Application of Solution Plasma Surface Modification Technology to the Formation of Thin Hydroxyapatite Film on Titanium Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Preparation of the Mineralizing Solution
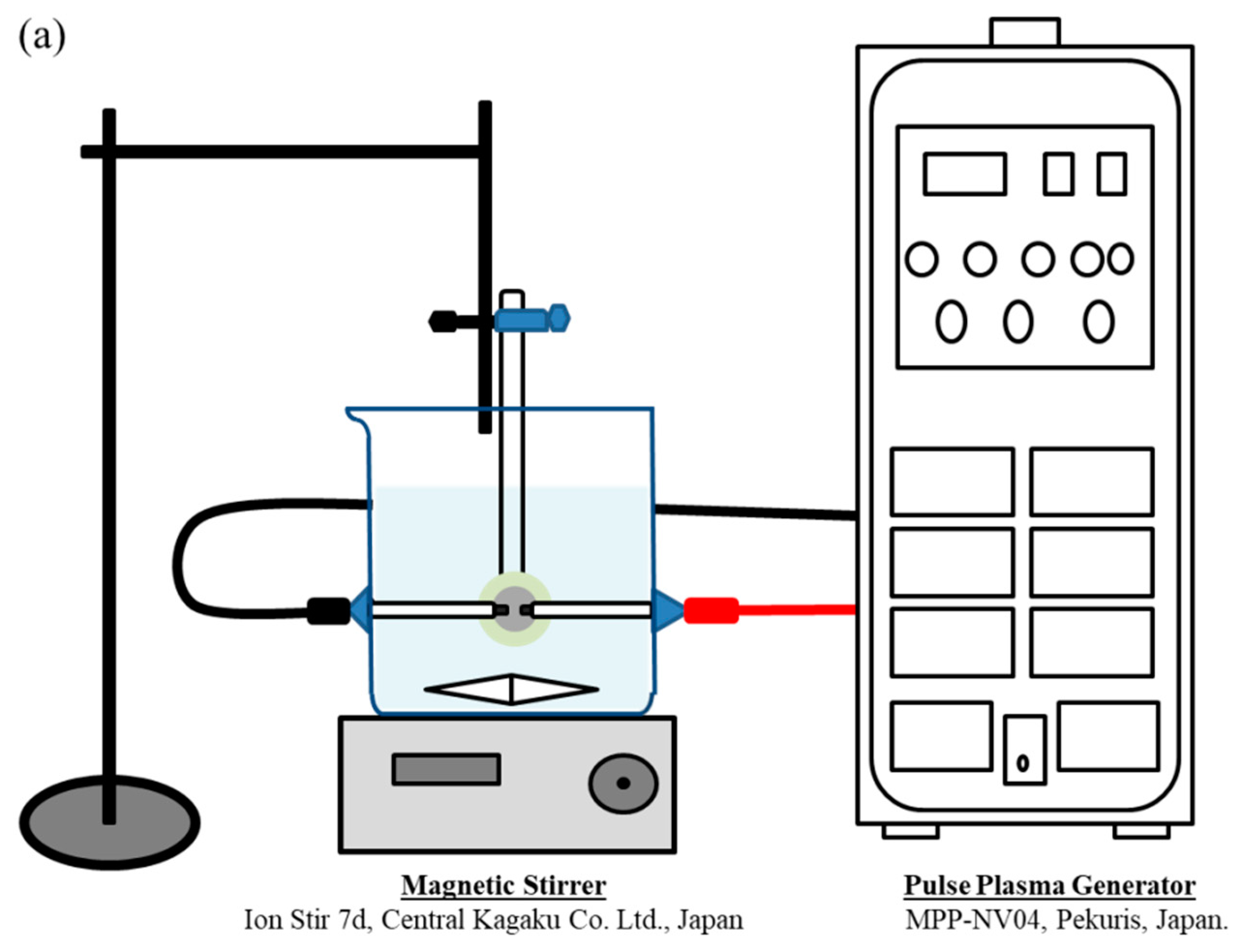
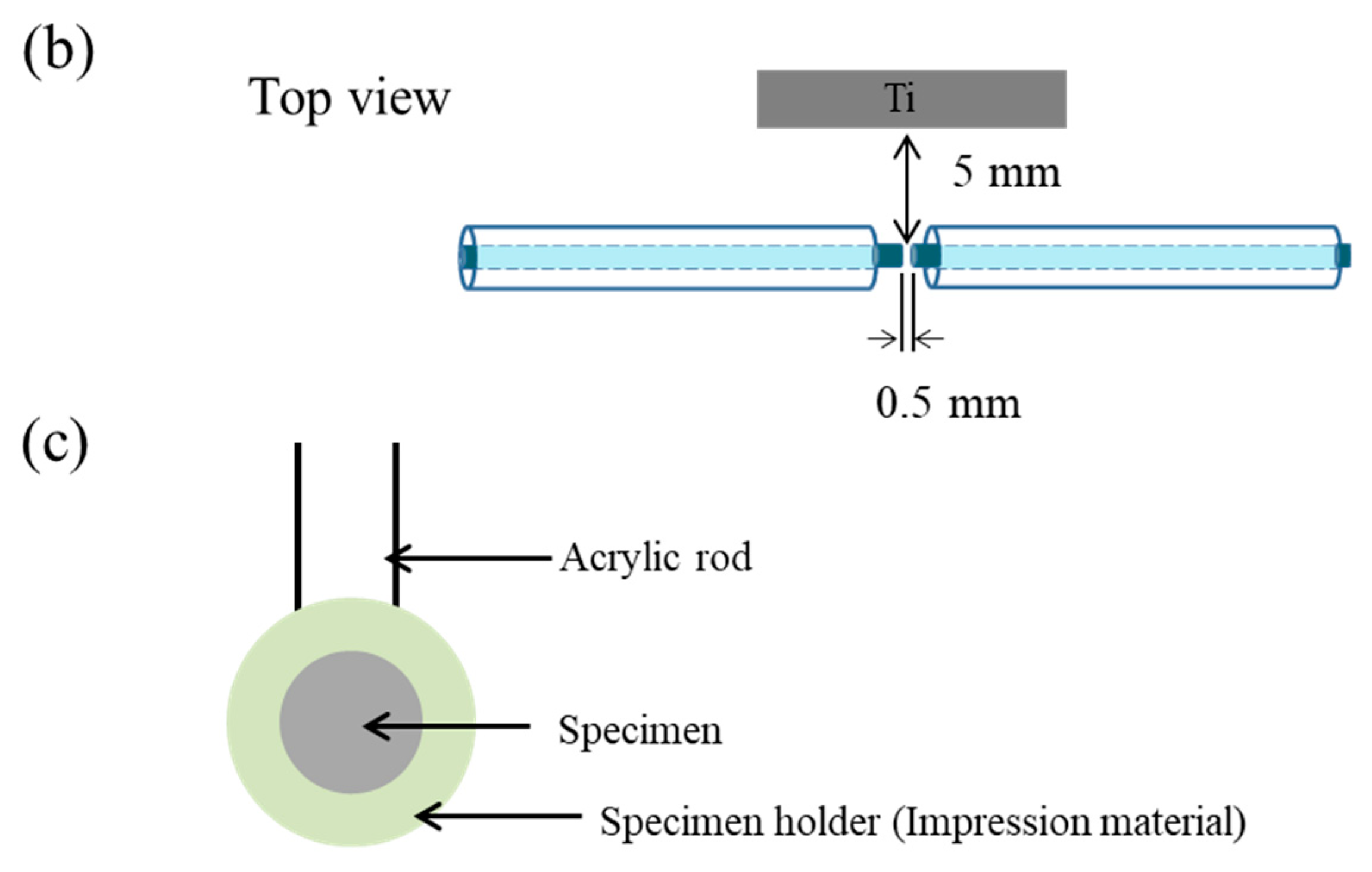
2.3. Solution Plasma Treatment
2.4. Characterization of the HA Layer Formed on Various Substrates
2.4.1. SEM Observation
2.4.2. X-ray Diffraction
2.4.3. Measurement of the Contact Angle of Water Droplets on the Surfaces
2.5. Evaluation of Cytocompatibility
2.5.1. Cell Line
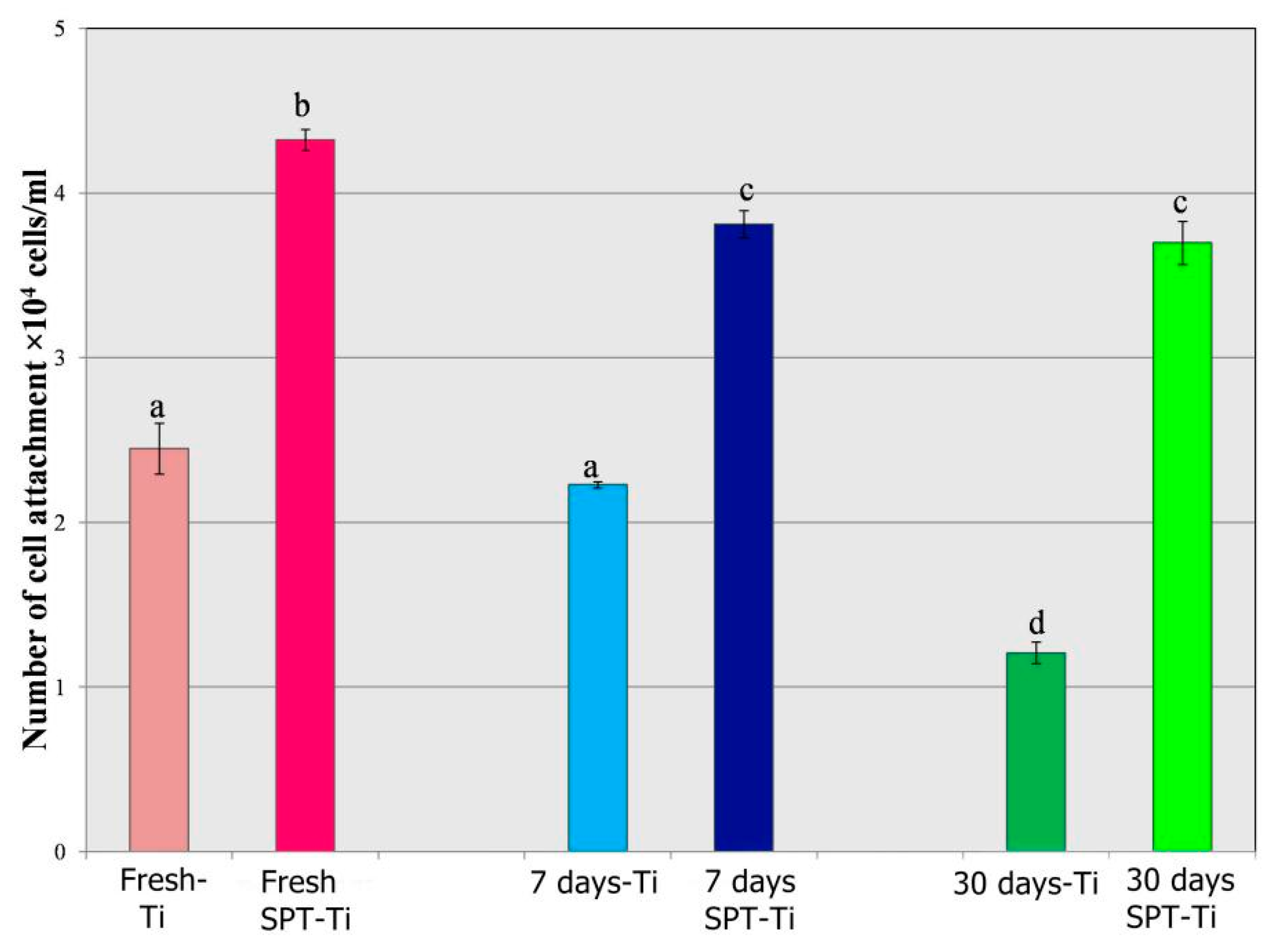
2.5.2. Attachment of Cells on Differently Modified Titanium Surfaces after Four Hours of Incubation
2.5.3. Statistical Analysis
3. Results
3.1. Variation of Solution Temperature in the Vicinity of the Sample with Time during SPT
3.2. Characterization of Titanium Surfaces Subjected to SPT and IT in the Calcium Phosphate Solution
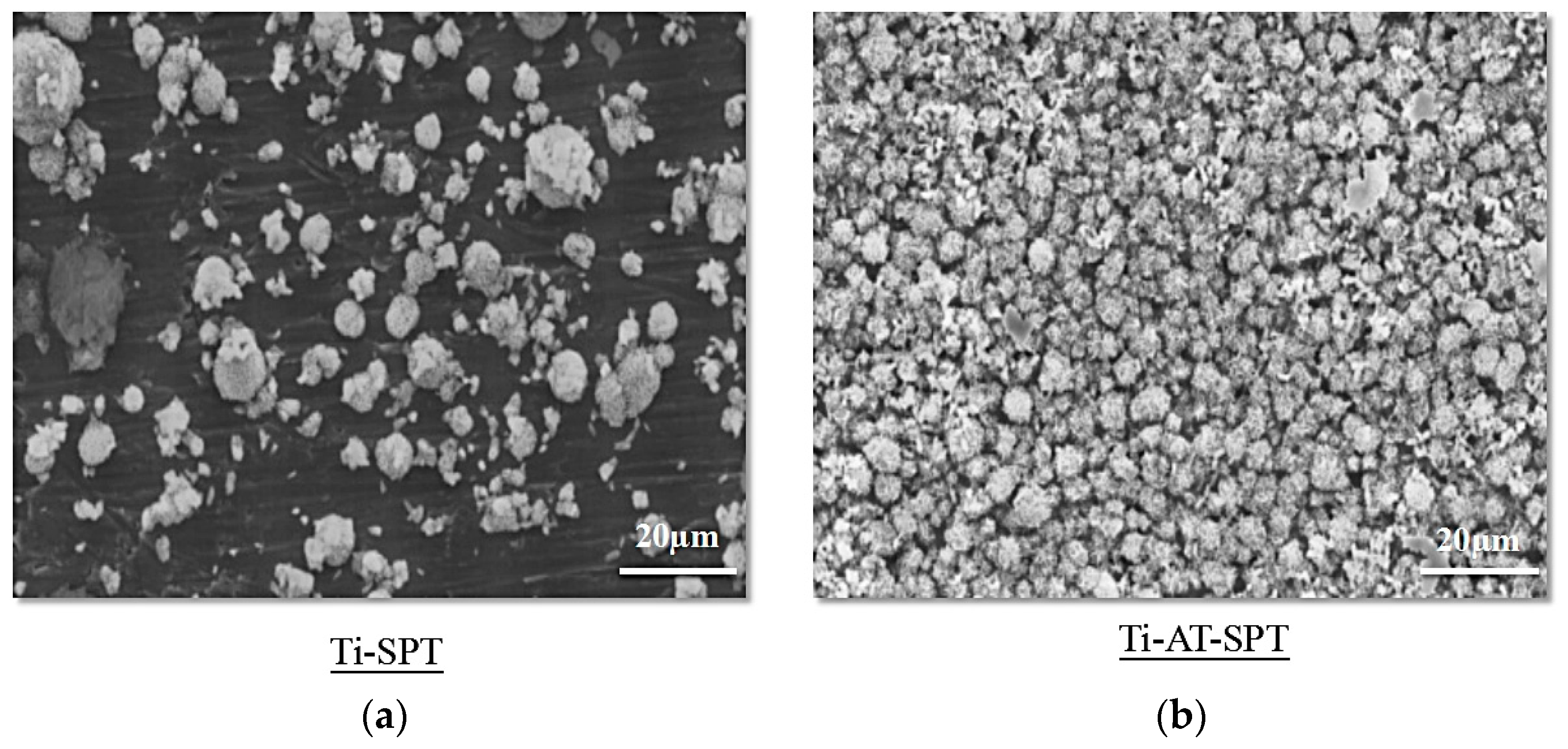
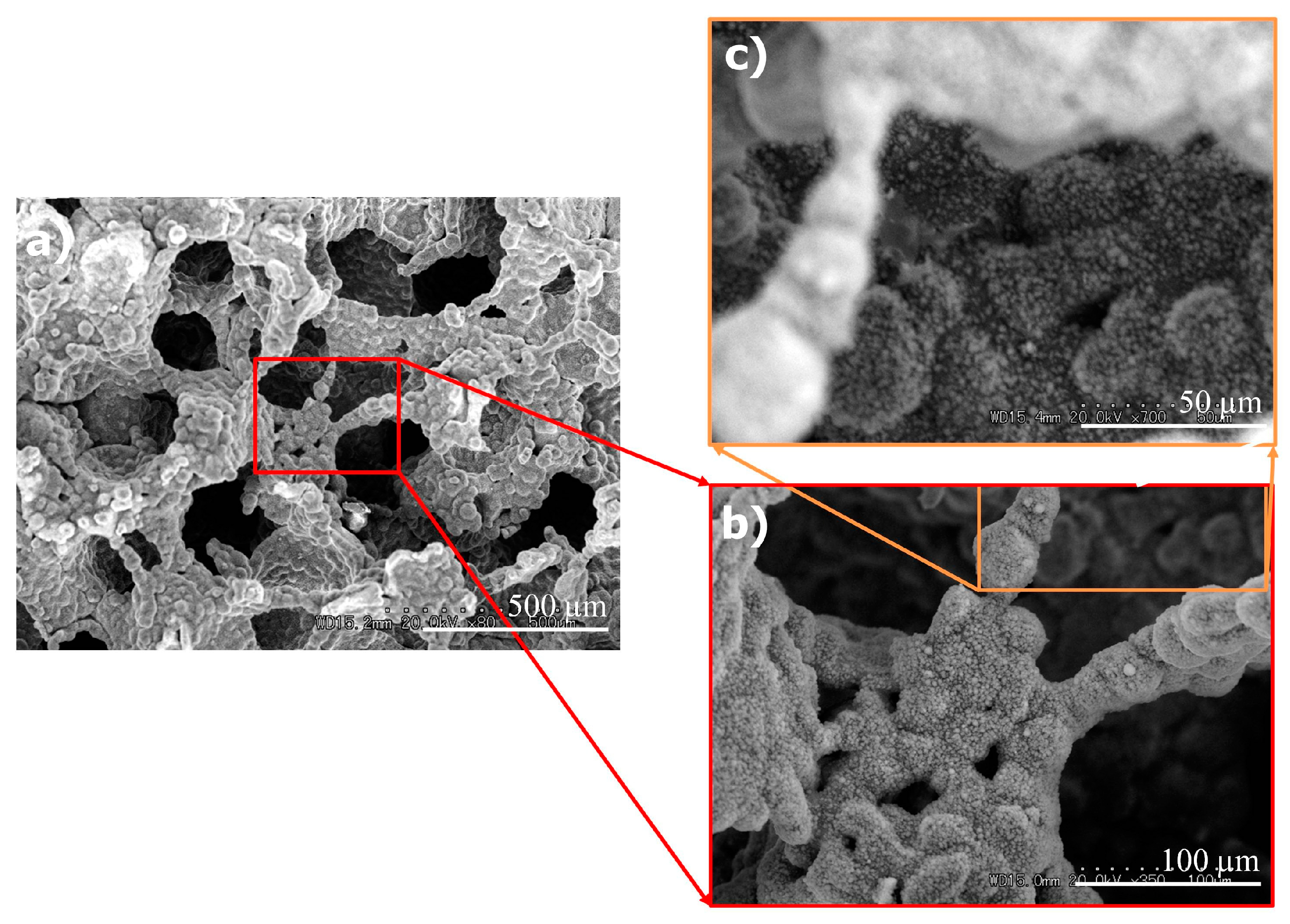
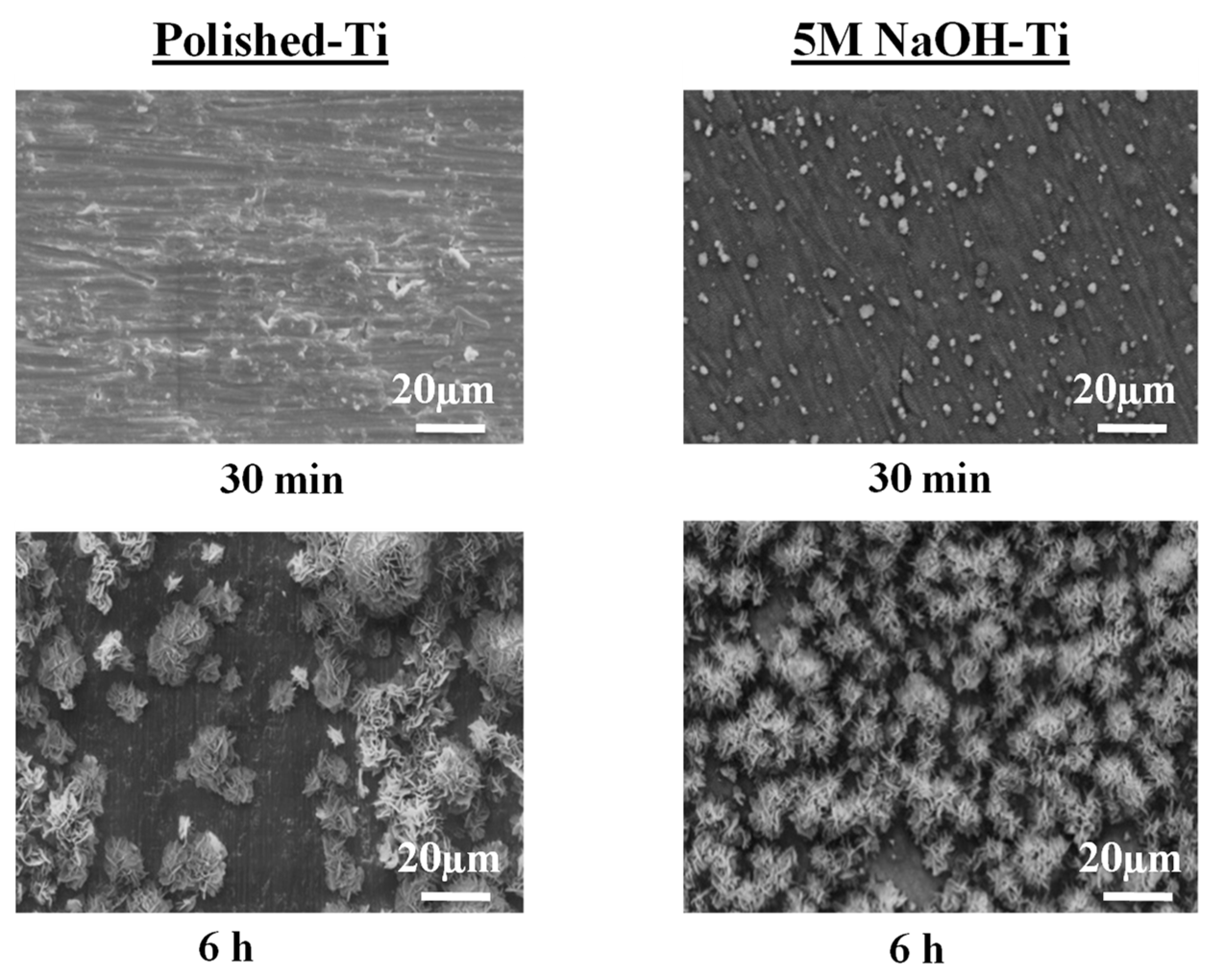
3.2.1. SEM Observation
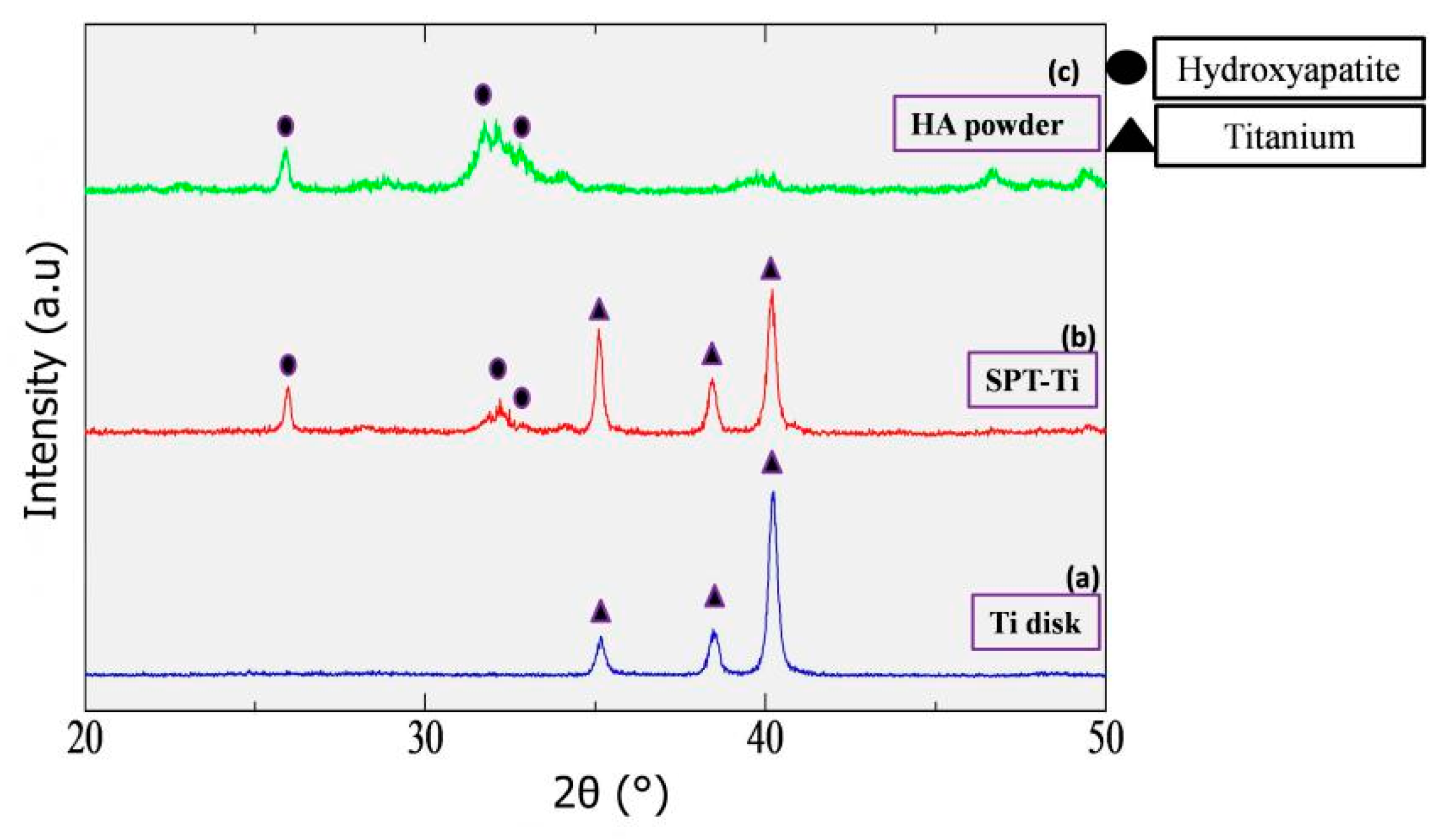
3.2.2. Analysis of Precipitated Particles on Ti-AT-SPT and Ti-AT-IT60° by XRD and EDX
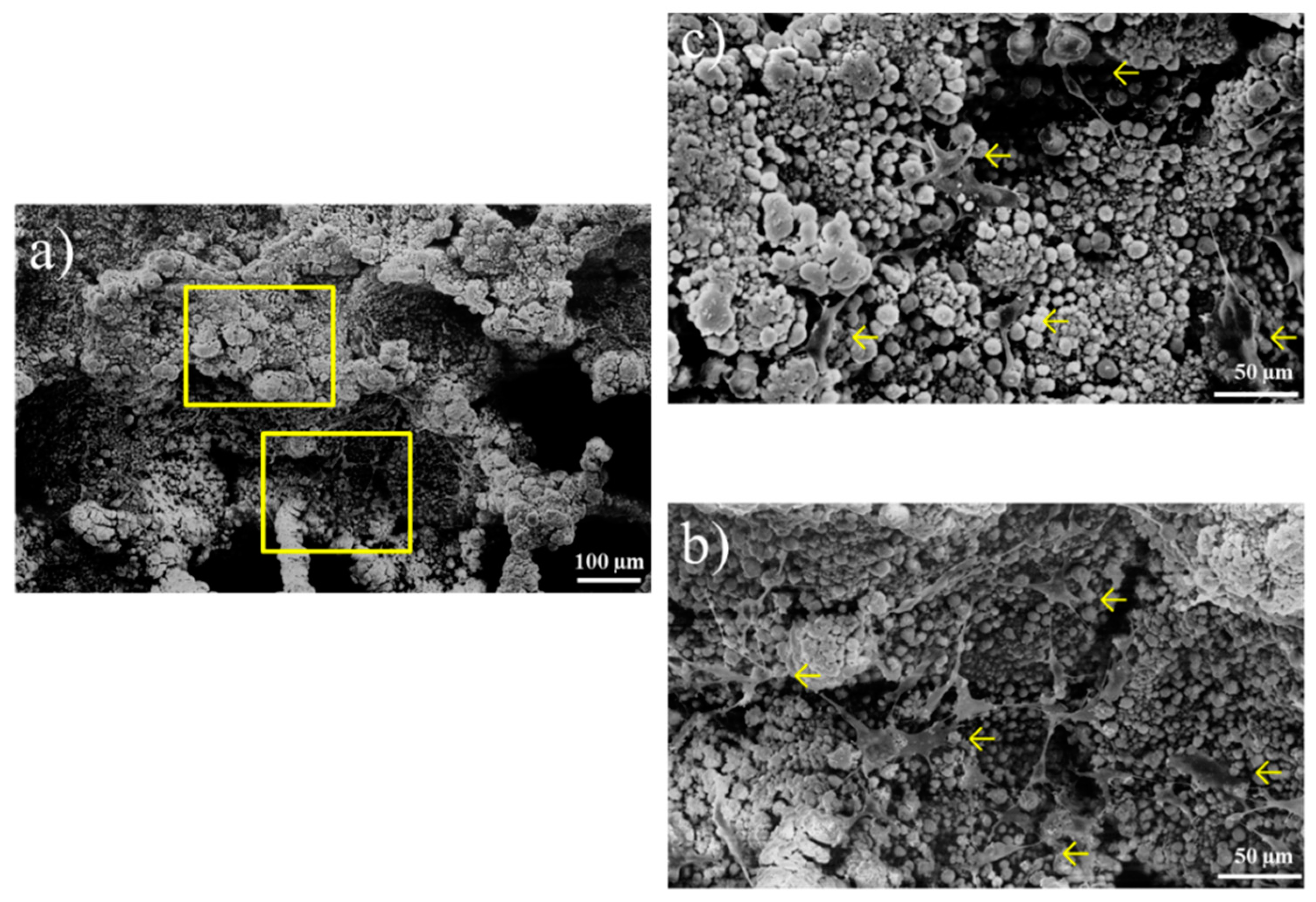
3.3. Crystals on the Titanium Surfaces after Immersion in the Calcium Phosphate Solution
3.4. Change in the Contact Angle of Water Droplets on Polished-Ti and Ti-AT-SPT with Aging Time
3.5. Bioactivity Analysis
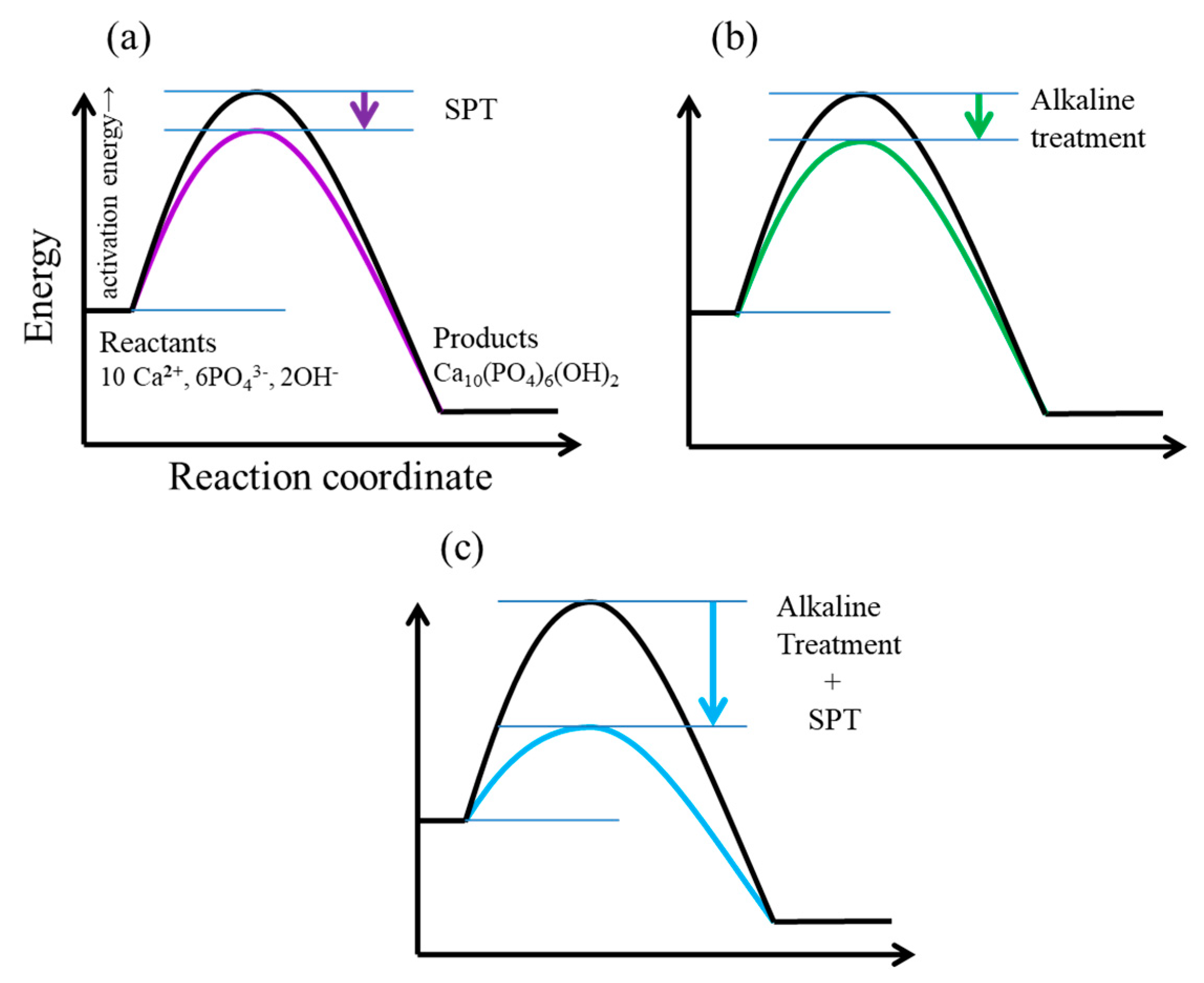
4. Discussion
4.1. Process of Rapid Formation of Thin and Uniform HA Film Consisting of Fine Spherical Particles on Ti-AT-SPT
4.2. Chemical and Biological Properties of HA Film Formed by SPT
4.3. HA Film Coating on Titanium by SPT to Develop an Osteoconductive Porous-Surfaced Dental Implant Body
4.4. Features of the SPT for HA Film Coating
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dental Implants Facts and Figures. Available online: https://www.aaid.com/about/Press_Room/Dental_Implants_FAQ.html (accessed on 1 December 2018).
- Herman, H. Plasma spray deposition processes. MRS Bull. 1988, 12, 60–67. [Google Scholar] [CrossRef]
- Dasarathy, H.; Riley, C.; Coble, H.D. Analysis of apatite deposits on substrates. J. Biomed. Mater. Res. 1993, 27, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Harris, L.A.; Lucas, L.C.; Lacefield, W.R.; Rigney, D. X-ray photoelectron spectroscopy characterization of ion-beam sputter-deposited calcium phosphate coatings. J. Am. Ceram. Soc. 1991, 74, 2301–2304. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nishiguchi, S.; Nakamura, T. Graded surface structure of bioactive titanium prepared by chemical treatment. J. Biomed. Mater. Res. 1999, 45, 100–107. [Google Scholar] [CrossRef]
- Ban, S.; Maruno, S.; Harada, A.; Hattori, M.; Narita, K.; Hasegawa, J. Effect of temperature on morphology of electrochemically deposit calcium phosphates. Dent. Mater. J. 1996, 15, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Miyamoto, Y.; Nagayoma, M.; Asaoka, K. Blast coating method: New method of coating titanium surface with hydroxyapatite at room temperature. J. Biomed. Mater. Res. 1997, 38, 129–134. [Google Scholar] [CrossRef]
- Kuroda, K.; Ichino, R.; Okido, M.; Takai, O. Hydroxyapatite coation on titanium by thermal substrate method in aqueous solution. J. Biomed. Mater. Res. 2001, 59, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Endo, K.; Maida, T.; Ohno, H. HA film coating by the thermally induced liquid-phase deposition method for titanium implants. Dent. Mater. J. 2006, 25, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Nakasugi, Y.; Akiyama, T. Generation of solution plasma over a large electrode surface area. J. Appl. Phys. 2015, 118, 23303. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Kim, H.M.; Kawashita, M.; Nakamura, T. Bioactive metals: Preparation and properties. J. Mater. Sci. Mater. Med. 2004, 15, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Arsenault, A.L.; Yamauchi, M.; Kuboki, Y.; Crenshaw, M.A. Mineral Induction by immobilized phosphoproteins. Bone 1997, 21, 305–311. [Google Scholar] [CrossRef]
- Nygren, H. Initial reactions of whole blood with hydrophilic and hydrophobictitanium surface. Colloids Surf. B Biointerfaces 1996, 6, 329–333. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, Y.; Ma, T.; Chen, H.; Lin, Y. Enhanced hydrophilicity and protein adsorption of titanium surface by sodium bicarbonate solution. J. Nanomater. 2015, 2015, 5. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef] [PubMed]





| Component | Concentration (mM) |
|---|---|
| CaCl2 | 2.24 |
| K2HPO4 | 1.04 |
| KH2PO4 | 0.30 |
| HEPES | 10.00 |
| KCl | 150.00 |
| C/P | 1.67 |
| pH | 7.40 |
| Degree of supersaturation with respect to HA | 3.85 × 107 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badruddoza Dithi, A.; Nezu, T.; Nagano-Takebe, F.; Hasan, M.R.; Saito, T.; Endo, K. Application of Solution Plasma Surface Modification Technology to the Formation of Thin Hydroxyapatite Film on Titanium Implants. Coatings 2019, 9, 3. https://doi.org/10.3390/coatings9010003
Badruddoza Dithi A, Nezu T, Nagano-Takebe F, Hasan MR, Saito T, Endo K. Application of Solution Plasma Surface Modification Technology to the Formation of Thin Hydroxyapatite Film on Titanium Implants. Coatings. 2019; 9(1):3. https://doi.org/10.3390/coatings9010003
Chicago/Turabian StyleBadruddoza Dithi, Akashlynn, Takashi Nezu, Futami Nagano-Takebe, Md Riasat Hasan, Takashi Saito, and Kazuhiko Endo. 2019. "Application of Solution Plasma Surface Modification Technology to the Formation of Thin Hydroxyapatite Film on Titanium Implants" Coatings 9, no. 1: 3. https://doi.org/10.3390/coatings9010003






