Outdoors: Influence of Coating Pigmentation, Cardinal Direction, and Inclination of Wood Surfaces" Coatings 9, no. 1: 27. https://doi.org/10.3390/coatings9010027
APA StylePodgorski, L., Reynaud, C., & Montibus, M. (2019). Fungal Growth on Coated Wood Exposed Outdoors: Influence of Coating Pigmentation, Cardinal Direction, and Inclination of Wood Surfaces. Coatings, 9(1), 27. https://doi.org/10.3390/coatings9010027




