Electrodeposition of Nanocrystalline Fe-P Coatings: Influence of Bath Temperature and Glycine Concentration on Structure, Mechanical and Corrosion Behavior
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. Potentiodynamic Investigations
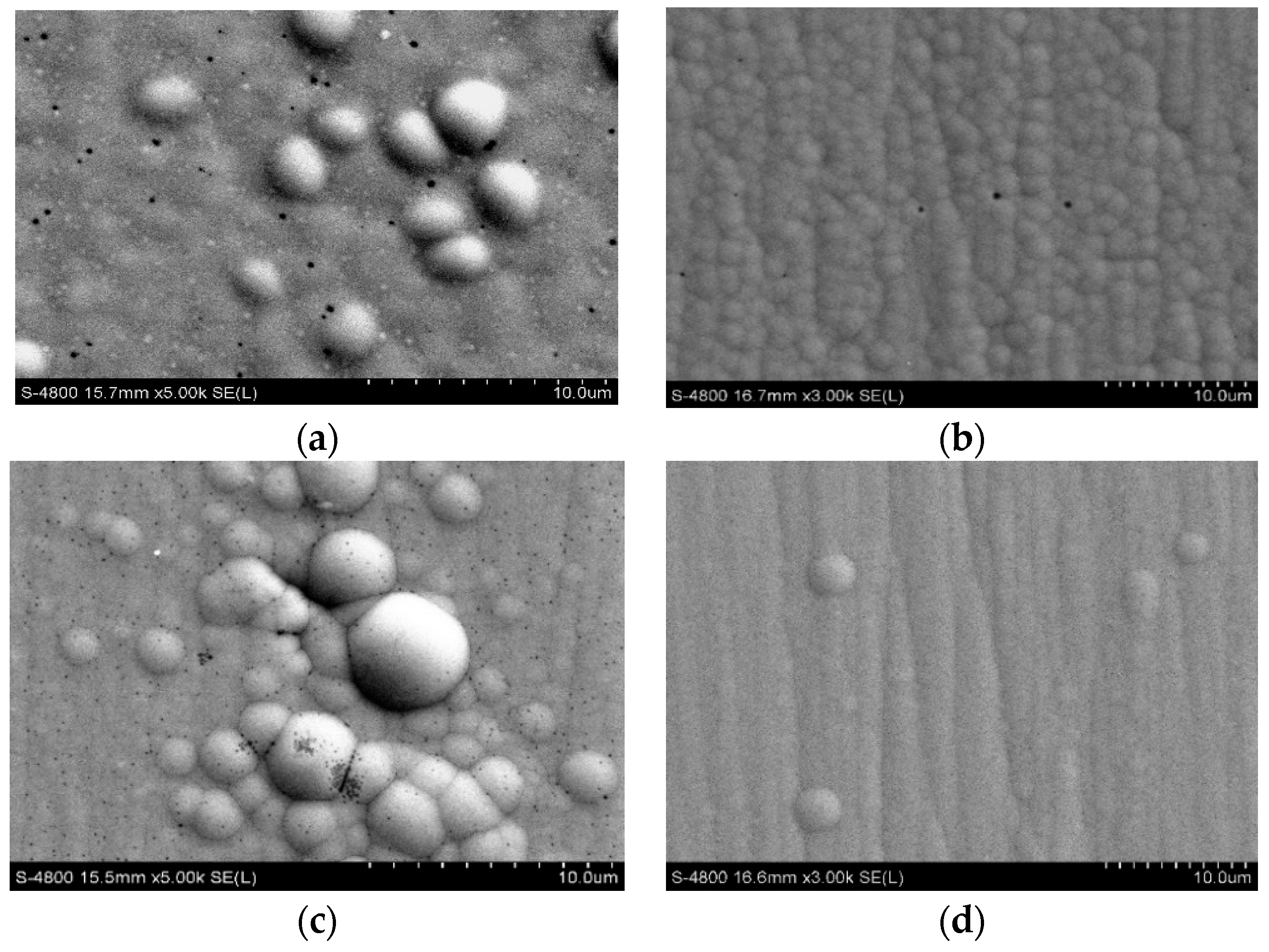
3.2. Morphological and Structural Investigation
3.3. Corrosion Properties
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jafari, S.; Beitollahi, A.; Eftekhariyekta, B.; Kanada, K.; Ohkubo, T.; Herzer, G. Microstructural and magnetic properties study of Fe–P rolled sheet alloys. J. Magn. Magn. Mater. 2014, 358, 38–43. [Google Scholar] [CrossRef]
- Manna, S.K.; Prabhu, D.B.; Gopalan, R.; Srinivas, V. AC magnetic properties and core loss behavior of Fe–P soft magnetic sheets. IEEE Trans. Magn. 2014, 50, 4–7. [Google Scholar] [CrossRef]
- Mikó, A.; Kuzmann, E.; Lakatos-Varsányi, M.; Kákay, A.; Nagy, F.; Varga, L.K. Mössbauer and XRD study of pulse plated Fe-P and Fe-Ni thin layers. In ICAME 2005, Proceedings of the 28th International Conference on the Applications of the Mössbauer Effect (ICAME 2005) held in Montpellier, France, 4–9 September 2005; Lippens, P.-E., Jumas, J.-C., Génin, J.-M.R., Eds.; Springer: Berlin, Germany, 2006; pp. 195–201. [Google Scholar]
- Suzuki, M.; Piccone, T.J.; Flemings, M.C.; Brody, H.D. Solidification of highly undercooled Fe-P alloys. Metall. Trans. A 1991, 22, 2761–2768. [Google Scholar] [CrossRef]
- Hong, M.; Jin, D.; Soo, J. The effects of pH and temperature on Ni–Fe–P alloy electrodeposition from a sulfamate bath and the material properties of the deposits. Thin Solid Films 2005, 489, 122–129. [Google Scholar] [CrossRef]
- Sequeira, C.A.C.; Santos, D.M.F.; Brito, P.S.D. Electrocatalytic activity of simple and modified Fe-P electrodeposits for hydrogen evolution from alkaline media. Energy 2011, 36, 847–853. [Google Scholar] [CrossRef]
- Hrubovčakova, M.; Kupkova, M.; Džupon, M. Fe and Fe-P foam for biodegradable bone replacement material: Morphology, corrosion behaviour, and mechanical properties. Adv. Mater. Sci. Eng. 2016, 2016, 6257368. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, X.M.; Wu, Y.S.; Xue, L.J.; Ke, F.S. Electrodeposition and lithium storage performance of novel three-dimensional porous Fe–Sb–P amorphous alloy electrode. Electrochem. Commun. 2009, 11, 585–588. [Google Scholar] [CrossRef]
- Zheng, X.M.; Zhang, P.Y.; Wang, L.K.; Tao, S.; Wang, Y.X.; Li, J.T. Superior Li storage anode based on novel Fe–Sn–P alloy prepared by electroplating. Electrochim. Acta 2017, 247, 314–320. [Google Scholar] [CrossRef]
- Shervedani, R.; Alinoori, A.; Madram, A. Electrocatalytic activities of nickel-phosphorous composite coating reinforced with codeposited graphite carbon for hydrogen evolution reaction in alkaline solution. J. New Mater. Electrochem. Syst. 2008, 11, 259–265. [Google Scholar]
- Safizadeh, F.; Sorour, N.; Ghali, E.; Houlachi, G. Study of the hydrogen evolution reaction on Fe-Mo-P coatings as cathodes for chlorate production. Int. J. Hydrog. Energy 2016, 42, 5455–5463. [Google Scholar] [CrossRef]
- Bala, H. The acid corrosion of Fe-P alloys. Mater. Corros. 1987, 38, 25–31. [Google Scholar] [CrossRef]
- Cserei, A.; Kuzmann, E. Study of the crystallization kinetics in amorphous Fe83P17 alloy. J. Radional Nucl. Chem. Lett. 1994, 187, 33–45. [Google Scholar] [CrossRef]
- Logan, J.; Yung, M. The low-temperature electrical resistivity of amorphous iron-phosphorus alloys. J. Non-Cryst. Solids 1976, 21, 151–154. [Google Scholar] [CrossRef]
- Vitkova, S.; Kjuchukova, M.; Raichevski, G. Electrochemical preparation of amorphous Fe–P alloys. J. Appl. Electrochem. 1988, 18, 673–678. [Google Scholar] [CrossRef]
- Kamei, K.; Maehara, Y. Structure and magnetic properties of pulse-plated Fe–P and Fe–Cu–P amorphous alloys. Mater. Sci. Eng. A 1994, 181–182, 906–910. [Google Scholar] [CrossRef]
- Armyanov, S.; Vitkova, S.; Blajiev, O. Internal stress and magnetic properties of electrodeposited amorphous Fe-P alloys. J. Appl. Electrochem. 1997, 27, 185–191. [Google Scholar] [CrossRef]
- Zecevic, S.K.; Gojkovic, S.L.; Radmilovic, V. Electrochemically deposited thin films of amorphous Fe–P alloy: Part I. Chemical composition and phase structure characterization. J. Electroanal. Chem. 1998, 448, 245–252. [Google Scholar] [CrossRef]
- Yanai, T.; Yamaguchi, T.; Akiyoshi, T.; Takashima, K.; Nakano, M.; Fukunaga, H. Effects of glycine in DES-based plating baths on structural and magnetic properties of Fe-Ni films. IEEE Trans. Magn. 2017, 53, 2004404. [Google Scholar] [CrossRef]
- Piz, I.; Branica, M. Voltammetric determination of stability constants of iron(III)—Glycine complexes in water solution. J. Electroanal.Chem. 2005, 583, 140–147. [Google Scholar] [CrossRef]
- Kuzmann, E.; Vértes, A.; Kiss, L.; Raichewski, G.; Vitkova, S. Mössbauer study of electrochemically deposited Fe-P amorphous alloys. Electrochim. Acta 1988, 33, 1455–1458. [Google Scholar] [CrossRef]
- Kamei, K.; Maehara, Y. Magnetic properties and microstructure of electrodeposited Fe–P amorphous alloy. J. Appl. Electrochem. 1996, 26, 529–535. [Google Scholar] [CrossRef]
- Varsanyi, M.; Kuzmann, E.; Vértes, C.; Vértes, A.; Vitkova, S.; Raichewski, G. Electrochemical behaviour of electrodeposited Fe–8P amorphous alloys. Hypcrfinc Interact. 1991, 69, 735–738. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Jahan, S.M.; Rajam, K.S. Studies on autocatalytic deposition of ternary Ni-W-P alloys using nickel sulphamate bath. Surf. Coat. Technol. 2006, 201, 507–512. [Google Scholar] [CrossRef]
- Kozlowski, W.; Flis, J. Effect of oxyanions of phosphorus on the anodic behaviour of iron and iron + phosphorus alloy in borate solution. J. Electroanal. Chem. 1994, 374, 53–61. [Google Scholar] [CrossRef]
- Das, J.; Chandra, K.; Misra, P.S.; Sarma, B. Hardness and tensile properties of Fe–P based alloys made through powder forging technique. Mater. Sci. Eng. A 2008, 479, 164–170. [Google Scholar] [CrossRef]
- Forker, W.; Worth, H.; Rahner, D. Kinetische und kristallografische Modellbetrachtungen zur Korrosion und Passivitat von Metallen. Werkstoffe und Korrosion 1981, 32, 545–551. (In German) [Google Scholar] [CrossRef]
- Kozlowski, W.; Flis, J. An ellipsometric study of anodic films formed on iron-phosphorous alloys in borate solution. Corros. Sci. 1988, 28, 787–798. [Google Scholar] [CrossRef]
- Viefhaus, H.; Möller, R.; Janik-Czachor, M. Effects of phosphorus on the stability of the passive state of Fe and Fe–Ni base glasses. Mater. Corros. 1988, 39, 453–462. [Google Scholar] [CrossRef]
- Sawyer, D.T.; Sobkowiak, A. Electrochemistry for Chemists, 2nd ed.; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Gamburg, Y.D.; Zangari, G. Theory and Practice of Metal Electrodeposition; Springer: New York, NY, USA, 2011. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Fundamentals and Fundamentals and Applications; A K Peters/CRC Press: New York, NY, USA, 2015. [Google Scholar]
- Critelli, R.A.J.; Sumodjo, P.T.A.; Bertotti, M.; Torresi, R.M. Influence of glycine on Co electrodeposition: IR spectroscopy and near-surface pH investigations. Electrochim. Acta 2018, 260, 762–771. [Google Scholar] [CrossRef]
- Nicolenco, A.; Tsyntsaru, N.; Cesiulis, H. Fe(III)-based ammonia-free bath for electrodeposition of Fe-W alloys. J. Electrochem. Soc. 2017, 164, D590–D596. [Google Scholar] [CrossRef]
- Diegle, R.B.; Sorensen, N.R.; Clayton, C.R.; Helfand, M.A.; Yu, Y.C. An XPS investigation into the passivity of an amorphous Ni-20P alloy. J. Electrochem. Soc. 1988, 135, 1085–1092. [Google Scholar] [CrossRef]
- Lu, G.; Zangari, G. Corrosion resistance of ternary Ni-P based alloys in sulfuric acid solutions. Electrochim. Acta 2002, 47, 2969–2979. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef] [Green Version]








| Concentration Gly/M | Potential, U (vs. SHE)/V |
|---|---|
| 0 | −0.67 |
| 0.11 | −0.71 |
| 0.21 | −0.75 |
| 0.64 | −0.85 |
| c[Gly]/M | T/°C | −jtotal/mA·cm−2 | −je/mA·cm−2 | ||
|---|---|---|---|---|---|
| Fe | P | H2 | |||
| 0.11 | 20 | 15 | 11.1 | 0.4 | 3.5 |
| 30 | 26.5 | 0.9 | 2.6 | ||
| 60 | 15 | 9.3 | 0.7 | 5.0 | |
| 30 | 18.9 | 1.5 | 9.5 | ||
| 0.64 | 20 | 15 | 8.8 | 0.9 | 5.4 |
| 30 | 20.6 | 2.8 | 6.6 | ||
| 60 | 15 | 3.5 | 0.6 | 11.0 | |
| 30 | 18.8 | 1.8 | 9.3 | ||
| Phosphorous Content/at.% (±1) | –Ucorr/V vs. SHE (±0.5) | jcorr/mA cm−2 (±1) | ρcorr/Ω·cm2 (±0.5) |
|---|---|---|---|
| Fe | 0.22 | 0.24 | 0.49 |
| Fe-6 P | 0.20 | 0.30 | 0.62 |
| Fe-12 P | 0.15 | 0.27 | 0.59 |
| Fe-16 P | 0.12 | 0.16 | 0.67 |
| Elements Content/at.% (±2) | |
|---|---|
| Before Corrosion Test | After Corrosion Test |
| 6 P-<10 O | 16 P-74 O |
| 12 P-<10 O | 23 P-48 O |
| 16 P-<10 O | 24 P-41 O |
| P Content/at.% | H/GPa | Er/GPa |
|---|---|---|
| Fe-6 P | 8.04 ± 0.42 | 167.0 ± 8.6 |
| Fe-12 P | 8.57 ± 0.27 | 142.3 ± 3.9 |
| Fe-16 P | 9.01 ± 0.16 | 146.6 ± 2.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalska, N.; Tsyntsaru, N.; Cesiulis, H.; Gebert, A.; Fornell, J.; Pellicer, E.; Sort, J.; Hansal, W.; Kautek, W. Electrodeposition of Nanocrystalline Fe-P Coatings: Influence of Bath Temperature and Glycine Concentration on Structure, Mechanical and Corrosion Behavior. Coatings 2019, 9, 189. https://doi.org/10.3390/coatings9030189
Kovalska N, Tsyntsaru N, Cesiulis H, Gebert A, Fornell J, Pellicer E, Sort J, Hansal W, Kautek W. Electrodeposition of Nanocrystalline Fe-P Coatings: Influence of Bath Temperature and Glycine Concentration on Structure, Mechanical and Corrosion Behavior. Coatings. 2019; 9(3):189. https://doi.org/10.3390/coatings9030189
Chicago/Turabian StyleKovalska, Natalia, Natalia Tsyntsaru, Henrikas Cesiulis, Annet Gebert, Jordina Fornell, Eva Pellicer, Jordi Sort, Wolfgang Hansal, and Wolfgang Kautek. 2019. "Electrodeposition of Nanocrystalline Fe-P Coatings: Influence of Bath Temperature and Glycine Concentration on Structure, Mechanical and Corrosion Behavior" Coatings 9, no. 3: 189. https://doi.org/10.3390/coatings9030189





