Self-Ordered Orientation of Crystalline Hexagonal Boron Nitride Nanodomains Embedded in Boron Carbonitride Films for Band Gap Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Deposition
2.2. Film Characterization
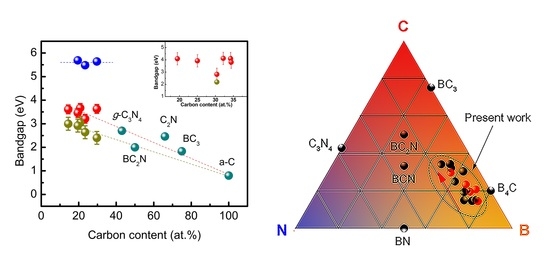
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, L.; Liu, Z.; Reddy, A.L.; Narayanan, N.T.; Taha-Tijerina, J.; Peng, J.; Gao, G.; Lou, J.; Vajtai, R.; Ajayan, P.M. Binary and ternary atomic layers built from carbon, boron, and nitrogen. Adv. Mater. 2012, 24, 4878–4895. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Wang, H.; Fu, W.; Gong, Y.; Zhou, W.; Ajayan, P.M.; Lou, J.; Liu, Z. Band engineering for novel two-dimensional atomic layers. Small 2015, 11, 1868–1884. [Google Scholar] [CrossRef]
- Byon, E.; Son, M.; Lee, K.-H.; Nam, K.-S.; Hara, N.; Sugimoto, K. Electrochemical properties of boron–carbon–nitride films formed by magnetron sputtering. Electrochim. Acta 2006, 51, 2662–2668. [Google Scholar] [CrossRef]
- Zhao, Y.; He, D.W.; Daemen, L.L.; Shen, T.D.; Schwarz, R.B.; Zhu, Y.; Bish, D.L.; Huang, J.; Zhang, J.; Shen, G.; et al. Superhard B–C–N materials synthesized in nanostructured bulks. J. Mater. Res. 2011, 17, 3139–3145. [Google Scholar] [CrossRef]
- Prakash, A.; Todi, V.; Sundaram, K.B.; Ross, L.; Xu, G.H.; French, M.; Henry, P.; King, S.W. Investigation of the dielectric and mechanical properties for magnetron sputtered BCN thin films. ECS J. Solid State Sci. Technol. 2015, 4, N3122–N3126. [Google Scholar] [CrossRef]
- Yue, J.S.; Cheng, W.J.; Zhang, X.W.; He, D.Y.; Chen, G.H. Ternary BCN thin films deposited by reactive sputtering. Thin Solid Films 2000, 375, 247–250. [Google Scholar] [CrossRef]
- Beniwal, S.; Hooper, J.; Miller, D.P.; Costa, P.S.; Chen, G.; Liu, S.Y.; Dowben, P.A.; Sykes, E.C.; Zurek, E.; Enders, A. Graphene-like boron-carbon-nitrogen monolayers. ACS Nano 2017, 11, 2486–2493. [Google Scholar] [CrossRef]
- Umeda, S.; Yuki, T.; Sugiyama, T.; Sugino, T. Boron carbon nitride film with low dielectric constant as passivation film for high speed electronic devices. Diam. Relat. Mater. 2004, 13, 1135–1138. [Google Scholar] [CrossRef]
- Prakash, A.; Sundaram, K.B.; Campiglia, A.D. Photoluminescence studies on BCN thin films synthesized by RF magnetron sputtering. Mater. Lett. 2016, 183, 355–358. [Google Scholar] [CrossRef]
- Prakash, A.; Nehate, S.D.; Sundaram, K.B. Boron carbon nitride based metal-insulator-metal UV detectors for harsh environment applications. Opt. Lett. 2016, 41, 4249–4252. [Google Scholar] [CrossRef]
- Wan, S.; Yu, Y.; Pu, J.; Lu, Z. Facile fabrication of boron nitride nanosheets–amorphous carbon hybrid film for optoelectronic applications. RSC Adv. 2015, 5, 19236–19240. [Google Scholar] [CrossRef]
- Prakash, A.; Sundaram, K.B. Optical and XPS studies of BCN thin films by co-sputtering of B4C and BN targets. Appl. Surf. Sci. 2017, 396, 484–491. [Google Scholar] [CrossRef]
- Todi, V.O.; Shantheyanda, B.P.; Sundaram, K.B. Influence of annealing on the optical properties of reactively sputtered BCN thin films. Mater. Chem. Phys. 2013, 141, 596–601. [Google Scholar] [CrossRef]
- Todi, V.O.; Shantheyanda, B.P.; Todi, R.M.; Sundaram, K.B.; Coffey, K. Optical characterization of BCN films deposited at various N2/Ar gas flow ratios by RF magnetron sputtering. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2011, 176, 878–882. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.; Choi, H.C.; Ahn, J.P.; Hou, J.Q.; Kang, H.S. X-ray photoelectron spectroscopy and first principles calculation of BCN nanotubes. J. Am. Chem. Soc. 2007, 129, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Ci, L.; Song, L.; Jin, C.H.; Jariwala, D.; Wu, D.X.; Li, Y.J.; Srivastava, A.; Wang, Z.F.; Storr, K.; Balicas, L.; et al. Atomic layers of hybridized boron nitride and graphene domains. Nat. Mater. 2010, 9, 430–435. [Google Scholar] [CrossRef]
- Krivanek, O.L.; Chisholm, M.F.; Nicolosi, V.; Pennycook, T.J.; Corbin, G.J.; Dellby, N.; Murfitt, M.F.; Own, C.S.; Szilagyi, Z.S.; Oxley, M.P.; et al. Atom-by-atom structural and chemical analysis by annular dark-field electron microscopy. Nature 2010, 464, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Shirodkar, S.N.; Waghmare, U.V.; Fisher, T.S.; Grau-Crespo, R. Engineering the electronic bandgaps and band edge positions in carbon-substituted 2D boron nitride: a first-principles investigation. Phys. Chem. Chem. Phys. 2015, 17, 13547–13552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Gao, G.Y.; Kutana, A.; Wang, Y.C.; Zou, X.L.; Tse, J.S.; Yakobson, B.I.; Li, H.D.; Liu, H.Y.; Ma, Y.M. Two-dimensional boron-nitrogen-carbon monolayers with tunable direct band gaps. Nanoscale 2015, 7, 12023–12029. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Pati, S.K. Criticality of surface topology for charge-carrier transport characteristics in two-dimensional borocarbonitrides: design principles for an efficient electronic material. Nanoscale 2014, 6, 13430–13434. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.F.; Bello, I.; Lei, M.K.; Li, K.Y.; Lee, C.S.; Lee, S.T. Synthesis and characterization of boron carbon nitride films by radio frequency magnetron sputtering. Surf. Coat. Technol. 2000, 128, 334–340. [Google Scholar] [CrossRef]
- Wada, Y.; Yap, Y.K.; Yoshimura, M.; Mori, Y.; Sasaki, T. The control of B–N and B–C bonds in BCN films synthesized using pulsed laser deposition. Diam. Relat. Mater. 2000, 9, 620–624. [Google Scholar] [CrossRef]
- Sugino, T.; Hieda, H. Field emission characteristics of boron carbon nitride films synthesized by plasma-assisted chemical vapor deposition. Diam. Relat. Mater. 2000, 9, 1233–1237. [Google Scholar] [CrossRef]
- Mannan, M.A.; Baba, Y.; Kida, T.; Nagano, M.; Shimoyama, I.; Hirao, N.; Noguchi, H. Orientation of B–C–N hybrid films deposited on Ni (111) and polycrystalline Ti substrates explored by X-ray absorption spectroscopy. Thin Solid Films 2011, 519, 1780–1786. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamamoto, K.; Kakudate, Y. Synthesis of B–C–N thin films by electron beam excited plasma CVD. Diam. Relat. Mater. 2002, 11, 1290–1294. [Google Scholar] [CrossRef]
- Caretti, I.; Jiménez, I.; Gago, R.; Cáceres, D.; Abendroth, B.; Albella, J.M. Tribological properties of ternary BCN films with controlled composition and bonding structure. Diam. Relat. Mater. 2004, 13, 1532–1537. [Google Scholar] [CrossRef]
- Laidani, N.; Anderle, M.; Canteri, R.; Elia, L.; Luches, A.; Martino, M.; Micheli, V.; Speranza, G. Structural and compositional study of B–C–N films produced by laser ablation of B4C targets in N2 atmosphere. Appl. Surf. Sci. 2000, 157, 135–144. [Google Scholar] [CrossRef]
- Kosaka, M.; Urakami, N.; Hashimoto, Y. Formation of graphitic carbon nitride and boron carbon nitride film on sapphire substrate. Jpn. J. Appl. Phys. 2018, 57, 02CB09. [Google Scholar] [CrossRef]
- Tsai, P.-C. The deposition and characterization of BCN films by cathodic arc plasma evaporation. Surf. Coat. Technol. 2007, 201, 5108–5113. [Google Scholar] [CrossRef]
- Bengu, E.; Genisel, M.F.; Gulseren, O.; Ovali, R. Theoretical and spectroscopic investigations on the structure and bonding in B–C–N thin films. Thin Solid Films 2009, 518, 1459–1464. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhuang, C.Q.; Li, X.Q.; Sai, L.W.; Zhao, J.J.; Jiang, X. Mapping distributions of mechanical properties and formation ability on the ternary B–C–N phase diagram. Diam. Relat. Mater. 2011, 20, 891–895. [Google Scholar] [CrossRef]
- Park, J.K.; Ko, J.S.; Baik, Y.J. Effect of hydrogen addition on the residual stress of B–C–N films with cubic boron nitride phase prepared by r.f. magnetron sputtering of a B4C target. Surf. Coat. Technol. 2013, 215, 104–109. [Google Scholar] [CrossRef]
- Tavsanoglu, T.; Jeandin, M.; Addemir, O. Synthesis and characterisation of thin films in the B–C–N triangle. Surf. Eng. 2016, 32, 755–760. [Google Scholar] [CrossRef]
- Prakash, A.; Sundaram, K.B. Deposition and XPS studies of dual sputtered BCN thin films. Diam. Relat. Mater. 2016, 64, 80–88. [Google Scholar] [CrossRef]
- Pakdel, A.; Wang, X.; Zhi, C.; Bando, Y.; Watanabe, K.; Sekiguchi, T.; Nakayama, T.; Golberg, D. Facile synthesis of vertically aligned hexagonal boron nitride nanosheets hybridized with graphitic domains. J. Mater. Chem. 2012, 22, 4818. [Google Scholar] [CrossRef]
- Giovannetti, G.; Khomyakov, P.A.; Brocks, G.; Kelly, P.J.; van den Brink, J. Substrate-induced band gap in graphene on hexagonal boron nitride: Ab initio density functional calculations. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef] [Green Version]
- Bepete, G.; Voiry, D.; Chhowalla, M.; Chiguvare, Z.; Coville, N.J. Incorporation of small BN domains in graphene during CVD using methane, boric acid and nitrogen gas. Nanoscale 2013, 5, 6552–6557. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Todi, R.M.; Warren, A.P.; Sundaram, K.B. Influence of N2/Ar gas mixture ratio and annealing on optical properties of SiCBN thin films prepared by rf sputtering. Diam. Relat. Mater. 2008, 17, 944–948. [Google Scholar] [CrossRef]
- Zhi, C.Y.; Bando, Y.; Tang, C.C.; Kuwahara, H.; Golberg, D. Large-scale fabrication of boron nitride nanosheets and their utilization in polymeric composites with improved thermal and mechanical properties. Adv. Mater. 2009, 21, 2889. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef]
- Yu, J.; Wang, E.G.; Ahn, J.; Yoon, S.F.; Zhang, Q.; Cui, J.; Yu, M.B. Turbostratic boron carbonitride films produced by bias-assisted hot filament chemical vapor deposition. J. Appl. Phys. 2000, 87, 4022–4025. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Li, F.; Lannin, J.S. Radial distribution function of amorphous carbon. Phys. Rev. Lett. 1990, 65, 1905–1908. [Google Scholar] [CrossRef]
- Beckel, C.L.; Yousaf, M.; Fuka, M.Z.; Raja, S.Y.; Lu, N. Lattice vibrations of the icosahedral solid alpha-boron. Phys. Rev. B 1991, 44, 2535. [Google Scholar] [CrossRef]
- Linss, V.; Hermann, I.; Schwarzer, N.; Kreissig, U.; Richter, F. Mechanical properties of thin films in the ternary triangle B–C–N. Surf. Coat. Technol. 2003, 163, 220–226. [Google Scholar] [CrossRef]
- Qu, J.; Li, Q.; Luo, C.; Cheng, J.; Hou, X. Characterization of flake boron nitride prepared from the low temperature combustion synthesized precursor and its application for dye adsorption. Coatings 2018, 8, 214. [Google Scholar] [CrossRef]
- Cometto, O.; Sun, B.; Tsang, S.H.; Huang, X.; Koh, Y.K.; Teo, E.H.T. Vertically self-ordered orientation of nanocrystalline hexagonal boron nitride thin films for enhanced thermal characteristics. Nanoscale 2015, 7, 18984–18991. [Google Scholar] [CrossRef]
- Pascual, E.; MartíNez, E.; Esteve, J.; Lousa, A. Boron carbide thin films deposited by tuned-substrate RF magnetron sputtering. Diam. Relat. Mater. 1999, 8, 402–405. [Google Scholar] [CrossRef]
- Yap, Y.K.; Kida, S.; Aoyama, T.; Mori, Y. Influence of negative dc bias voltage on structural transformation of carbon nitride at 600 °C. Appl. Phys. Lett. 1998, 73, 915–917. [Google Scholar] [CrossRef]
- Nakao, S.; Sonoda, T.; Tsugawa, K.; Choi, J.; Kato, T. Effects of nitrogen gas ratio on composition and microstructure of BCN films prepared by RF magnetron sputtering. Vacuum 2009, 84, 642–647. [Google Scholar] [CrossRef]
- Higashi, G.S.; Chabal, Y.J.; Trucks, G.W.; Raghavachari, K. Ideal hydrogen termination of the Si (111) surface. Appl. Phys. Lett. 1990, 56, 656–658. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Q.; Fang, C.; Chen, J.; Su, J.; Xu, K.; Ai, L.; Liu, D. Preparation, structure, and properties of surface modified graphene/epoxy resin composites for potential application in conductive ink. Coatings 2018, 8, 387. [Google Scholar] [CrossRef]
- Chang, C.K.; Kataria, S.; Kuo, C.C.; Ganguly, A.; Wang, B.Y.; Hwang, J.Y.; Huang, K.J.; Yang, W.H.; Wang, S.B.; Chuang, C.H. Band gap engineering of chemical vapor deposited graphene by in situ BN doping. ACS Nano 2013, 7, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Cancado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Li, Q.; Marks, L.D.; Lifshitz, Y.; Lee, S.T.; Bello, I. Controlling the nucleation environment of c-BN films and their related properties. Phys. Rev. B 2002, 65. [Google Scholar] [CrossRef]
- Mckenzie, D.R.; Mcfall, W.D.; Sainty, W.G.; Davis, C.A.; Collins, R.E. Compressive stress induced formation of cubic boron nitride. Diam. Relat. Mater. 1993, 2, 970–976. [Google Scholar] [CrossRef]
- Lifshitz, Y.; Meng, X.M.; Lee, S.T.; Akhveldiany, R.; Hoffman, A. Visualization of diamond nucleation and growth from energetic species. Phys. Rev. Lett. 2004, 93. [Google Scholar] [CrossRef]
- Anutgan, M.; Anutgan, T.A.; Ozkol, E.; Atilgan, I.; Katircioglu, B. Influence of the nitrogen flow rate on the order and structure of PECVD boron nitride thin films. J. Non-Cryst. Solids 2009, 355, 1622–1629. [Google Scholar] [CrossRef]
- Hofsäss, H.; Feldermann, H.; Eyhusen, S.; Ronning, C. Fundamental role of ion bombardment for the synthesis of cubic boron nitride films. Phys. Rev. B 2002, 65, 115410. [Google Scholar] [CrossRef]
- Linss, V.; Rodil, S.E.; Reinke, P.; Garnier, M.G.; Oelhafen, P.; Kreissig, U.; Richter, F. Bonding characteristics of DC magnetron sputtered B–C–N thin films investigated by Fourier-transformed infrared spectroscopy and X-ray photoelectron spectroscopy. Thin Solid Films 2004, 467, 76–87. [Google Scholar] [CrossRef]
- Puyoo, G.; Teyssandier, F.; Pailler, R.; Labrugere, C.; Chollon, G. Boron carbonitride coatings synthesized by LPCVD, structure and properties. Carbon 2017, 122, 19–46. [Google Scholar] [CrossRef]
- Jia, F.; Zhuang, C.; Guan, C.; Zhao, J.; Bai, Y.; Jiang, X. Preparation of B–C–N films by magnetron sputtering with different N2/Ar flow ratio. Vacuum 2011, 85, 887–891. [Google Scholar] [CrossRef]
- Kang, Y.; Chu, Z.Y.; Zhang, D.J.; Li, G.Y.; Jiang, Z.H.; Cheng, H.F.; Li, X.D. Incorporate boron and nitrogen into graphene to make BCN hybrid nanosheets with enhanced microwave absorbing properties. Carbon 2013, 61, 200–208. [Google Scholar] [CrossRef]
- Lei, M.K.; Li, Q.; Zhou, Z.F.; Bello, I.; Lee, C.S.; Lee, S.T. Characterization and optical investigation of BCN film deposited by RF magnetron sputtering. Thin Solid Films 2001, 389, 194–199. [Google Scholar] [CrossRef]
- Xu, S.; Ma, X.; Tang, G.; Zhang, Q. Air annealing effect on scratch behaviour of BCN films. Surf. Eng. 2015, 31, 549–555. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Yang, Q.; Tu, R.; Wang, C.; Shen, Q.; Zhang, L. Stoichiometric controlling of boroncarbonitride thin films with using BN-C dual-targets. AIP Adv. 2015, 5, 047125. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Keunecke, M.; Bewilogua, K.; Czigany, Z.; Hultman, L. Structural features of thick c-boron nitride coatings deposited via a graded B–C–N interlayer. Surf. Coat. Technol. 2001, 142, 881–888. [Google Scholar] [CrossRef]
- Yuan, Y.W.; Zhang, L.L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.Z.; Li, Y.M.; Hu, X.; Wang, S.J.; Genc, A.; et al. High-yield synthesis and optical properties of g-C3N4. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef]
- Watanabe, K.; Taniguchi, T.; Kanda, H. Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal. Nat. Mater. 2004, 3, 404–409. [Google Scholar] [CrossRef]
- Ech-chamikh, E.; Essafti, A.; Azizan, M.; Ijdiyaou, Y. Optical characterization of a-C:N thin films deposited by RF sputtering. Sol. Energy Mater. Sol. Cells 2006, 90, 1424–1428. [Google Scholar] [CrossRef]
- Shi, J.R.; Wang, J.P. Diamond-like carbon films prepared by facing-target sputtering. Thin Solid Films 2002, 420, 172–176. [Google Scholar] [CrossRef]








| Sample (Temperature) | ID/IG | Defect Density (×1011 cm−2) | h-BN Nanodomain Crystallite Size (nm) |
|---|---|---|---|
| 400 °C | 2.1 | 7.4 | 42.9 |
| 500 °C | 1.432 | 5.1 | 62.9 |
| 600 °C | 0.987 | 3.5 | 91.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Gao, W.; Wang, F.; Zhao, D.; Zhang, Y.; Yin, H. Self-Ordered Orientation of Crystalline Hexagonal Boron Nitride Nanodomains Embedded in Boron Carbonitride Films for Band Gap Engineering. Coatings 2019, 9, 185. https://doi.org/10.3390/coatings9030185
Li Y, Gao W, Wang F, Zhao D, Zhang Y, Yin H. Self-Ordered Orientation of Crystalline Hexagonal Boron Nitride Nanodomains Embedded in Boron Carbonitride Films for Band Gap Engineering. Coatings. 2019; 9(3):185. https://doi.org/10.3390/coatings9030185
Chicago/Turabian StyleLi, Yujing, Wei Gao, Fei Wang, Dehe Zhao, Yuyuan Zhang, and Hong Yin. 2019. "Self-Ordered Orientation of Crystalline Hexagonal Boron Nitride Nanodomains Embedded in Boron Carbonitride Films for Band Gap Engineering" Coatings 9, no. 3: 185. https://doi.org/10.3390/coatings9030185