Generative Noise Reduction in Dental Cone-Beam CT by a Selective Anatomy Analytic Iteration Reconstruction Algorithm
Abstract
:1. Introduction
2. Methodology
2.1. Equipment Configuration of the Duplicated Simulation
- (1)
- Use a flat-panel imaging detector (FPID) to determine the CBCT modality.
- (2)
- Set the number of the received projections to sparse.
- (3)
- Scan the region of interest in dental anatomy structures for various diagnoses such as head-and-neck, oral, and dentition anatomy.
2.2. Selective Anatomy Analytic Iteration Reconstruction (SA2IR)
| Algorithm 1: “Densification enrichment process” or Pseudo-Set Interpolation |
| Input: Initial projection set captured by FPID, as follows: Output: Interpolated pseudo-set for the reconstruction completeness availability, as follows: Begin Initialize necessary parameters. For k = 1 to k = maximum number of iterations: 1: , , and: . Find the common projection angle coincidences of two sets: and 2: Assign a kernel to remove the non-attenuated outer-cored region of interest (ROI). Implement the iteration loop by times. Then, assign the targeted pseudo-set with a denser viewed set, implanted with more interpolated projections in each interval of , between and . 3: Correct iteratively the implanted interpolated projections’ sinograms quality by structural similarity and mean squared errors with the given number of iterations. Assign the corrected ones as the final interpolated pseudo-set . End |
| Algorithm 2: “Quality enhancement process” or Pseudo-Set Trio Subset Enhancement Operator |
| Input: Interpolated pseudo-set, as followed: Output: Regenerative pseudo-set for reconstruction, as follows: Begin 1: , and: 2: Apply the trio subset’s “estimating–filtering–verifying” processing loop per each projection in the interpolated pseudo-set iteratively.
End |
| Algorithm 3: SA2IT algorithm |
| Input: Regenerative pseudo-set, as follows: Output: The SA2IT results Begin Initialize necessary parameters. For k = 1 to k = maximum number of iterations: 1: Apply the conventional SIRT algorithm to compute forward projection , correction computation (), back-projection, and the reconstructed volume , by Equations (8) and (9). 2: Apply the gradient descent to the reconstructed 3: Assign the corrected ones, adapted . End |
3. Experimental Setup
3.1. Phantom Reconstruction Experiments
3.2. Comparison with the Other Reconstruction Algorithms
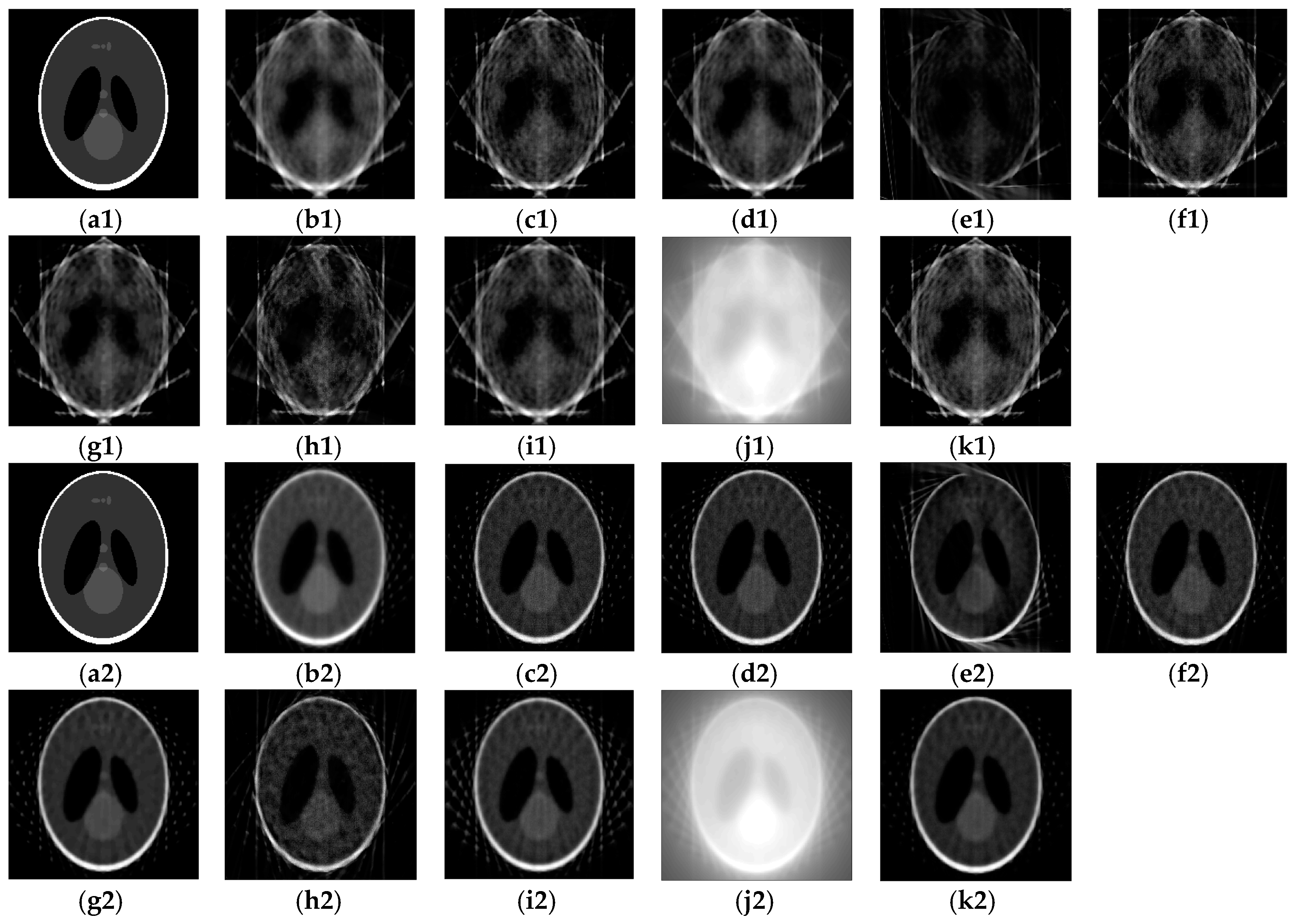
4. Results and Discussions
4.1. Results of the SA2IR Algorithm’s Reconstruction Simulation
4.2. Comparison to the Other Reconstruction Algorithms
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALADA | As Low As Diagnostically Acceptable |
| ALARA | As Low As Reasonably Achievable |
| (OS)-ASD-POCS | (Order-Subset) Adaptive Steepest Descent Projection On Convex Sets |
| CAD/CAM | Computer-Aided Design/ Computer -Aided Manufacturing |
| CBCT | Cone-Beam Computed Tomography |
| CGLS | Conjugated Gradient Least Square |
| CT | Computed Tomography |
| DICOM | Digital Imaging and Communication in Medicine |
| FISTA | Fast Iterative Shrinkage-Thresholding Algorithm |
| FPID | Flat Panel Imaging Detector |
| FDK | Feldkamp - Davis - Kress |
| GPU | Graphical Processing Unit |
| NCRP | National Council on Radiation Protection (United States) |
| RMSE | Root Mean Square Error |
| SA2IR | Selective Anatomy Analytic Iteration Reconstruction |
| SA2IT | Selective Anatomy Analytic Iteration Technique |
| (OS)-SART | (Order-Subset) Simultaneous Algebraic Reconstruction Technique |
| SIRT | Simultaneous Iterative Reconstruction Technique |
| SNR | Signal-To-Noise Ratio |
| PSNR | Peak Signal-To-Noise Ratio |
| SSIM | Structural Similarity |
| TSC | Total Similarity Coefficient |
References
- Fokas, G.; Vaughn, V.N.; Scarfe, W.C.; Bornstein, M.M. Acccuracy of linear measurement on CBCT images related to presurgical implant treatment: A systematic review. Clin. Oral Implant. Res 2018, 29, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Jorner, K.; Jacobs, R. Use of cone beam computed tomography in implant dentistry: Current concepts, indications, and limitations for clinical practice and research. Periodontology 2017, 73, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Singer, S.R.; Mupparapu, M. Review of cone beam computed tomography guidelines in North America. Quintessence Int. 2019, 50, 136–145. [Google Scholar]
- Hayashi, T.; Arai, Y.; Chikui, T.; Hayashi-Sakai, S.; Honda, K.; Indo, H.; Kawai, T.; Kobayashi, K.; Murakami, S.; Nagasawa, M.; et al. Committee on Clinical Practice Guidelines Japanese Society for, Oral Maxillofacial, Radiology. Clinical guidelines for dental cone-beam computed tomography. Oral Radiol. 2018, 34, 89–104. [Google Scholar] [CrossRef]
- Alghazzawi, T.F. Advancements in CAD/CAM technology: Options for practical implementation. J. Prosthodont. Res. 2016, 60, 72–84. [Google Scholar] [CrossRef]
- Dao-Ngoc, L. The review of RP (Rapid Prototyping application in maxillofacial surgeries in Vietnam from 2010 to 2016: In the manufacturing engineer’s view. Cập nhật nha khoa–Tài liệu tham khảo và đào tạo liên tục 2017, 22, 121–142. [Google Scholar]
- Qin, Z.; Zhang, X.; Li, Y.; Wang, P.; Li, J. One-stage treatment for maxillofacial asymmetry with orthognathic and contouring surgery using virtual surgical planning and 3D-printed surgical templates. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 97–106. [Google Scholar] [CrossRef]
- Bushberg, J.T. Eleventh annual Warren K. Sinclair keynote address-science, radiation protection and NCRP: Building on the past, looking to the future. Health Phys. 2015, 108, 115–123. [Google Scholar] [CrossRef]
- White, S.C.; Scarfe, W.C.; Schulze, R.K.W.; Lurie, A.G.; Douglass, J.M.; Farman, A.G.; Law, C.S.; Levin, M.D.; Sauer, R.A.; Valachovic, R.W.; et al. The Image Gently in Dentistry campaign: Promotion of responsible use of maxillofacial radiology in dentistry for children. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 257–261. [Google Scholar] [CrossRef]
- Fernandes, K.; Levin, T.L.; Miller, T.; Schoenfeld, A.H.; Amis, E.S., Jr. Evaluating an Image Gently and Image Wisely Campaign in a Multihospital Health Care System. J. Am. Coll. Radiol. 2016, 13, 1010–1017. [Google Scholar] [CrossRef]
- Jaju, P.P.; Jaju, S.P. Cone-beam computed tomography: Time to move from ALARA to ALADA. Imaging Sci. Dent. 2015, 45, 263–265. [Google Scholar] [CrossRef]
- Matenine, D.; Goussard, Y.; Despres, P. GPU-accelerated regularized iterative reconstruction for few-view cone beam CT. Med. Phys. 2015, 42, 1505–1517. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zeng, D.; Tao, X.; Ma, J. Regularization strategies in statistical image reconstruction of low-dose X-ray CT: A review. Med. Phys. 2018, 45, e886–e907. [Google Scholar] [CrossRef]
- Jacobs, R.; Vranckx, M.; Vanderstuyft, T.; Quirynen, M.; Salmon, B. CBCT vs. other imaging modalities to assess peri-implant bone and diagnose complications: A systematic review. Eur. J. Oral Implant. 2018, 11, 77–92. [Google Scholar]
- Katsumata, A.; Hirukawa, A.; Okumura, S.; Naitoh, M.; Fujishita, M.; Ariji, E.; Langlais, R.P. Effects of image artifacts on gray-value density in limited-volume cone-beam computerized tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, 829–836. [Google Scholar] [CrossRef]
- Stefanelli, L.V.; DeGroot, B.S.; Lipton, D.I.; Mandelaris, G.A. Accuracy of Dynamic Dental Implant Navigation System in a Private Practice. Int. J. Oral Maxillofac. Implant. 2019, 34, 205–2013. [Google Scholar] [CrossRef]
- Harris, B.T.; Montero, D.; Grant, G.T.; Morton, D.; Llop, D.R.; Lin, W.S. Creation of a 3D dimensional virtual dental patient for computer-guided surgery and CAD-CAM interim complete removable and fixed dental prostheses: A clinical report. J. Prosthet. Dent. 2017, 117, 197–204. [Google Scholar] [CrossRef]
- Patel, S.; Brown, J.; Pimentel, T.; Kelly, R.D.; Abella, F.; Durack, C. Cone beam computed tomography in Endodontics—A review of the literature. Int. Endod. J. 2019, 52, 1138–1152. [Google Scholar] [CrossRef]
- Tchorz, J.P. 3D Endo: Three-dimensional endodontic treatment planning. Int. J. Comput. Dent. 2017, 20, 87–92. [Google Scholar]
- Woelber, J.P.; Fleiner, J.; Rau, J.; Ratka-Kruger, P.; Hannig, C. Accuracy and Usefulness of CBCT in Periodontology: A Systematic Review of the Literature. Int. J. Periodontics Restor. Dent. 2018, 38, 289–297. [Google Scholar] [CrossRef]
- Pozzi, A.; Arcuri, L.; Moy, P.K. The smiling scan technique: Facially driven guided surgery and prosthetics. J. Prosthodont. Res. 2018, 62, 514–517. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, M.B.; Duncan, H.F.; Horner, K. An analysis of effective dose optimization and its impact on image quality and diagnostic efficacy relating to dental cone beam computed tomography (CBCT). Swiss Dent. J. 2018, 128, 297–316. [Google Scholar]
- Karimi, D.; Ward, R.K. Sinogram denoising via simultaneous sparse representation in learned dictionaries. Phys. Med. Biol. 2016, 61, 3536–3553. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Wahid, K.; Babyn, P.; Cooper, D.; Pratt, I.; Carter, Y. Improved Compressed Sensing Based Algorithm for Sparse—View CT Image Reconstruction. Comput. Math. Methods Med. 2013, 2013, 185750. [Google Scholar] [CrossRef]
- Zhang, H.M.; Li, L.; Wang, L.Y.; Sun, Y.M.; Yan, B.; Cai, A.L.; Hu, G.E. Computed Tomography Sinogram Inpainting With Compound Prior Modelling Both Sinogram and Image Sparsity. IEEE Trans. Nucl. Sci. 2016, 63, 2567–2576. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.; Zheng, J.; Li, M.; Lu, Y.; You, J.; Guan, Y. A Model of Regularization Parameter Determination in Low-Dose X-Ray CT Reconstruction Based on Dictionary Learning. Comput. Math. Methods Med. 2015, 2015, 831790. [Google Scholar] [CrossRef]
- Du, D.; Pan, Z.; Zhang, P.; Li, Y.; Ku, W. Compressive sensing image recovery using dictionary learning and shape-adaptive DCT thresholding. Magn. Reson. Imaging 2019, 55, 60–71. [Google Scholar] [CrossRef]
- Kim, G.; Park, S.; Je, U.; Cho, H.; Park, C.; Kim, K.; Lim, H.; Lee, D.; Lee, H.; Park, Y.; et al. A New Voxelization Strategy in Compressed-Sensing (CS)-Based Iterative CT Reconstruction for Reducing Computational Cost: Simulation and Experimental Studies. J. Med. Biol. Eng. 2018, 38, 129–137. [Google Scholar] [CrossRef]
- Byrne, C. A unified treatment of some iterative algorithms in signal processing and image reconstruction. Inverse Probl. 2003, 20, 103–120. [Google Scholar] [CrossRef]
- Shepp, L.A.; Logan, B.F. The Fourier reconstruction of a head section. IEEE Trans. Nucl. Sci. 1974, 21, 21–43. [Google Scholar] [CrossRef]
- Aichert, A.; Manhart, M.T.; Navalpakkam, B.K.; Grimm, R.; Hutter, J.; Maier, A.; Hornegger, J.; Doerfler, A. A realistic digital phantom for perfusion C-arm CT based on MRI data. In Proceedings of the 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC), Seoul, Korea, 27 October–2 November 2013. [Google Scholar]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical Cone-Beam Algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Kak, A.C.; Slaney, M. Principles of Computerized Tomographic Imaging, Classics in Applied Mathematics; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2001. [Google Scholar] [CrossRef]
- Andersen, A.H.; Kak, A.C. Simultaneous Algebraic Reconstruction Technique (SART)—A Superior Implementation of the ART Algorithm. Ultrason. Imaging 1984, 6, 81–94. [Google Scholar] [CrossRef]
- Censor, Y.; Elfving, T. Block-iterative algorithms with diagonally scaled oblique projections for the linear feasibility problem. SIAM J. Matrix Anal. Appl. 2002, 24, 40–58. [Google Scholar] [CrossRef]
- Sidky, E.Y.; Pan, X. Image reconstruction in circular cone-beam computed tomography by constrained, total-variation minimization. Phys. Med. Biol. 2008, 53, 47–77. [Google Scholar] [CrossRef]
- Björck, Å. Numerical Methods for Least Squares Problems; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1996. [Google Scholar] [CrossRef]
- Biguri, A.; Dosanjh, M.; Hancock, S.; Soleimani, M. TIGRE: A MATLAB-GPU toolbox for CBCT image reconstruction. Biomed. Phys. Eng. Express 2016, 2, 055010. [Google Scholar] [CrossRef]
- Perona, P.; Malik, J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans. Pattern Anal. Mach. Intell. 1990, 16, 629–639. [Google Scholar] [CrossRef] [Green Version]




| Technical Parameters | Information | - | ||
|---|---|---|---|---|
| Notation | Unit | Value | ||
| Source-to-Detector Distance | SDD | cm | 72 | Term of “Patient” position means “Rotation Axis” position |
| Source-to-Patient Distance | SPD | cm | 50 | |
| Patient-to-Detector Distance | PDD | cm | 22 | |
| X-ray beam exposure size | WE × HE | cm × cm | 12.8 × 12.8 | Width × Height |
| Cone-beam opening of FPID | βx βz | (o) | 9.15° 9.15° | Respected to xx and yy axis |
| Detector’s size (Width × Length) | W × L | cm × cm | 13 × 13 | Amorphous Silicon Receptor |
| Voxel size | Vx | mm | 0.125 and 0.200 | - |
| Rotation degree | Α | (o) | 180° or 360° | Projection arch per scan |
| Field-of-View (Diameter × Height) | FOV (D × H) | cm × cm | 9 × 9 and 15 × 9 | - |
| Projection rotation angular step | αpst | (o/step) | 0.6 | - |
| Number of projections | Np | projections | 300 or 600 | np = α/pp |
| Simulated Results Based on the Modified Shepp–Logan Phantom | ||||||
|---|---|---|---|---|---|---|
| Processes | Estimation Process | Verification Process | ||||
| Studied Segments | - | |||||
| Ω1 | A 1,6,12 | 0.1634 | 0.8366 | 2.4442 | 2.4442 ± 2.0901 × 10−14 | 0.3215 |
| 0.0050 | 0.9950 | 2.4575 | 2.4575 ± 1.3341 × 10−14 | |||
| 0.0063 | 0.9937 | 2.4577 | 2.4577 ± 1.4675 × 10−14 | |||
| B 1,9,18 | 0.1283 | 0.8717 | 2.4576 | 2.4576 ± 2.2235 × 10−14 | 0.2139 | |
| 0.0052 | 0.9948 | 2.4575 | 2.4575 ± 4.4470 × 10−16 | |||
| 0.0046 | 0.9954 | 2.4575 | 2.4575 ± 2.1790 × 10−14 | |||
| C 1,11,21 | 0.1008 | 0.8992 | 2.4575 | 2.4576 ± 1.2451 × 10−14 | 0.1833 | |
| 0.0028 | 0.9972 | 2.4575 | 2.4575 ± 8.4492 × 10−15 | |||
| 0.0023 | 0.9977 | 2.4574 | 2.4575 ± 6.6704 × 10−15 | |||
| Ω2 | A 12,18,24 | 0.0063 | 0.9937 | 2.4577 | 2.4577 ± 1.4675 × 10−14 | 0.3215 |
| 0.0041 | 0.9959 | 2.4577 | 2.4577 ± 2.3569 × 10−14 | |||
| 0.0019 | 0.9981 | 2.4577 | 2.4577 ± 2.0011 × 10−14 | |||
| B 18,27,36 | 0.0046 | 0.9954 | 2.4575 | 2.4575 ± 2.1790 × 10−14 | 0.2139 | |
| 0.9324 | 0.9991 | 2.4575 | 2.4575 ± 7.5598 × 10−15 | |||
| 0.1033 | 0.9999 | 2.4575 | 2.4575 ± 8.4492 × 10−15 | |||
| C 21,32,42 | 0.0023 | 0.9977 | 2.4575 | 2.4575 ± 6.6704 × 10−15 | 0.1833 | |
| 0.0041 | 0.9959 | 2.4575 | 2.4575 ± 1.4230 × 10−14 | |||
| 0.3650 | 1.0000 | 2.4575 | 2.4575 ± 1.5120 × 10−14 | |||
| Ω3 | A 24,30,36 | 0.0019 | 0.9981 | 2.4577 | 2.4577 ± 2.0011 × 10−14 | 0.3215 |
| 0.3053 | 0.9997 | 2.4576 | 2.4576 ± 2.2401 × 10−14 | |||
| 0.9441 | 0.9999 | 2.4576 | 2.4576 ± 1.9122 × 10−14 | |||
| B 36,45,54 | 0.1033 | 0.9999 | 2.4575 | 2.4575 ± 8.4492 × 10−15 | 0.2139 | |
| 0.0016 | 0.9984 | 2.4575 | 2.4575 ± 5.7810 × 10−15 | |||
| 0.0056 | 0.9944 | 2.4575 | 2.4575 ± 1.6009 × 10−14 | |||
| C 42,53,63 | 0.3650 | 1.0000 | 2.4575 | 2.4575 ± 1.5120 × 10−14 | 0.1833 | |
| 0.1337 | 0.9999 | 2.4575 | 2.4575 ± 1.2451 × 10−14 | |||
| 0.0125 | 0.9875 | 2.4575 | 2.4575 ± 2.0901 × 10−14 | |||
| Ω4 | A 36,42,48 | 0.9441 | 0.9999 | 2.4576 | 2.4576 ± 1.9122 × 10−14 | 0.3215 |
| 0.0015 | 0.9985 | 2.4576 | 2.4576 ± 1.2451 × 10−14 | |||
| 0.0036 | 0.9964 | 2.4576 | 2.4576 ± 2.3124 × 10−14 | |||
| B 54,63,72 | 0.0056 | 0.9944 | 2.4575 | 2.4575 ± 1.6009 × 10−14 | 0.2139 | |
| 0.0102 | 0.9898 | 2.4575 | 2.4575 ± 1.9122 × 10−14 | |||
| 0.0060 | 0.9940 | 2.4575 | 2.4575 ± 1.0228 × 10−14 | |||
| C 63,74,84 | 0.0125 | 0.9875 | 2.4575 | 2.4575 ± 2.0901 × 10−14 | 0.1833 | |
| 0.6270 | 0.3730 | 2.4575 | 2.4575 ± 1.1562 × 10−14 | |||
| 0.0022 | 0.9978 | 2.4575 | 2.4575 ± 1.7343 × 10−14 | |||
| Ω5 | A 48,54,60 | 0.0036 | 0.9964 | 2.4576 | 2.4576 ± 2.3124 × 10−14 | 0.3215 |
| 0.0055 | 0.9945 | 2.4576 | 2.4576 ± 9.3386 × 10−15 | |||
| 0.0050 | 0.9950 | 2.4576 | 2.4576 ± 1.3786 × 10−14 | |||
| B 72,81,90 | 0.0060 | 0.9940 | 2.4575 | 2.4575 ± 1.0228 × 10−14 | 0.2139 | |
| 0.0045 | 0.9955 | 2.4575 | 2.4575 ± 1.3341 × 10−14 | |||
| 0.0013 | 0.9987 | 2.4575 | 2.4575 ± 1.5120 × 10−14 | |||
| C 84,95,105 | 0.0022 | 0.9978 | 2.4575 | 2.4575 ± 1.7343 × 10−14 | 0.1833 | |
| 0.0055 | 0.9945 | 2.4575 | 2.4575 ± 5.3363 × 10−15 | |||
| 0.1044 | 0.9999 | 2.4575 | 2.4575 ± 1.0228 × 10−14 | |||
| Ω6 | A 60,66,72 | 0.0050 | 0.9950 | 2.4576 | 2.4576 ± 1.3786 × 10−14 | 0.3215 |
| 0.0086 | 0.9914 | 2.4576 | 2.4576 ± 1.1562 × 10−14 | |||
| 0.0054 | 0.9946 | 2.4576 | 2.4576 ± 1.2007 × 10−14 | |||
| B 90,99,108 | 0.0013 | 0.9987 | 2.4575 | 2.4575 ± 1.5120 × 10−14 | 0.2139 | |
| 0.8497 | 0.9999 | 2.4575 | 2.4575 ± 1.8677 × 10−14 | |||
| 0.0022 | 0.9978 | 2.4575 | 2.4575 ± 9.3386 × 10−15 | |||
| C 105,116,126 | 0.1044 | 0.9999 | 2.4575 | 2.4575 ± 1.0228 × 10−14 | 0.1833 | |
| 0.3272 | 0.9997 | 2.4575 | 2.4575 ± 1.2007 × 10−14 | |||
| 0.0108 | 0.9892 | 2.4575 | 2.4575 ± 1.8677 × 10−14 | |||
| Ω7 | A 72,78,84 | 0.0054 | 0.9946 | 2.4576 | 2.4576 ± 1.2007 × 10−14 | 0.3215 |
| 0.0027 | 0.9973 | 2.4576 | 2.4576 ± 2.9456 × 10−14 | |||
| 0.0019 | 0.9981 | 2.4576 | 2.4576 ± 8.8939 × 10−15 | |||
| B 108,117,126 | 0.0022 | 0.9978 | 2.4575 | 2.4575 ± 9.3386 × 10−15 | 0.2139 | |
| 0.0056 | 0.9944 | 2.4575 | 2.4575 ± 6.2257 × 10−15 | |||
| 0.0077 | 0.9923 | 2.4575 | 2.4575 ± 6.2257 × 10−15 | |||
| C 126,137,147 | 0.0108 | 0.9892 | 2.4575 | 2.4575 ± 1.8677 × 10−14 | 0.1833 | |
| 0.0031 | 0.9969 | 2.4575 | 2.4575 ± 6.2257 × 10−15 | |||
| 0.0057 | 0.9943 | 2.4575 | 2.4575 ± 3.5576 × 10−15 | |||
| Ω8 | A 84,90,96 | 0.0019 | 0.9981 | 2.4576 | 2.4576 ± 8.8939 × 10−15 | 0.3215 |
| 0.0012 | 0.9988 | 2.4576 | 2.4576 ± 1.4657 × 10−14 | |||
| - | - | 2.4532 | 2.4532 ± 6.2257 × 10−15 | |||
| B 126, 135,144 | 0.0077 | 0.9923 | 2.4575 | 2.4575 ± 6.2257 × 10−15 | 0.2139 | |
| 0.0060 | 0.9940 | 2.4575 | 2.4575 ± 4.8917 × 10−15 | |||
| - | - | 2.4576 | 2.4575 ± 2.0011 × 10−14 | |||
| C 147,158,168 | 0.0057 | 0.9943 | 2.4575 | 2.4575 ± 3.5576 × 10−15 | 0.1833 | |
| 0.0054 | 0.9946 | 2.4575 | 2.4575 ± 1.6898 × 10−14 | |||
| - | - | 2.4575 | 2.4575 ± 1.0228 × 10−15 | |||
| Segment Ωi | Case A | Case B | Case C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First | Middle | Last | First | Middle | Last | First | Middle | Last | |
| Ω1 | 1 | 6 | 12 | 1 | 9 | 18 | 1 | 11 | 21 |
| Ω2 | 12 | 18 | 24 | 18 | 27 | 36 | 21 | 32 | 42 |
| Ω3 | 24 | 30 | 36 | 36 | 45 | 54 | 42 | 53 | 63 |
| Ω4 | 36 | 42 | 48 | 54 | 63 | 72 | 63 | 74 | 84 |
| Ω5 | 48 | 54 | 60 | 72 | 81 | 90 | 84 | 95 | 105 |
| Ω6 | 60 | 66 | 72 | 90 | 99 | 108 | 105 | 116 | 126 |
| Ω7 | 72 | 78 | 84 | 108 | 117 | 126 | 126 | 137 | 147 |
| Ω8 | 84 | 90 | 96 | 126 | 135 | 144 | 147 | 158 | 168 |
| Algorithms | RMSE | SSIM | PSNR | SNR | Entropy |
|---|---|---|---|---|---|
| SIRT | 0.0082 * | 0.6401 * | 20.8486 * | −1.7900 | 5.1575 |
| SART | 0.0057 * | 0.6517 * | 22.4720 * | −0.9387 | 5.3968 * |
| OS-SART | 0.0055 | 0.7024 | 22.6087 * | −1.0994 | 5.1386 * |
| TV-SART | 0.0156 | 0.5216 | 18.0633 | −1.8817 | 5.8063 |
| ASD-POCS | 0.0063 * | 0.5802 | 21.9976 * | −0.9116 | 5.3289 * |
| OS-ASD-POCS | 0.0053 | 0.7150 | 22.7878 * | −0.91174 | 4.7335 |
| β-ASD-POCS | 0.0081 | 0.4037 | 20.8905 | −0.5470 | 5.8491 |
| CGLS | 0.0058 | 0.6488 | 22.3796 | −1.3787 | 5.6202 |
| FISTA | 0.0509 | 0.1795 | 12.9291 | −25.0662 | 1.7108 |
| SA2IR | 0.0079 * | 0.6452 * | 21.0202 * | −1.9962 | 5.0969 * |
| Algorithms | RMSE | SSIM | PSNR | SNR | Entropy | Computation Time (secs.) |
|---|---|---|---|---|---|---|
| Upper Jaw Arch Region–Head | ||||||
| SIRT | 0.0013 * | 0.6139 | 29.0292 | −0.2467 | 4.1147 | 3.00 |
| SART | 0.0013 * | 0.5423 | 28.9282 | −0.0218 | 4.2795 * | 30.20 * |
| OS-SART | 0.0009 * | 0.6005 * | 30.5914 * | −0.0514 | 4.1238 | 5.40 |
| ASD-POCS | 0.0011 * | 0.5625 * | 29.6087 * | −0.0906 | 4.1928 * | 32.00 * |
| OS-ASD-POCS | 0.0006 | 0.6747 | 32.4155 | −0.0827 | 3.712 | 5.30 * |
| CGLS | 0.0022 * | 0.4164 | 26.5544 | −0.0671 | 5.6606 | 1.80 |
| SA2IR | 0.0009 * | 0.5988 * | 30.2784 * | −0.1543 | 4.2824 * | 4.80 * |
| Lower Jaw Arch Region–Head | ||||||
| SIRT | 0.0012 * | 0.6003 | 29.254 | −0.2533 | 3.8695 | 2.80 |
| SART | 0.0012 * | 0.5412 | 29.22 | −0.0383 | 4.0492 * | 30.20 * |
| OS-SART | 0.0008 * | 0.5976 * | 30.7944 * | −0.0614 | 3.9003 * | 5.30 * |
| ASD-POCS | 0.0010 * | 0.5557 | 29.8809 * | −0.1041 | 4.072 * | 35.90 * |
| OS-ASD-POCS | 0.0006 | 0.6596 | 32.2566 | −0.0931 | 3.5123 | 5.30 * |
| CGLS | 0.0020 * | 0.4042 | 26.8865 | −0.0846 | 5.4896 | 1.90 |
| SA2IR | 0.0009 * | 0.5865 * | 30.3070 * | −0.1638 | 4.0506 * | 5.00 * |
| Mid-Facial Region–Head | ||||||
| SIRT | 0.0011 * | 0.6161 | 29.7068 | −0.2359 | 3.885 | 2.80 |
| SART | 0.0011 * | 0.5509 | 29.4622 * | −0.0267 | 4.0768 * | 29.40 * |
| OS-SART | 0.0008 * | 0.6062 * | 31.108 * | −0.0526 | 3.9013 * | 5.40 * |
| ASD-POCS | 0.0010 * | 0.5662 | 30.1793 * | −0.0919 | 3.9817 * | 31.60 * |
| OS-ASD-POCS | 0.0005 | 0.6764 | 32.8669 | −0.0823 | 3.5258 | 5.20 * |
| CGLS | 0.0019 * | 0.4185 | 27.162 | −0.0628 | 5.5153 | 1.80 |
| SA2IR | 0.0008 * | 0.6037 * | 30.8624 * | −0.1472 | 4.0744 * | 4.80 * |
| Temporomandibular joint Region–Head | ||||||
| SIRT | 0.0040 * | 0.5173 | 23.9879 | −0.5294 | 4.7749 | 3.00 |
| SART | 0.0027 | 0.5377 * | 25.6691 * | −0.0972 | 4.9691 * | 35.10 * |
| OS-SART | 0.0021 | 0.5889 | 26.8414 | −0.1208 | 4.7041 | 5.80 * |
| ASD-POCS | 0.0026 * | 0.5494 * | 25.8782 * | −0.1777 | 4.9102 * | 37.10 * |
| OS-ASD-POCS | 0.0018 | 0.6166 | 27.4522 | −0.1851 | 4.6107 | 5.60 * |
| CGLS | 0.0053 * | 0.3314 | 22.7355 | −0.2227 | 6.1786 | 1.90 |
| SA2IR | 0.0030 * | 0.5151 * | 25.1978 * | −0.3472 | 5.0687 * | 5.30 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao-Ngoc, L.; Du, Y.-C. Generative Noise Reduction in Dental Cone-Beam CT by a Selective Anatomy Analytic Iteration Reconstruction Algorithm. Electronics 2019, 8, 1381. https://doi.org/10.3390/electronics8121381
Dao-Ngoc L, Du Y-C. Generative Noise Reduction in Dental Cone-Beam CT by a Selective Anatomy Analytic Iteration Reconstruction Algorithm. Electronics. 2019; 8(12):1381. https://doi.org/10.3390/electronics8121381
Chicago/Turabian StyleDao-Ngoc, Lam, and Yi-Chun Du. 2019. "Generative Noise Reduction in Dental Cone-Beam CT by a Selective Anatomy Analytic Iteration Reconstruction Algorithm" Electronics 8, no. 12: 1381. https://doi.org/10.3390/electronics8121381





