Exploring Skin Interactions with 5G Millimeter-Wave through Fluorescence Lifetime Imaging Microscopy
Abstract
:1. Introduction
2. Fluorescence Lifetime Imaging Microscopy
2.1. Fluorescence Lifetime
2.2. Determining Lifetime through Phase-Modulation Method
2.3. Skin Anatomy and Fluorophores
| Fluorophore | 1-P Peak Excitation Wavelength (nm) | 2-P Peak Excitation Wavelength (nm) | Emission Wavelength (nm) | Fluorescence Lifetime (ns) |
|---|---|---|---|---|
| Bound FAD | 360–465 | 725–760, 850–950 | 520–530 | 0.003–4.55 |
| Free FAD | 360–465 | 725–760, 850–950 | 520–530 | 2.3–2.9 |
| Bound NAD(P)H | 330–360 | 760 | 440–470 | 1.9–5.7 |
| Free NAD(P)H | 330–360 | 760 | 440–470 | 0.3–0.4 |
3. Materials and Methods
3.1. Skin Samples
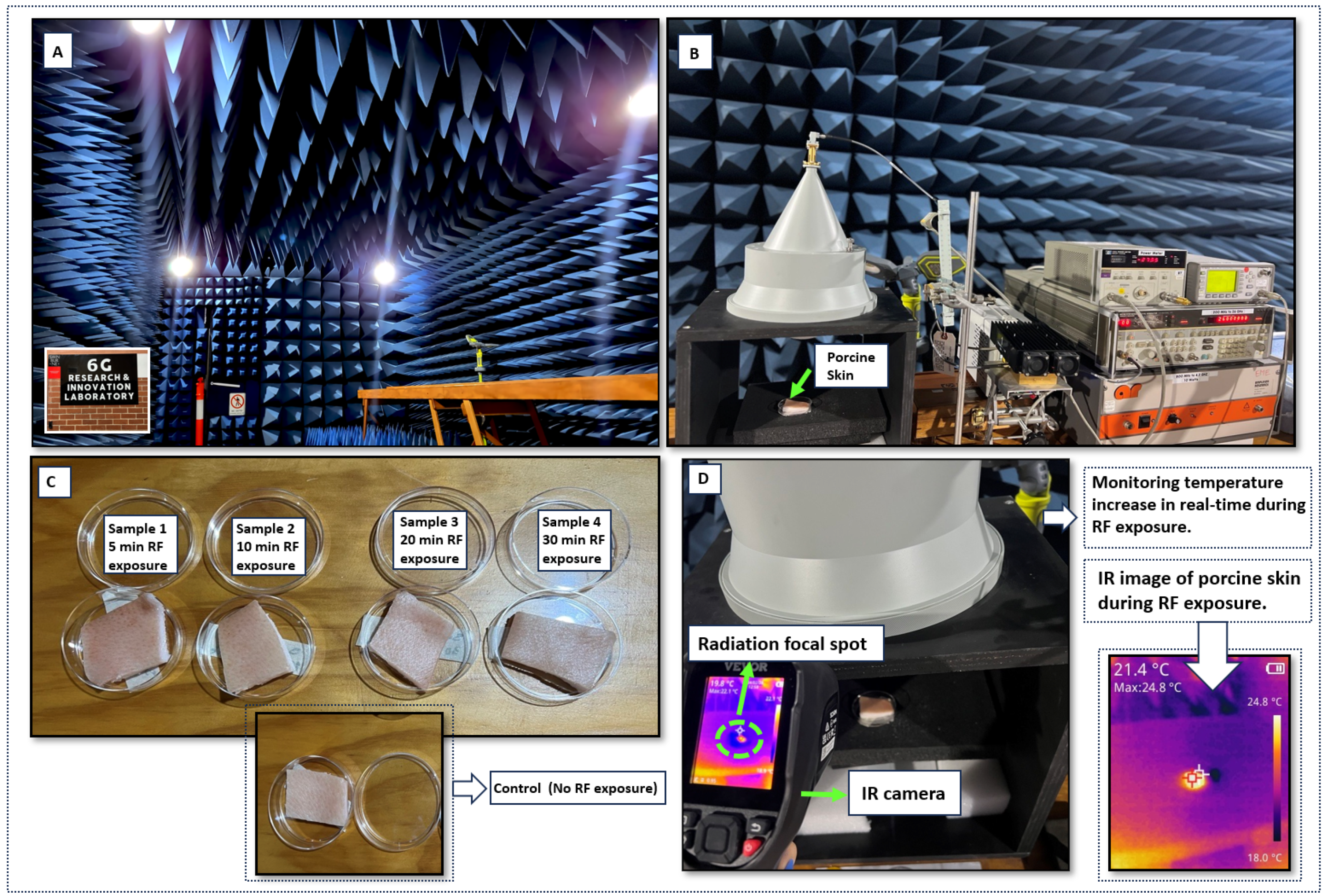
3.2. mmWave Exposure
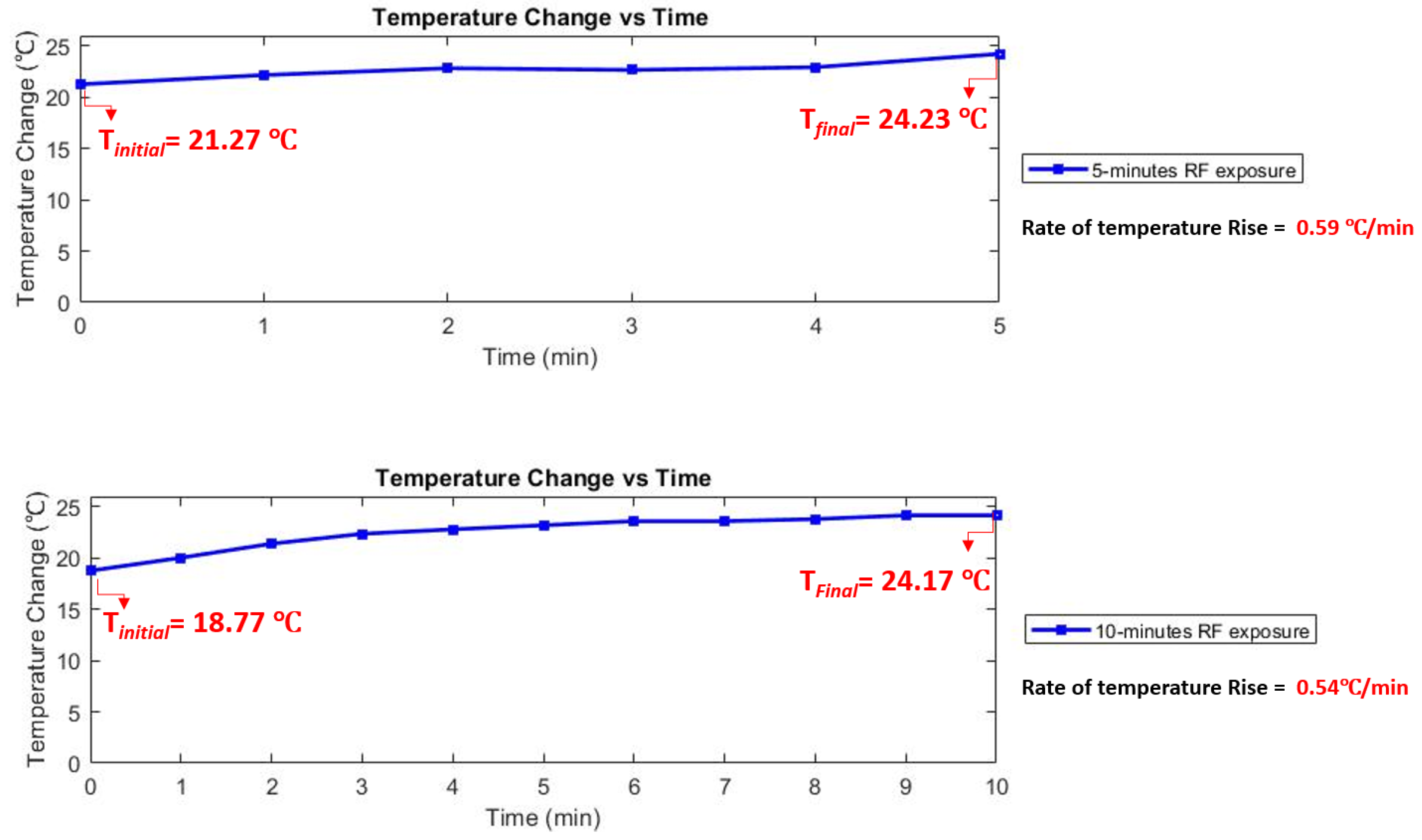
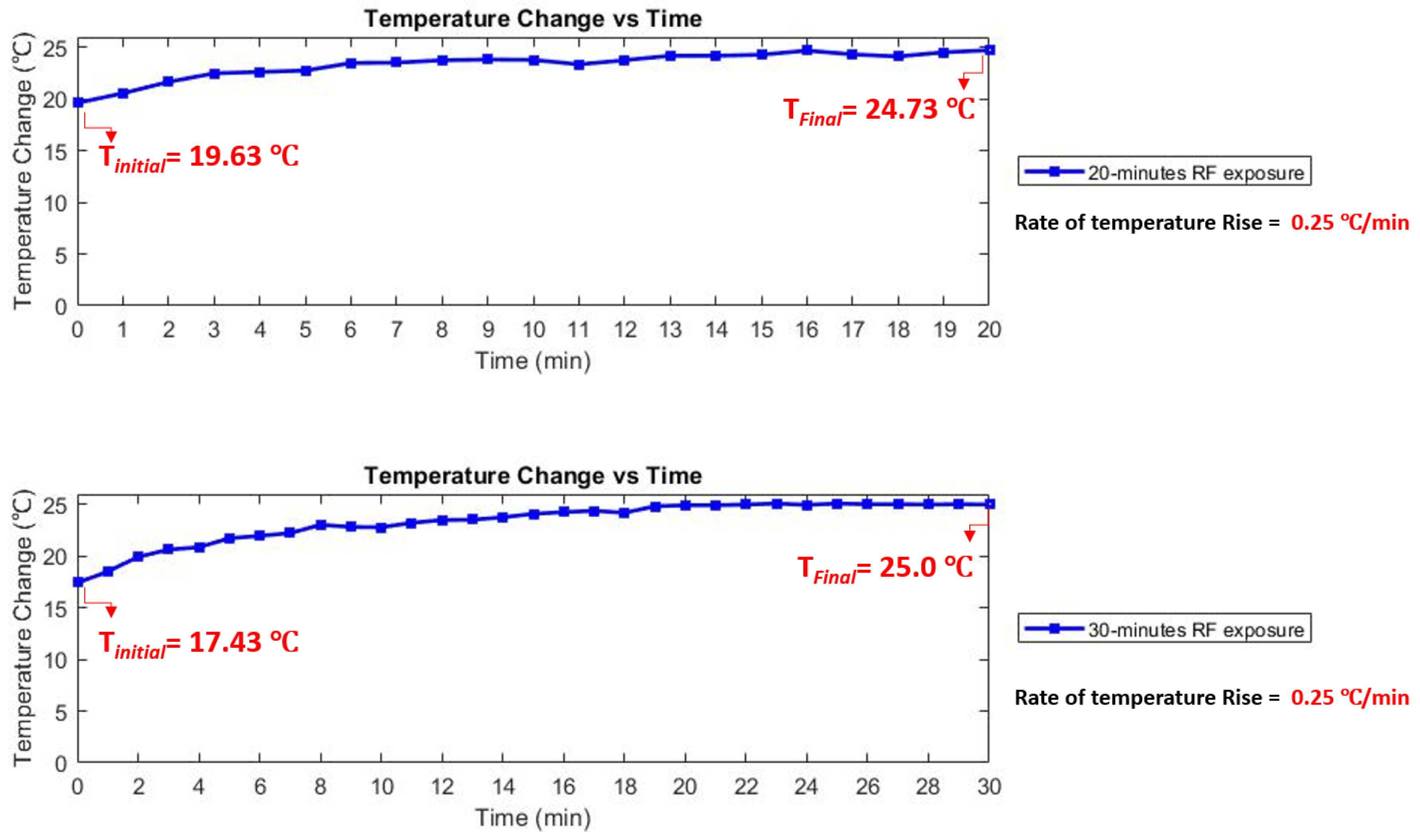
3.3. Temperature Rise Measurement
3.4. FLIM Imaging and Data Analysis
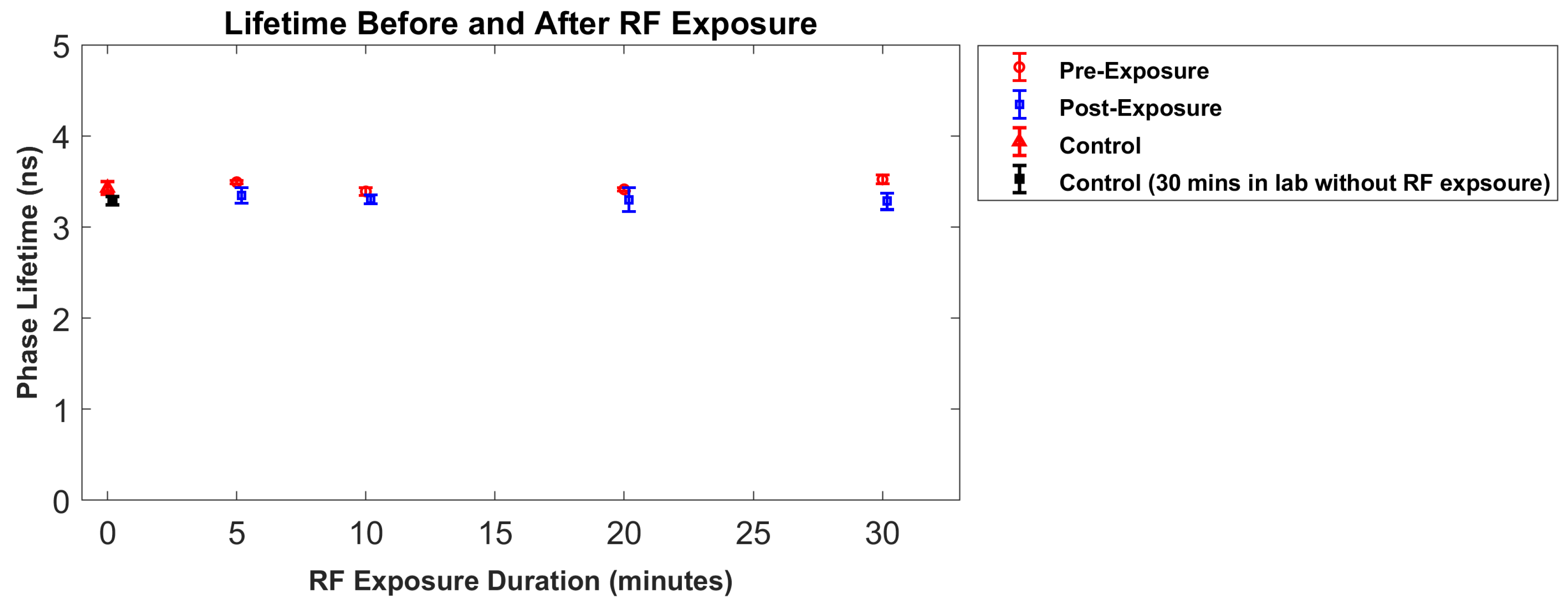
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (NAD(P)H) | Adenine Dinucleotide Phosphate |
| 6G | Sixth generation |
| 5G | Fifth generation |
| EM | Electromagnetic |
| FAD | Flavin Adenine Dinucleotide |
| FLIM | Fluorescence Lifetime Imaging Microscopy |
| FLT | Fluorescence lifetime |
| FR1 | Frequency Range 1 |
| FR2 | Frequency Range 2 |
| GB | Gigabytes |
| GHz | Gigahertz |
| ICNIRP | International Commission on Non-ionizing Radiation Protection |
| IR | Infrared |
| MHz | Megahertz |
| ms | Millisecond |
| mmWave | Millimeter-wave |
| ns | Nanosecond |
| RF | Radiofrequency |
| ROI | Region of Interest |
References
- Foroughimehr, N.; Vilagosh, Z.; Yavari, A.; Wood, A. The Impact of Base Cell Size Setup on the Finite Difference Time Domain Computational Simulation of Human Cornea Exposed to Millimeter Wave Radiation at Frequencies above 30 GHz. Sensors 2022, 22, 5924. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Jiang, Y.; Ren, H.; Deng, S.; Sun, J.; Cheng, F.; Jing, J.; Chen, Y. 3D-printed carbon-based conformal electromagnetic interference shielding module for integrated electronics. Nano-Micro Lett. 2024, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Segelstein, D.J. The Complex Refractive Index of Water. Ph.D. Thesis, University of Missouri-Kansas City, Kansas City, MO, USA, 1981. [Google Scholar]
- ICNIRP. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef] [PubMed]
- Foroughimehr, N.; Wood, A.; McKenzie, R.; Karipidis, K.; Yavari, A. Design and Implementation of a Specialised Millimetre-Wave Exposure System for Investigating the Radiation Effects of 5G and Future Technologies. Sensors 2024, 24, 1516. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hanazawa, M.; Yamashiro, Y.; Sasaki, H.; Watanabe, S.; Taki, M.; Suzuki, Y.; Hirata, A.; Kamimura, Y.; Sasaki, K. Acute ocular injuries caused by 60-GHz millimeter-wave exposure. Health Phys. 2009, 97, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sakai, T.; Nagaoka, T.; Wake, K.; Watanabe, S.; Kojima, M.; Hasanova, N.; Sasaki, H.; Sasaki, K.; Suzuki, Y.; et al. Dosimetry using a localized exposure system in the millimeter-wave band for in vivo studies on ocular effects. IEEE Trans. Microw. Theory Tech. 2014, 62, 1554–1564. [Google Scholar] [CrossRef]
- Diao, Y.; Rashed, E.A.; Hirata, A. Assessment of absorbed power density and temperature rise for nonplanar body model under electromagnetic exposure above 6 GHz. Phys. Med. Biol. 2020, 65, 224001. [Google Scholar] [CrossRef]
- Foster, K.R.; Laakso, I.; Chalfin, S. Nonuniform exposure to the cornea from millimeter waves. Health Phys. 2021, 120, 525–531. [Google Scholar] [CrossRef]
- Karampatzakis, A.; Samaras, T. Numerical modeling of heat and mass transfer in the human eye under millimeter wave exposure. Bioelectromagnetics 2013, 34, 291–299. [Google Scholar] [CrossRef]
- Foroughimehr, N.; Vilagosh, Z.; Wood, A.W. The reflectance of sepia melanin at THz frequencies. In Proceedings of the 2023 5th Australian Microwave Symposium (AMS), Melbourne, Australia, 16–17 February 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 1–2. [Google Scholar]
- Foroughimehr, N.; Vilagosh, Z.; Yavari, A.; Wood, A. The effects of mmW and THz radiation on dry eyes: A finite-difference time-domain (FDTD) computational simulation using XFdtd. Sensors 2023, 23, 5853. [Google Scholar] [CrossRef]
- Foroughimehr, N.; Vilagosh, Z.; Yavari, A.; Wood, A. Investigating the Impact of Synchrotron THz Radiation on the Corneal Hydration Using Synchrotron THz-Far Infrared Beamline. Sensors 2022, 22, 8261. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, D. Physiological effects of millimeter-waves on skin and skin cells: An overview of the to-date published studies. Rev. Environ. Health 2020, 35, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Samaras, T.; Neufeld, E.; Kuster, N. RF-induced temperature increase in a stratified model of the skin for plane-wave exposure at 6–100 GHz. Radiat. Prot. Dosim. 2020, 188, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Patrignoni, L.; Hurtier, A.; Orlacchio, R.; Joushomme, A.; Poulletier de Gannes, F.; Lévêque, P.; Arnaud-Cormos, D.; Revzani, H.R.; Mahfouf, W.; Garenne, A.; et al. Evaluation of mitochondrial stress following ultraviolet radiation and 5G radiofrequency field exposure in human skin cells. Bioelectromagnetics 2024, 45, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Feldman, Y.; Puzenko, A.; Ishai, P.B.; Caduff, A.; Davidovich, I.; Sakran, F.; Agranat, A.J. The electromagnetic response of human skin in the millimetre and submillimetre wave range. Phys. Med. Biol. 2009, 54, 3341. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, S.; Radzievsky, A.; Szabo, I.; Ziskin, M. Local heating of human skin by millimeter waves: Effect of blood flow. Bioelectromagnetics 2005, 26, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Millenbaugh, N.J.; Roth, C.; Sypniewska, R.; Chan, V.; Eggers, J.S.; Kiel, J.L.; Blystone, R.V.; Mason, P.A. Gene expression changes in the skin of rats induced by prolonged 35 GHz millimeter-wave exposure. Radiat. Res. 2008, 169, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Foroughimehr, N. Millimeter Wave Absorption by the Cornea. Ph.D. Thesis, Swinburne University of Technology, Melbourne, VIC, Australia, 2023. [Google Scholar]
- Skala, M.C.; Riching, K.M.; Bird, D.K.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; Keely, P.J.; Ramanujam, N. In vivo multiphoton fluorescence lifetime imaging of protein-bound and free NADH in normal and pre-cancerous epithelia. J. Biomed. Opt. 2007, 12, 024014. [Google Scholar] [CrossRef]
- Dancik, Y.; Favre, A.; Loy, C.J.; Zvyagin, A.V.; Roberts, M.S. Use of multiphoton tomography and fluorescence lifetime imaging to investigate skin pigmentation in vivo. J. Biomed. Opt. 2013, 18, 026022. [Google Scholar]
- Roberts, M.S.; Dancik, Y.; Prow, T.W.; Thorling, C.A.; Lin, L.L.; Grice, J.E.; Robertson, T.A.; König, K.; Becker, W. Non-invasive imaging of skin physiology and percutaneous penetration using fluorescence spectral and lifetime imaging with multiphoton and confocal microscopy. Eur. J. Pharm. Biopharm. 2011, 77, 469–488. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Q.; Zhang, M.; Wu, Z.; Xue, P. Fluorescence lifetime imaging microscopy and its applications in skin cancer diagnosis. J. Innov. Opt. Health Sci. 2019, 12, 1930004. [Google Scholar] [CrossRef]
- Masters, B.R. Three-dimensional confocal microscopy of human skin in vivo: Autofluorescence of normal skin. Bioimages 1996, 4, 13–19. [Google Scholar]
- Koenig, K.; Riemann, I. High-resolution multiphoton tomography of human skin with subcellular spatial resolution and picosecond time resolution. J. Biomed. Opt. 2003, 8, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Kolenc, O.I.; Quinn, K.P. Evaluating cell metabolism through autofluorescence imaging of NAD (P) H and FAD. Antioxidants Redox Signal. 2019, 30, 875–889. [Google Scholar] [CrossRef]
- Laiho, L.H.; Pelet, S.; Hancewicz, T.M.; Kaplan, P.D.; So, P.T. Two-photon 3-D mapping of ex vivo human skin endogenous fluorescence species based on fluorescence emission spectra. J. Biomed. Opt. 2005, 10, 024016. [Google Scholar] [CrossRef]
- Eskandari, V.; Kordzadeh, A.; Zeinalizad, L.; Sahbafar, H.; Aghanouri, H.; Hadi, A.; Ghaderi, S. Detection of molecular vibrations of atrazine by accumulation of silver nanoparticles on flexible glass fiber as a surface-enhanced Raman plasmonic nanosensor. Opt. Mater. 2022, 128, 112310. [Google Scholar] [CrossRef]
- Zeng, H. Human Skin Optical Properties and Autofluorescence Decay Dynamics. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1993. [Google Scholar]
- Meerwaldt, R.; Links, T.; Graaff, R.; Thorpe, S.R.; Baynes, J.W.; Hartog, J.; Gans, R.; Smit, A. Simple noninvasive measurement of skin autofluorescence. Ann. N. Y. Acad. Sci. 2005, 1043, 290–298. [Google Scholar] [CrossRef] [PubMed]
- De Beule, P.; Dunsby, C.; Galletly, N.; Stamp, G.; Chu, A.; Anand, U.; Anand, P.; Benham, C.; Naylor, A.; French, P. A hyperspectral fluorescence lifetime probe for skin cancer diagnosis. Rev. Sci. Instrum. 2007, 78, 123101. [Google Scholar] [CrossRef]
- Karinen, A.; Heinävaara, S.; Nylund, R.; Leszczynski, D. Mobile phone radiation might alter protein expression in human skin. BMC Genom. 2008, 9, 77. [Google Scholar] [CrossRef]
- Sanchez, S.; Milochau, A.; Ruffie, G.; Poulletier de Gannes, F.; Lagroye, I.; Haro, E.; Surleve-Bazeille, J.E.; Billaudel, B.; Lassegues, M.; Veyret, B. Human skin cell stress response to GSM-900 mobile phone signals: In vitro study on isolated primary cells and reconstructed epidermis. FEBS J. 2006, 273, 5491–5507. [Google Scholar] [CrossRef]
- Straume, A.; Oftedal, G.; Johnsson, A. Skin temperature increase caused by a mobile phone: A methodological infrared camera study. Bioelectromagnetics 2005, 26, 510–519. [Google Scholar] [CrossRef]
- Keshvari, J.; Lang, S. Comparison of radio frequency energy absorption in ear and eye region of children and adults at 900, 1800 and 2450 MHz. Phys. Med. Biol. 2005, 50, 4355. [Google Scholar] [CrossRef]
- Kodera, S.; Gomez-Tames, J.; Hirata, A. Temperature elevation in the human brain and skin with thermoregulation during exposure to RF energy. Biomed. Eng. Online 2018, 17, 1. [Google Scholar] [CrossRef]
- Hirata, A.; Asano, T.; Fujiwara, O. FDTD analysis of human body-core temperature elevation due to RF far-field energy prescribed in the ICNIRP guidelines. Phys. Med. Biol. 2007, 52, 5013. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foroughimehr, N.; Clayton, A.H.A.; Yavari, A. Exploring Skin Interactions with 5G Millimeter-Wave through Fluorescence Lifetime Imaging Microscopy. Electronics 2024, 13, 1630. https://doi.org/10.3390/electronics13091630
Foroughimehr N, Clayton AHA, Yavari A. Exploring Skin Interactions with 5G Millimeter-Wave through Fluorescence Lifetime Imaging Microscopy. Electronics. 2024; 13(9):1630. https://doi.org/10.3390/electronics13091630
Chicago/Turabian StyleForoughimehr, Negin, Andrew H. A. Clayton, and Ali Yavari. 2024. "Exploring Skin Interactions with 5G Millimeter-Wave through Fluorescence Lifetime Imaging Microscopy" Electronics 13, no. 9: 1630. https://doi.org/10.3390/electronics13091630








