Effect of Multiple-Pass Friction Stir Processing on Hardness and Corrosion Resistance of Martensitic Stainless Steel
Abstract
:1. Introduction
2. Experimental details
2.1. Materials
2.2. Friction Stir Processing (FSP)
2.3. Metallographic and Microstructural Studies
2.4. Hardness Test
2.5. Corrosion Test
3. Results and Discussion
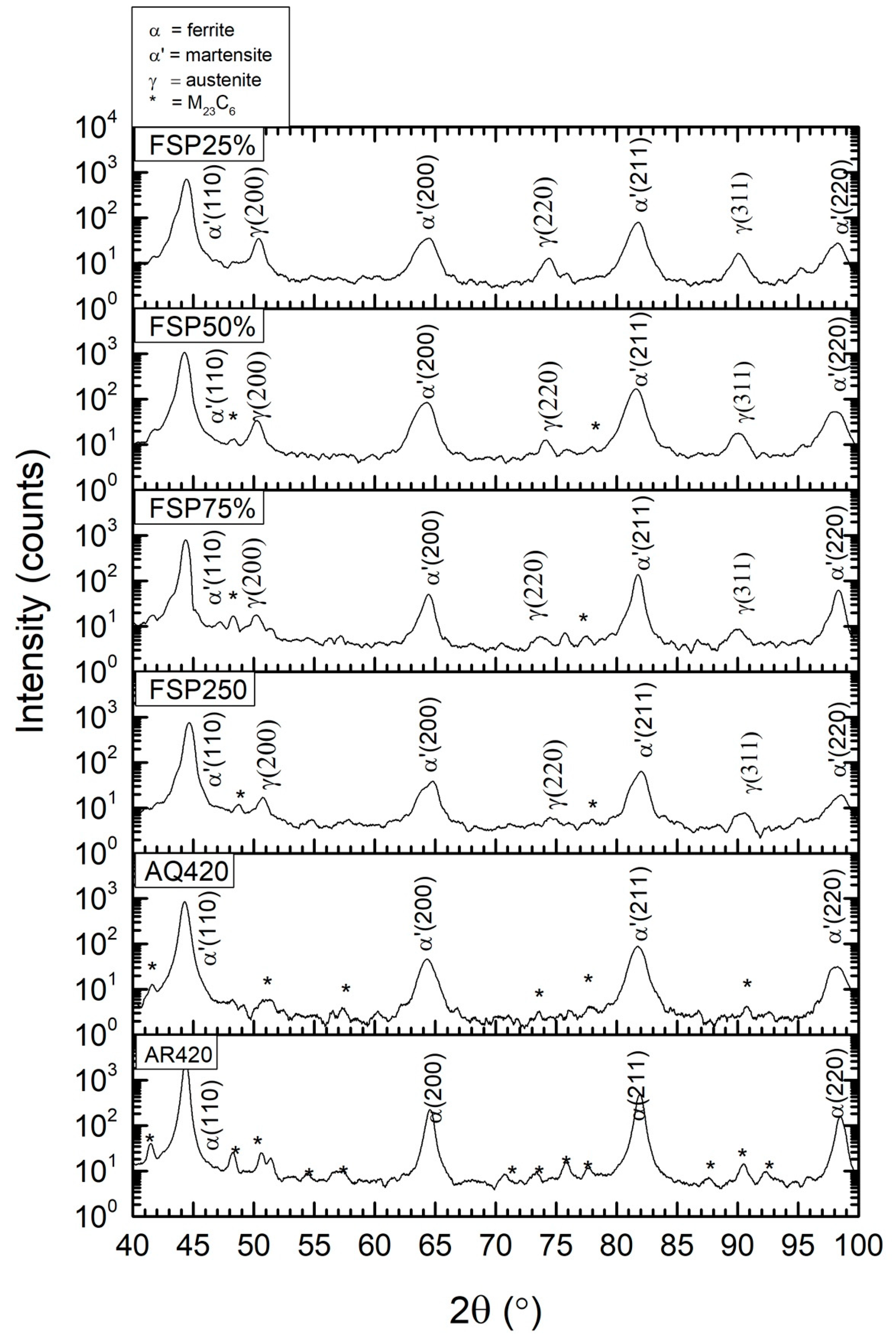
3.1. Microstructure
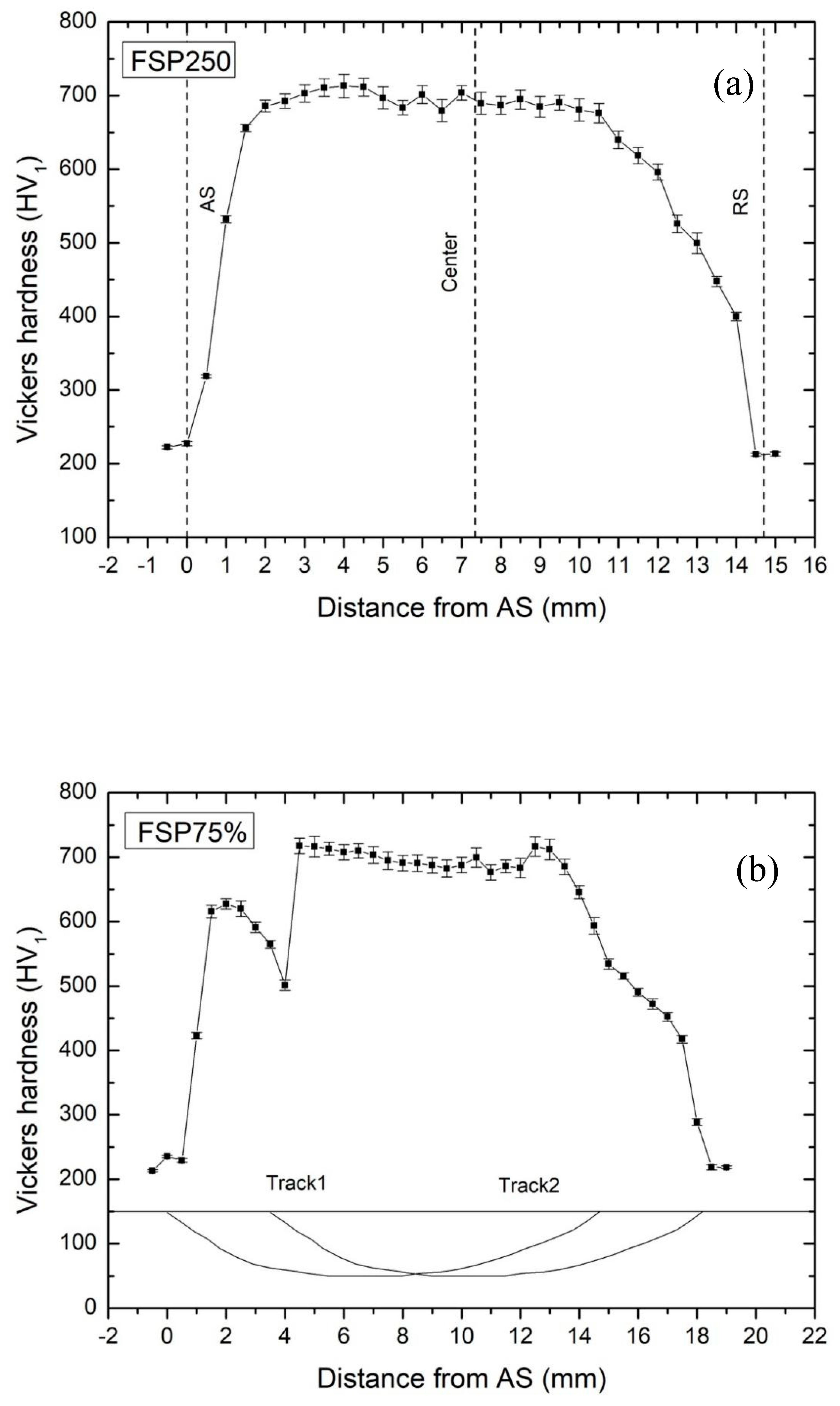
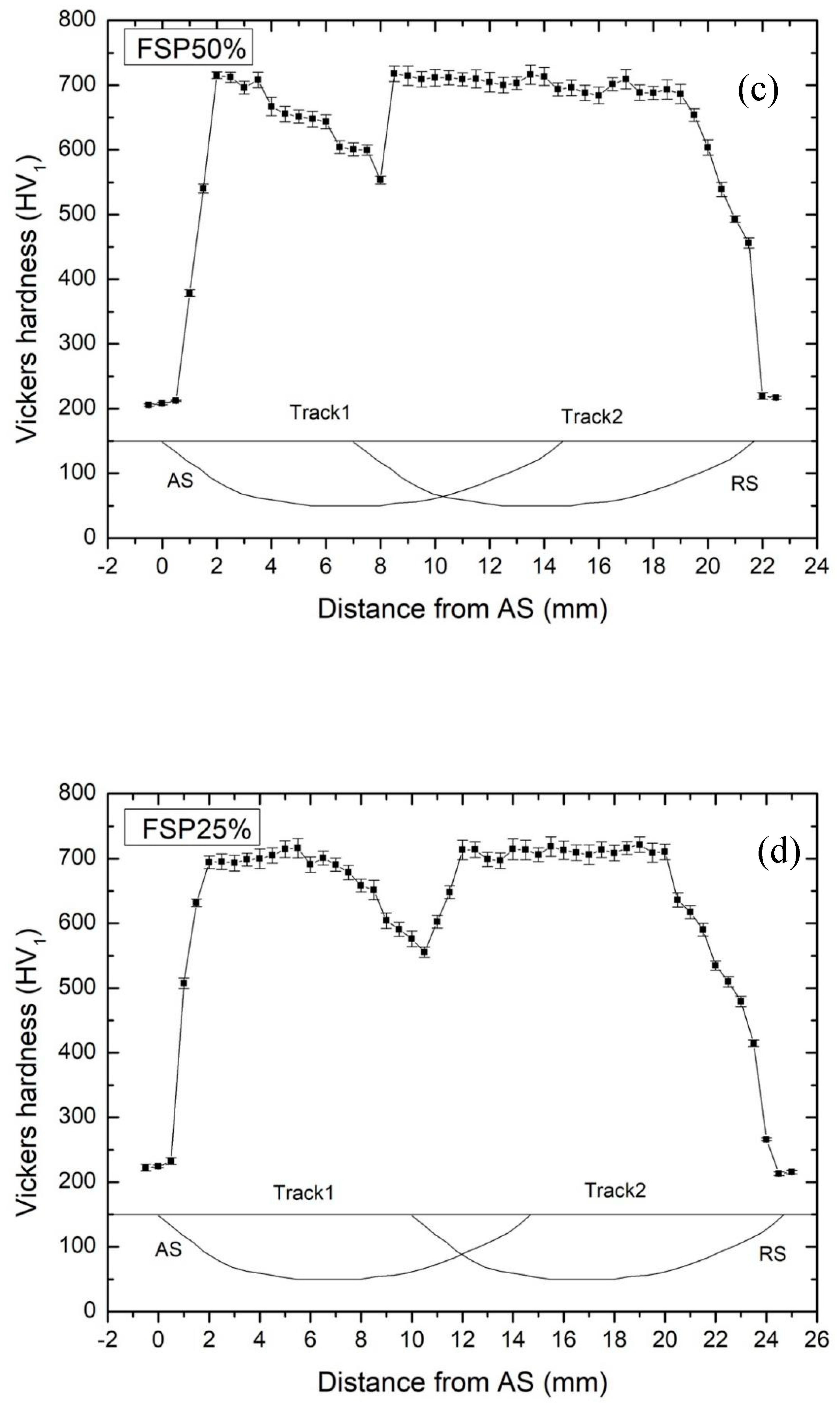
3.2. Hardness
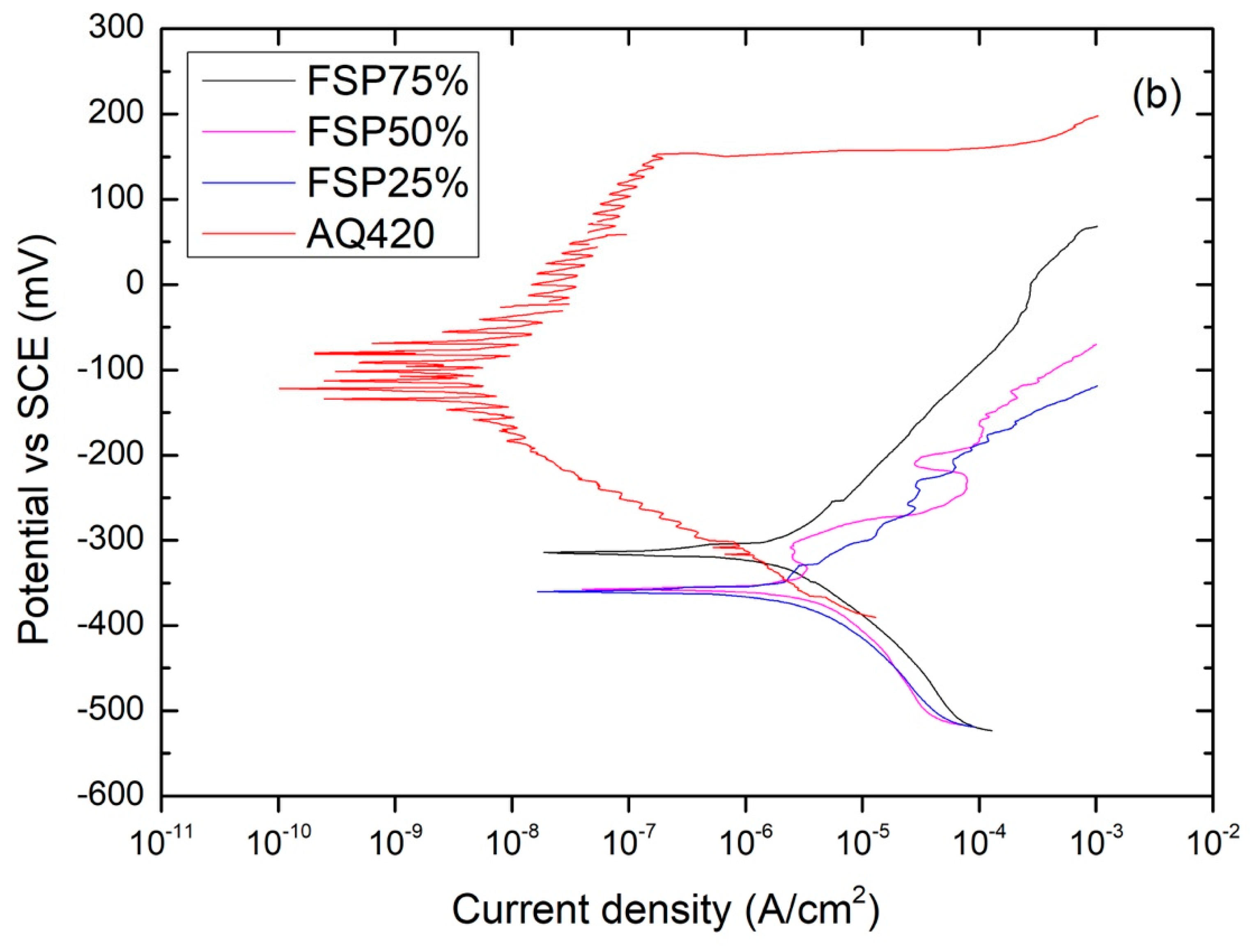
3.3. Electrochemical Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, W.M.; Nicholas, E.D.; Needham, J.C.; Murch, M.G.; Templesmith, P.; Dawes, C.J. Friction Stir Butt Welding. International Patent PCT/GB92/02203, GB Patent 9125978, December 1991. [Google Scholar]
- Mishra, R.S.; Ma, Z.Y. Friction stir welding and processing. Mater. Sci. Eng. R Rep. 2005, 50, 1–78. [Google Scholar] [CrossRef]
- Mishra, R.S.; Mahoney, M.W.; McFadden, S.X.; Mara, N.A.; Mukherjee, A.K. High strain rate superplasticity in a friction stir processed 7075 Al alloy. Scr. Mater. 1999, 42, 163–168. [Google Scholar] [CrossRef]
- Mironov, S.; Sato, Y.S.; Kokawa, H. Microstructural evolution during friction stir-processing of pure iron. Acta Mater. 2008, 56, 2602–2614. [Google Scholar] [CrossRef]
- Chainarong, S.; Muangjunburee, P.; Suthummanon, S. Friction Stir Processing of SSM356 Aluminium Alloy. Procedia Eng. 2014, 97, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Morisada, Y.; Fujii, H.; Nagaoka, T.; Fukusumi, M. Nanocrystallized magnesium alloy—Uniform dispersion of C60 molecules. Scr. Mater. 2006, 55, 1067–1070. [Google Scholar] [CrossRef]
- Berbon, P.B.; Bingel, W.H.; Mishra, R.S.; Bampton, C.C.; Mahoney, M.W. Friction stir processing: A tool to homogenize nanocomposite aluminum alloys. Scr. Mater. 2001, 44, 61–66. [Google Scholar] [CrossRef]
- Grewal, H.S.; Arora, H.S.; Singh, H.; Agrawal, A. Surface modification of hydroturbine steel using friction stir processing. Appl. Surf. Sci. 2013, 268, 547–555. [Google Scholar] [CrossRef]
- Ni, D.R.; Xue, P.; Ma, Z.Y. Effect of Multiple-Pass Friction Stir Processing Overlapping on Microstructure and Mechanical Properties of As-Cast NiAl Bronze. Metall. Mater. Trans. A 2011, 42, 2125–2135. [Google Scholar] [CrossRef]
- Isfahany, A.N.; Saghafian, H.; Borhani, G. The effect of heat treatment on mechanical properties and corrosion behavior of AISI420 martensitic stainless steel. J. Alloy. Compd. 2011, 509, 3931–3936. [Google Scholar] [CrossRef]
- Gholi, A.R.; Lindwall, G.; Jönsson, M. Effects of tempering on corrosion properties of high nitrogen alloyed tooling steels in pyrolysis oil. Corros. Sci. 2011, 318, 1298. [Google Scholar]
- Dossett, J.L.; Totten, G.E.; ASM Handbook Committee. ASM Handbook, Volume 4A: Steel Heat Treating Fundamentals and Processes; ASM International: Almere, The Netherlands, 2013. [Google Scholar]
- Mahmoudi, B.; Torkamany, M.J.; Aghdam, A.S.R.; Sabbaghzade, J. Laser surface hardening of AISI 420 stainless steel treated by pulsed Nd:YAG laser. Mater. Des. (1980–2015) 2010, 31, 2553–2560. [Google Scholar] [CrossRef]
- Dodds, S.; Jones, A.H.; Cater, S. Tribological enhancement of AISI 420 martensitic stainless steel through friction-stir processing. Wear 2013, 302, 863–877. [Google Scholar] [CrossRef]
- Aldajah, S.H.; Ajayi, O.O.; Fenske, G.R.; David, S. Effect of friction stir processing on the tribological performance of high carbon steel. Wear 2009, 267, 350–355. [Google Scholar] [CrossRef]
- Puli, R.; Janaki Ram, G.D. Microstructures and properties of friction surfaced coatings in AISI 440C martensitic stainless steel. Surf. Coat. Technol. 2012, 207, 310–318. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Attarzadeh, F.R.; Vakili-Azghandi, M. Effect of Multi-pass Friction Stir Processing on the Electrochemical and Corrosion Behavior of Pure Titanium in Strongly Acidic Solutions. Metall. Mater. Trans. A 2017, 48, 403–411. [Google Scholar] [CrossRef]
- Pan, L.; Kwok, C.T.; Lo, K.H. Enhancement in hardness and corrosion resistance of AISI 420 martensitic stainless steel via friction stir processing. Surf. Coat. Technol. 2019, 357, 339–347. [Google Scholar] [CrossRef]
- Leal, R.M.; Loureiro, A. Effect of overlapping friction stir welding passes in the quality of welds of aluminium alloys. Mater. Des. 2008, 29, 982–991. [Google Scholar] [CrossRef]
- Van Ingelgem, Y.; Vandendael, I.; Van den Broek, D.; Hubin, A.; Vereecken, J. Influence of laser surface hardening on the corrosion resistance of martensitic stainless steel. Electrochim. Acta 2007, 52, 7796–7801. [Google Scholar] [CrossRef]
- Nascimento, F.; Santos, T.; Vilaça, P.; Miranda, R.M.; Quintino, L. Microstructural modification and ductility enhancement of surfaces modified by FSP in aluminium alloys. Mater. Sci. Eng. A 2009, 506, 16–22. [Google Scholar] [CrossRef]
- ASTM. ASTM Standard CE384-17. Standard Test Method for Microindentation Hardness of Materials; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM. ASTM Standard G5-14. Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements; ASTM: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Gandra, J.; Miranda, R.M.; Vilaça, P. Effect of overlapping direction in multipass friction stir processing. Mater. Sci. Eng. A 2011, 528, 5592–5599. [Google Scholar] [CrossRef]
- Avila, J.A.; Giorjao RA, R.; Rodriguez, J.; Fonseca, E.B.; Ramirez, A.J. Modeling of thermal cycles and microstructural analysis of pipeline steels processed by friction stir processing. Int. J. Adv. Manuf. Technol. 2018, 98, 2611–2618. [Google Scholar] [CrossRef] [Green Version]
- Hajian, M.; Abdollah-zadeh, A.; Rezaei-Nejad, S.S.; Assadi, H.; Hadavi, S.M.M.; Chung, K.; Shokouhimehr, M. Microstructure and mechanical properties of friction stir processed AISI 316L stainless steel. Mater. Des. 2015, 67, 82–94. [Google Scholar] [CrossRef]
- Baghjari, S.H.; Akbari Mousavi SA, A. Effects of pulsed Nd:YAG laser welding parameters and subsequent post-weld heat treatment on microstructure and hardness of AISI 420 stainless steel. Mater. Des. 2013, 43, 1–9. [Google Scholar] [CrossRef]
- Lakhkar, R.S.; Shin, Y.C.; Krane, M.J.M. Predictive modeling of multi-track laser hardening of AISI 4140 steel. Mater. Sci. Eng. A 2008, 480, 209–217. [Google Scholar] [CrossRef]
- Giorleo, L.; Previtali, B.; Semeraro, Q. Modelling of back tempering in laser hardening. Int. J. Adv. Manuf. Technol. 2010, 54, 969–977. [Google Scholar] [CrossRef]
- Iino, Y.; Shimoda, K. Effect of overlap pass tempering on hardness and fatigue behaviour in laser heat treatment of carbon steel. J. Mater. Sci. Lett. 1987, 6, 1193–1194. [Google Scholar] [CrossRef]
- Sunil Kumar, B.; Kain, V.; Vishwanadh, B. Effect of Tempering Treatments on Microstructure and Intergranular Corrosion of 13 wt% Cr Martensitic Stainless Steel. Corrosion 2017, 73, 362–378. [Google Scholar] [CrossRef]
- Hufenbach, J.; Giebeler, L.; Hoffmann, M.; Kohlar, S.; Kühn, U.; Gemming, T.; Eckert, J. Effect of short-term tempering on microstructure and mechanical properties of high-strength FeCrMoVC. Acta Mater. 2012, 60, 4468–4476. [Google Scholar] [CrossRef]
- Santhanakrishnan, S.; Kong, F.; Kovacevic, R. An experimentally based thermo-kinetic phase transformation model for;multi-pass laser heat treatment by using high power direct diode laser. Int. J. Adv. Manuf. Technol. 2013, 64, 219–238. [Google Scholar] [CrossRef]
- Abbasi-Khazaei, B.; Mollaahmadi, A. Rapid Tempering of Martensitic Stainless Steel AISI420: Microstructure, Mechanical and Corrosion Properties. J. Mater. Eng. Perform. 2017, 26, 1626–1633. [Google Scholar] [CrossRef]
- Lippold, J.C.; Kotecki, D.J. Welding Metallurgy and Weldability of Stainless Steels; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Salih, A.A.; Omar, M.Z.; Junaidi, S.; Sajuri, Z. Effect of Different Heat Treatment on the SS440C Martensitic Stainless Steel. Aust. J. Basic Appl. Sci. 2011, 5, 867–871. [Google Scholar]
- Hollomon, J.H.; Jaffe, L.D. Time-temperature relations in tempering steel. Trans. AIME 1945, 162, 223–249. [Google Scholar]
- Anantha, K.H.; Örnek, C.; Ejnermark, S.; Medvedeva, A.; Sjöström, J.; Pan, J. Correlative Microstructure Analysis and In Situ Corrosion Study of AISI 420 Martensitic Stainless Steel for Plastic Molding Applications. J. Electrochem. Soc. 2017, 164, C85–C93. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M.; Vines, S.P. Origins of pitting corrosion. Corros. Eng. Sci. Technol. 2004, 39, 25–30. [Google Scholar] [CrossRef]
- Baroux, B. Further insights on the pitting corrosion of stainless steels. Corros. Mech. Theory Pract. 1995, 265–310. [Google Scholar]
- Candelária, A.F.; Pinedo, C.E. Influence of the heat treatment on the corrosion resistance of the martensitic stainless steel type AISI 420. J. Mater. Sci. Lett. 2003, 22, 1151–1153. [Google Scholar] [CrossRef]
- Schneider, M.; Kremmer, K.; Lämmel, C.; Sempf, K.; Herrmann, M. Galvanic corrosion of metal/ceramic coupling. Corros. Sci. 2014, 80, 191–196. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Bouyiouri, E.; Kouloumbi, N.; Vassiliou, P.; Koutsomichalis, A. Wear and corrosion resistance of laser surface hardened structural steel. Surf. Coat. Technol. 2002, 161, 125–134. [Google Scholar] [CrossRef]
- Malheiros LR, C.; Rodriguez EA, P.; Arlazarov, A. Mechanical behavior of tempered martensite: Characterization and modeling. Mater. Sci. Eng. A 2017, 706, 38–47. [Google Scholar] [CrossRef]











| Material | C | Cr | Si | Mn | Fe |
|---|---|---|---|---|---|
| AISI 420 | 0.30 ± 0.05 | 15.4 ± 0.26 | 0.50 ± 0.07 | 0.45 ± 0.12 | Balance |
| Specimen | Ecorr (mV) vs. SCE | Epit (mV) vs. SCE |
|---|---|---|
| AR420 | −141 ± 8 | 4.0 ± 6 |
| AQ420 | −92 ± 4 | 154 ± 7 |
| FSP250 | −55 ± 10 | 161 ± 23 |
| FSP75% | −314 ± 17 | active |
| FSP50% | −357 ± 15 | active |
| FSP25% | −360 ± 17 | active |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Kwok, C.T.; Lo, K.H. Effect of Multiple-Pass Friction Stir Processing on Hardness and Corrosion Resistance of Martensitic Stainless Steel. Coatings 2019, 9, 620. https://doi.org/10.3390/coatings9100620
Pan L, Kwok CT, Lo KH. Effect of Multiple-Pass Friction Stir Processing on Hardness and Corrosion Resistance of Martensitic Stainless Steel. Coatings. 2019; 9(10):620. https://doi.org/10.3390/coatings9100620
Chicago/Turabian StylePan, Linlin, Chi Tat Kwok, and Kin Ho Lo. 2019. "Effect of Multiple-Pass Friction Stir Processing on Hardness and Corrosion Resistance of Martensitic Stainless Steel" Coatings 9, no. 10: 620. https://doi.org/10.3390/coatings9100620




