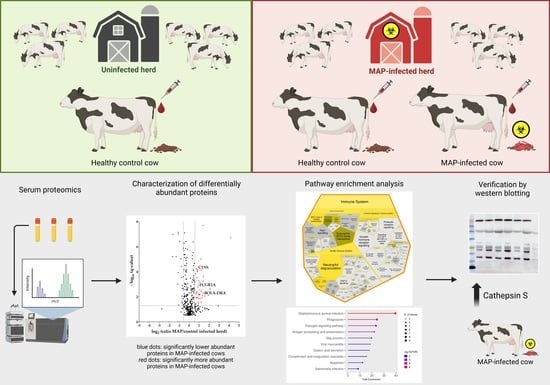
Cathepsin S Is More Abundant in Serum of Mycobacterium avium subsp. paratuberculosis-Infected Dairy Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Detection of MAP Infection Status
2.2. Sample Digestion for Differential Proteome Analysis
2.3. Mass Spectrometric Analysis and Label-Free Quantification
2.4. Data Analysis
2.5. Western Blots
3. Results
3.1. Serum Proteomics Reveals Differentially Abundant Proteins in Serum of MAP-Infected Dairy Cows When Compared to Two Healthy Control Groups from Farms with Divergent MAP Infection Status
3.2. Proteins with Significantly Higher Abundance in MAP-Infected Cows Associate with Immune System Pathways
3.3. CTSS Involved in Pathways with Strong Association to the Immune System
3.4. Detection of Increased Abundance of CTSS in Serum of MAP-Infected Dairy Cows Using LC-MS/MS and Western Blotting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richards Vincent, P.; Nigsch, A.; Pavinski Bitar, P.; Sun, Q.; Stuber, T.; Ceres, K.; Smith Rebecca, L.; Robbe Austerman, S.; Schukken, Y.; Grohn Yrjo, T.; et al. Evolutionary Genomic and Bacterial Genome-Wide Association Study of Mycobacterium avium subsp. paratuberculosis and Dairy Cattle Johne’s Disease Phenotypes. Appl. Environ. Microbiol. 2021, 87, e02570-20. [Google Scholar] [CrossRef] [PubMed]
- Rees, W.D.; Lorenzo-Leal, A.C.; Steiner, T.S.; Bach, H. Mycobacterium avium subspecies paratuberculosis Infects and Replicates within Human Monocyte-Derived Dendritic Cells. Microorganisms 2020, 8, 994. [Google Scholar] [CrossRef] [PubMed]
- Buergelt, C.D.; Hall, C.; McEntee, K.; Duncan, J.R. Pathological Evaluation of Paratuberculosis in Naturally Infected Cattle. Vet. Pathol. 1978, 15, 196–207. [Google Scholar] [CrossRef]
- Rasmussen, P.; Barkema, H.W.; Mason, S.; Beaulieu, E.; Hall, D.C. Economic losses due to Johne’s disease (paratuberculosis) in dairy cattle. J. Dairy Sci. 2021, 104, 3123–3143. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.; Mamo, G.; Zewude, A.; Sirak, A.; Gumi, B.; Ameni, G. Prevalence of paratuberculosis in cattle based on gross and microscopic lesions in Ethiopia. BMC Vet. Res. 2023, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.W. Transmission of Paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 305–312. [Google Scholar] [CrossRef]
- Windsor, P.A.; Whittington, R.J. Evidence for age susceptibility of cattle to Johne’s disease. Vet. J. 2010, 184, 37–44. [Google Scholar] [CrossRef]
- Nielsen, S.S. Transitions in diagnostic tests used for detection of Mycobacterium avium subsp. paratuberculosis infections in cattle. Vet. Microbiol. 2008, 132, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Whittington Richard, J.; Marshall, D.J.; Nicholls Paul, J.; Marsh Ian, B.; Reddacliff Leslie, A. Survival and Dormancy of Mycobacterium avium subsp. paratuberculosis in the Environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef]
- Field, N.L.; Mee, J.F.; McAloon, C.G. Characteristics (sensitivity and specificity) of herd-level diagnostic tests for Mycobacterium avium subspecies paratuberculosis in cattle—A systematic review. Vet. J. 2022, 279, 105786. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Toft, N. Ante mortem diagnosis of paratuberculosis: A review of accuracies of ELISA, interferon-γ assay and faecal culture techniques. Vet. Microbiol. 2008, 129, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Magombedze, G.; Shiri, T.; Eda, S.; Stabel, J.R. Inferring biomarkers for Mycobacterium avium subsp. paratuberculosis infection and disease progression in cattle using experimental data. Sci. Rep. 2017, 7, 44765. [Google Scholar] [CrossRef]
- Schwalm, A.K.; Obiegala, A.; Pfeffer, M.; Sting, R. Enhanced sensitivity and fast turnaround time in laboratory diagnosis for bovine paratuberculosis in faecal samples. J. Microbiol. Methods 2018, 152, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, Z.E.; Swift, B.M.C.; Botsaris, G.; Davidson, R.S.; Hutchings, M.R.; Huxley, J.N.; Rees, C.E.D. Survival of Mycobacterium avium subspecies paratuberculosis in retail pasteurised milk. Food Microbiol. 2018, 74, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Shankar, H.; Singh, S.V.; Singh, P.K.; Singh, A.V.; Sohal, J.S.; Greenstein, R.J. Presence, characterization, and genotype profiles of Mycobacterium avium subspecies paratuberculosis from unpasteurized individual and pooled milk, commercial pasteurized milk, and milk products in India by culture, PCR, and PCR-REA methods. Int. J. Infect. Dis. 2010, 14, e121–e126. [Google Scholar] [CrossRef]
- Botsaris, G.; Swift, B.M.C.; Slana, I.; Liapi, M.; Christodoulou, M.; Hatzitofi, M.; Christodoulou, V.; Rees, C.E.D. Detection of viable Mycobacterium avium subspecies paratuberculosis in powdered infant formula by phage-PCR and confirmed by culture. Int. J. Food Microbiol. 2016, 216, 91–94. [Google Scholar] [CrossRef]
- Okura, H.; Toft, N.; Pozzato, N.; Tondo, A.; Nielsen, S.S. Apparent Prevalence of Beef Carcasses Contaminated with Mycobacterium avium subsp. paratuberculosis Sampled from Danish Slaughter Cattle. Vet. Med. Int. 2011, 2011, 152687. [Google Scholar] [CrossRef] [PubMed]
- Noli, M.; Meloni, G.; Manca, P.; Cossu, D.; Palermo, M.; Sechi, L.A. HERV-W and Mycobacterium avium subspecies paratuberculosis Are at Play in Pediatric Patients at Onset of Type 1 Diabetes. Pathogens 2021, 10, 1135. [Google Scholar] [CrossRef]
- Moghadam, M.; Ghaemi, E.A.; Akbari, H.; Razavi Nikoo, H.; Zamani, S. Mycobacterium avium subsp. paratuberculosis and Hashimoto’s thyroiditis: Is MAP the trigger? Front. Cell. Infect. Microbiol. 2022, 12, 972929. [Google Scholar]
- Estevinho, M.M.; Cabeda, J.; Santiago, M.; Machado, E.; Silva, R.; Duro, M.; Pita, I.; Morais, R.; Macedo, G.; Bull, T.J.; et al. Viable Mycobacterium avium subsp. paratuberculosis Colonizes Peripheral Blood of Inflammatory Bowel Disease Patients. Microorganisms 2023, 11, 1520. [Google Scholar]
- Espeschit, I.F.; Bastos, D.S.S.; Fonseca Junior, A.; Cardoso, S.A.; Ferrari, M.L.A.; Moreira, M.A.S. Mycobacterium avium subsp. paratuberculosis and Crohn’s disease: Characterization of the interaction with different aspects of the disease. Braz. J. Microbiol. 2023, 54, 1239–1249. [Google Scholar] [CrossRef]
- Dow, C.T.; Alvarez, B.L. Mycobacterium paratuberculosis zoonosis is a One Health emergency. EcoHealth 2022, 19, 164–174. [Google Scholar] [CrossRef]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef]
- Matthews, C.; Cotter, P.A.-O.; O’Mahony, J. MAP, Johne’s disease and the microbiome; current knowledge and future considerations. Anim. Microbiome 2021, 3, 34. [Google Scholar] [CrossRef]
- Phanse, Y.; Wu, C.-W.; Venturino, A.J.; Hansen, C.; Nelson, K.; Broderick, S.R.; Steinberg, H.; Talaat, A.M. A Protective Vaccine against Johne’s Disease in Cattle. Microorganisms 2020, 8, 1427. [Google Scholar] [CrossRef] [PubMed]
- Köhler, H.; Gyra, H.; Zimmer, K.; Dräger, K.G.; Burkert, B.; Lemser, B.; Hausleithner, D.; Cußler, K.; Klawonn, W.; Heß, R.G. Immune Reactions in Cattle after Immunization with a Mycobacterium paratuberculosis Vaccine and Implications for the Diagnosis of M. paratuberculosis and M. bovis Infections. J. Vet. Med. Ser. B 2001, 48, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.M.; Vazquez, P.; Molina, E.; Plazaola, J.M.; Sevilla, I.A.; Geijo, M.V.; Alonso-Hearn, M.; Juste, R.A. Paratuberculosis Vaccination Causes Only Limited Cross-Reactivity in the Skin Test for Diagnosis of Bovine Tuberculosis. PLoS ONE 2013, 8, e80985. [Google Scholar] [CrossRef] [PubMed]
- Crociati, M.; Grispoldi, L.; Chalias, A.; Monaci, M.; Cenci-Goga, B.; Sylla, L. Effect of Culling Management Practices on the Seroprevalence of Johne’s Disease in Holstein Dairy Cattle in Central Italy. Vet. Sci. 2022, 9, 162. [Google Scholar] [CrossRef]
- Arango-Sabogal, J.C.; Paré, J.; Labrecque, O.; Côté, G.; Roy, J.P.; Buczinski, S.; Wellemans, V.; Fecteau, G. Incidence of fecal excretion of Mycobacterium avium subsp. paratuberculosis in dairy cows before and after the enrolment in the Québec voluntary program. Prev. Vet. Med. 2017, 148, 94–105. [Google Scholar] [CrossRef] [PubMed]
- McAloon, C.G.; Roche, S.; Ritter, C.; Barkema, H.W.; Whyte, P.; More, S.J.; O’Grady, L.; Green, M.J.; Doherty, M.L. A review of paratuberculosis in dairy herds—Part 2: On-farm control. Vet. J. 2019, 246, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.; Di Paolo, A.; Petrucci, L.; Torricelli, M.; Corneli, S.; Sebastiani, C.; Ciullo, M.; Sebastianelli, M.; Costarelli, S.; Scoccia, E.; et al. Evaluation of Single Nucleotide Polymorphisms (SNPs) Associated with Genetic Resistance to Bovine Paratuberculosis in Marchigiana Beef Cattle, an Italian Native Breed. Animals 2023, 13, 587. [Google Scholar] [CrossRef]
- Gopi, B.; Vir Singh, R.; Kumar, S.; Kumar, S.; Chauhan, A.; Sonwane, A.; Kumar, A.; Bharati, J.; Vir Singh, S. Effect of selected single nucleotide polymorphisms in SLC11A1, ANKRA2, IFNG and PGLYRP1 genes on host susceptibility to Mycobacterium avium subspecies paratuberculosis infection in Indian cattle. Vet. Res. Commun. 2022, 46, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Lutterberg, K.; Kleinwort, K.J.H.; Hobmaier, B.F.; Hauck, S.M.; Nüske, S.; Scholz, A.M.; Deeg, C.A. A Functionally Different Immune Phenotype in Cattle Is Associated with Higher Mastitis Incidence. Front. Immunol. 2018, 9, 2884. [Google Scholar] [CrossRef] [PubMed]
- Kleinwort, K.J.H.; Hauck, S.M.; Degroote, R.L.; Scholz, A.M.; Hölzel, C.; Maertlbauer, E.P.; Deeg, C. Peripheral blood bovine lymphocytes and MAP show distinctly different proteome changes and immune pathways in host-pathogen interaction. PeerJ 2019, 7, e8130. [Google Scholar] [CrossRef]
- Rodríguez-Gil, A.; Ritter, O.; Saul, V.V.; Wilhelm, J.; Yang, C.-Y.; Grosschedl, R.; Imai, Y.; Kuba, K.; Kracht, M.; Schmitz, M.L. The CCR4-NOT complex contributes to repression of Major Histocompatibility Complex class II transcription. Sci. Rep. 2017, 7, 3547. [Google Scholar] [CrossRef] [PubMed]
- Korbonits, L.; Kleinwort, K.J.H.; Amann, B.; Didier, A.; Märtlbauer, E.; Hauck, S.M.; Deeg, C.A. Mycobacterium avium subsp. paratuberculosis Infected Cows Reveal Divergent Immune Response in Bovine Peripheral Blood Derived Lymphocyte Proteome. Metabolites 2022, 12, 924. [Google Scholar]
- Dayon, L.; Cominetti, O.; Affolter, M. Proteomics of human biological fluids for biomarker discoveries: Technical advances and recent applications. Expert Rev. Proteom. 2022, 19, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Reddy, P.J.; Jain, R.; Gollapalli, K.; Moiyadi, A.; Srivastava, S. Proteomic technologies for the identification of disease biomarkers in serum: Advances and challenges ahead. Proteomics 2011, 11, 2139–2161. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, X.; Li, M.; Jia, H.; Lin, W.; Fang, L.; Jiang, Y.; Zhu, H.; Zhang, Z.; Ding, J.; et al. Interleukin 8 and Pentaxin (C-Reactive Protein) as Potential New Biomarkers of Bovine Tuberculosis. J. Clin. Microbiol. 2019, 57, e00274-19. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, M.-E.; Whitlock, R.H. Treatment and Chemoprophylaxis for Paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 547–557. [Google Scholar] [CrossRef]
- Machackova, M.; Svastova, P.; Lamka, J.; Parmova, I.; Liska, V.; Smolik, J.; Fischer, O.A.; Pavlik, I. Paratuberculosis in farmed and free-living wild ruminants in the Czech Republic (1999–2001). Vet. Microbiol. 2004, 101, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Rössler, T.; Berezhnoy, G.; Singh, Y.; Cannet, C.; Reinsperger, T.; Schäfer, H.; Spraul, M.; Kneilling, M.; Merle, U.; Trautwein, C. Quantitative Serum NMR Spectroscopy Stratifies COVID-19 Patients and Sheds Light on Interfaces of Host Metabolism and the Immune Response with Cytokines and Clinical Parameters. Metabolites 2022, 12, 1277. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, J.; Steadham, E.; Haynes, J.; Bailey, T.; Cheville, N. Phagosomal maturation and intracellular survival of Mycobacterium avium subspecies paratuberculosis in J774 cells. Comp. Immunol. Microbiol. Infect. Dis. 2003, 26, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Clemens, D.L.; Horwitz, M.A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 1995, 181, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Belhaouane, I.; Pochet, A.; Chatagnon, J.; Hoffmann, E.; Queval, C.J.; Deboosère, N.; Boidin-Wichlacz, C.; Majlessi, L.; Sencio, V.; Heumel, S.; et al. Tirap controls Mycobacterium tuberculosis phagosomal acidification. PLoS Pathog. 2023, 19, e1011192. [Google Scholar] [CrossRef] [PubMed]
- Gossner, A.; Watkins, C.; Chianini, F.; Hopkins, J. Pathways and Genes Associated with Immune Dysfunction in Sheep Paratuberculosis. Sci. Rep. 2017, 7, 46695. [Google Scholar] [CrossRef] [PubMed]
- González-Ruiz, S.; Strillacci, M.G.; Durán-Aguilar, M.; Cantó-Alarcón, G.J.; Herrera-Rodríguez, S.E.; Bagnato, A.; Guzmán, L.F.; Milián-Suazo, F.; Román-Ponce, S.I. Genome-Wide Association Study in Mexican Holstein Cattle Reveals Novel Quantitative Trait Loci Regions and Confirms Mapped Loci for Resistance to Bovine Tuberculosis. Animals 2019, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Jenum, S.; Bakken, R.; Dhanasekaran, S.; Mukherjee, A.; Lodha, R.; Singh, S.; Singh, V.; Haks, M.C.; Ottenhoff, T.H.M.; Kabra, S.K.; et al. BLR1 and FCGR1A transcripts in peripheral blood associate with the extent of intrathoracic tuberculosis in children and predict treatment outcome. Sci. Rep. 2016, 6, 38841. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Thirunavukkarasu, S.; Rosa, B.A.; Thomas, K.A.; Das, S.; Rangel-Moreno, J.; Lu, L.; Mehra, S.; Mbandi, S.K.; Thackray, L.B.; et al. Immune correlates of tuberculosis disease and risk translate across species. Sci. Transl. Med. 2020, 12, eaay0233. [Google Scholar] [CrossRef]
- Noble, A.; Paudyal, B.; Schwartz, J.C.; Mwangi, W.; Munir, D.; Tchilian, E.; Hammond, J.A.; Graham, S.P. Distinct effector functions mediated by Fc regions of bovine IgG subclasses and their interaction with Fc gamma receptors. Front. Immunol. 2023, 14, 1286903. [Google Scholar] [CrossRef] [PubMed]
- Mearelli, F.; Barbati, G.; Moras, C.; Ronco, C.; Biolo, G. Soluble FcγRIA expressed on monocytes (sCD64): A new serum biomarker of acute kidney injury in patients with suspected infection at emergency department admission. Cytokine 2021, 148, 155661. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Wang Taia, T.; Ravetch Jeffrey, V. The Role and Function of Fcγ Receptors on Myeloid Cells. Microbiol. Spectr. 2017, 4, 405–427. [Google Scholar] [CrossRef]
- Schiff, D.E.; Rae, J.; Martin, T.R.; Davis, B.H.; Curnutte, J.T. Increased Phagocyte FcγRI Expression and Improved Fcγ-Receptor–Mediated Phagocytosis After In Vivo Recombinant Human Interferon-γ Treatment of Normal Human Subjects. Blood 1997, 90, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Dudemaine, P.L.; Fecteau, G.; Lessard, M.; Labrecque, O.; Roy, J.P.; Bissonnette, N. Increased blood-circulating interferon-γ, interleukin-17, and osteopontin levels in bovine paratuberculosis. J. Dairy Sci. 2014, 97, 3382–3393. [Google Scholar] [CrossRef]
- Galon, J.; Paulet, P.; Galinha, A.; Lores, P.; Bonnerot, C.; Jami, J.; Fridman, W.-H.; Sautes, C. Soluble Fcγ Receptors: Interaction with Ligands and Biological Consequences. Int. Rev. Immunol. 1997, 16, 87–111. [Google Scholar] [CrossRef]
- Varin, N.; Sautès, C.; Galinha, A.; Even, J.; Hogarth, P.M.; Fridman, W.H. Recombinant soluble receptors for the Fcγ portion inhibit antibody production in vitro. Eur. J. Immunol. 1989, 19, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, Y.; Wei, P.; Shi, L.; Shi, L.; Li, J.; Zhao, Y.; Chen, Y.; Zhang, X.; Ye, F.; et al. Identification of hub genes for adult patients with sepsis via RNA sequencing. Sci. Rep. 2022, 12, 5128. [Google Scholar] [CrossRef] [PubMed]
- Farias, M.G.; de Lucena, N.P.; Bó, S.D.; de Castro, S.M. Neutrophil CD64 expression as an important diagnostic marker of infection and sepsis in hospital patients. J. Immunol. Methods 2014, 414, 65–68. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Nunes, J.S.; Figueiredo, J.F.; Lawhon, S.D.; Rossetti, C.A.; Gull, T.; Rice-Ficht, A.C.; Adams, L.G. Early Phase Morphological Lesions and Transcriptional Responses of Bovine Ileum Infected with Mycobacterium avium subsp. paratuberculosis. Vet. Pathol. 2009, 46, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Marete, A.; Ariel, O.; Ibeagha-Awemu, E.; Bissonnette, N. Identification of Long Non-coding RNA Isolated from Naturally Infected Macrophages and Associated with Bovine Johne’s Disease in Canadian Holstein Using a Combination of Neural Networks and Logistic Regression. Front. Vet. Sci. 2021, 8, 639053. [Google Scholar] [CrossRef] [PubMed]
- Ambruso, D.R.; Ellison, M.A.; Thurman, G.W.; Leto, T.L. Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 306–315. [Google Scholar] [CrossRef]
- Udby, L.; Calafat, J.; Sørensen, O.E.; Borregaard, N.; Kjeldsen, L. Identification of human cysteine-rich secretory protein 3 (CRISP-3) as a matrix protein in a subset of peroxidase-negative granules of neutrophils and in the granules of eosinophils. J. Leukoc. Biol. 2002, 72, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Klose, F.P.; Björnsdottir, H.; Dahlstrand Rudin, A.; Persson, T.; Khamzeh, A.; Sundqvist, M.; Thorbert-Mros, S.; Dieckmann, R.; Christenson, K.; Bylund, J. A rare CTSC mutation in Papillon-Lefèvre Syndrome results in abolished serine protease activity and reduced NET formation but otherwise normal neutrophil function. PLoS ONE 2021, 16, e0261724. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Pérez, V.; Arteche-Villasol, N.; Elguezabal, N.; Molina, E.; Benavides, J.; Gutiérrez-Expósito, D. Evaluation of the innate immune response of caprine neutrophils against Mycobacterium avium subspecies paratuberculosis in vitro. Vet. Res. 2023, 54, 61. [Google Scholar] [CrossRef] [PubMed]
- Ladero-Auñon, I.; Molina, E.; Oyanguren, M.; Barriales, D.; Fuertes, M.; Sevilla, I.A.; Luo, L.; Arrazuria, R.; De Buck, J.; Anguita, J.; et al. Oral vaccination stimulates neutrophil functionality and exerts protection in a Mycobacterium avium subsp. paratuberculosis infection model. NPJ Vaccines 2021, 6, 102. [Google Scholar] [CrossRef]
- Zang, X.; Dang, G.; Cai, Z.; Shao, M.; Tang, Y.; Cao, J.; Cui, Z.; Liu, S. Extracellular DNase MAP3916c attacks the neutrophil extracellular traps and is needed for Mycobacterium avium subsp. paratuberculosis virulence. Vet. Microbiol. 2022, 273, 109529. [Google Scholar] [CrossRef]
- Ladero-Auñon, I.; Molina, E.; Holder, A.; Kolakowski, J.; Harris, H.; Urkitza, A.; Anguita, J.; Werling, D.; Elguezabal, N. Bovine Neutrophils Release Extracellular Traps and Cooperate with Macrophages in Mycobacterium avium subsp. paratuberculosis clearance In Vitro. Front. Immunol. 2021, 12, 645304. [Google Scholar] [CrossRef]
- Smyth, P.; Sasiwachirangkul, J.; Williams, R.; Scott, C.J. Cathepsin S (CTSS) activity in health and disease—A treasure trove of untapped clinical potential. Mol. Asp. Med. 2022, 88, 101106. [Google Scholar] [CrossRef]
- Riese, R.J.; Wolf, P.R.; Brömme, D.; Natkin, L.R.; Villadangos, J.A.; Ploegh, H.L.; Chapman, H.A. Essential Role for Cathepsin S in MHC Class II–Associated Invariant Chain Processing and Peptide Loading. Immunity 1996, 4, 357–366. [Google Scholar] [CrossRef]
- Bania, J.; Gatti, E.; Lelouard, H.; David, A.; Cappello, F.; Weber, E.; Camosseto, V.; Pierre, P. Human cathepsin S, but not cathepsin L, degrades efficiently MHC class II-associated invariant chain in nonprofessional APCs. Proc. Natl. Acad. Sci. USA 2003, 100, 6664–6669. [Google Scholar] [CrossRef]
- Sendide, K.; Deghmane, A.-E.; Pechkovsky, D.; Av-Gay, Y.; Talal, A.; Hmama, Z. Mycobacterium bovis BCG Attenuates Surface Expression of Mature Class II Molecules through IL-10-Dependent Inhibition of Cathepsin S1. J. Immunol. 2005, 175, 5324–5332. [Google Scholar] [CrossRef]
- Soualhine, H.; Deghmane, A.-E.; Sun, J.; Mak, K.; Talal, A.; Av-Gay, Y.; Hmama, Z. Mycobacterium bovis Bacillus Calmette-Guérin Secreting Active Cathepsin S Stimulates Expression of Mature MHC Class II Molecules and Antigen Presentation in Human Macrophages1. J. Immunol. 2007, 179, 5137–5145. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J.; Evanson, O.A.; de Souza, C.; Abrahamsen, M.S. A critical role of interleukin-10 in the response of bovine macrophages to infection by Mycobacterium avium subsp. paratuberculosis. Am. J. Vet. Res. 2005, 66, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Weiss Douglas, J.; Evanson Oral, A.; McClenahan David, J.; Abrahamsen Mitchell, S.; Walcheck Bruce, K. Regulation of Expression of Major Histocompatibility Antigens by Bovine Macrophages Infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infect. Immun. 2001, 69, 1002–1008. [Google Scholar] [CrossRef]
- Ramachandra, L.; Qu, Y.; Wang, Y.; Lewis Colleen, J.; Cobb Brian, A.; Takatsu, K.; Boom, W.H.; Dubyak George, R.; Harding Clifford, V. Mycobacterium tuberculosis Synergizes with ATP To Induce Release of Microvesicles and Exosomes Containing Major Histocompatibility Complex Class II Molecules Capable of Antigen Presentation. Infect. Immun. 2010, 78, 5116–5125. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Walczak, M.J.; Aebi, M.; Wider, G. Posttranslational Modifications of Intact Proteins Detected by NMR Spectroscopy: Application to Glycosylation. Angew. Chem. Int. Ed. 2015, 54, 7096–7100. [Google Scholar] [CrossRef]
- Kleiner-Grote, G.R.M.; Risse, J.M.; Friehs, K. Secretion of recombinant proteins from E. coli. Eng. Life Sci. 2018, 18, 532–550. [Google Scholar] [CrossRef]
- Wiederanders, B.; Brömme, D.; Kirschke, H.; von Figura, K.; Schmidt, B.; Peters, C. Phylogenetic conservation of cysteine proteinases. Cloning and expression of a cDNA coding for human cathepsin S. J. Biol. Chem. 1992, 267, 13708–13713. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.-L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Petrera, A.; von Toerne, C.; Behler, J.; Huth, C.; Thorand, B.; Hilgendorff, A.; Hauck, S.M. Multiplatform Approach for Plasma Proteomics: Complementarity of Olink Proximity Extension Assay Technology to Mass Spectrometry-Based Protein Profiling. J. Proteome Res. 2021, 20, 751–762. [Google Scholar] [CrossRef]
- You, Q.; Verschoor, C.P.; Pant, S.D.; Macri, J.; Kirby, G.M.; Karrow, N.A. Proteomic analysis of plasma from Holstein cows testing positive for Mycobacterium avium subsp. paratuberculosis (MAP). Vet. Immunol. Immunopathol. 2012, 148, 243–251. [Google Scholar] [CrossRef]
- Navarro León, A.I.; Muñoz, M.; Iglesias, N.; Blanco-Vázquez, C.; Balseiro, A.; Milhano Santos, F.; Ciordia, S.; Corrales, F.J.; Iglesias, T.; Casais, R. Proteomic Serum Profiling of Holstein Friesian Cows with Different Pathological Forms of Bovine Paratuberculosis Reveals Changes in the Acute-Phase Response and Lipid Metabolism. J. Proteome Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Eckersall, P.D.; Young, F.J.; McComb, C.; Hogarth, C.J.; Safi, S.; Fitzpatrick, J.L.; Nolan, A.M.; Weber, A.; McDonald, T. Acute phase proteins in serum and milk from dairy cows with clinical mastitis. Vet. Rec. 2001, 148, 35–41. [Google Scholar] [CrossRef]
- Bean, L.D.; Wing, J.J.; Harris, R.E.; Smart, S.M.; Raman, S.V.; Milks, M.W. Transferrin predicts trimethylamine-N-oxide levels and is a potential biomarker of cardiovascular disease. BMC Cardiovasc. Disord. 2022, 22, 209. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Niu, J.-T.; Wu, H.-W.; Si, X.-L.; Zhang, S.-J.; Li, D.-H.; Bian, T.-T.; Li, Y.-F.; Yan, X.-K. Actin-Binding Proteins as Potential Biomarkers for Chronic Inflammation-Induced Cancer Diagnosis and Therapy. Anal. Cell. Pathol. 2021, 2021, 6692811. [Google Scholar] [CrossRef]
- Xu, H.; Cui, H.; Weng, S.; Zhang, Y.; Wang, L.; Xing, Z.; Han, X.; Liu, Z. Crosstalk of cell death pathways unveils an autophagy-related gene AOC3 as a critical prognostic marker in colorectal cancer. Commun. Biol. 2024, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Clempson, A.M.; Pollott, G.E.; Brickell, J.S.; Wathes, D.C. Associations Between Bovine IGFBP2 Polymorphisms with Fertility, Milk Production, and Metabolic Status in UK Dairy Cows. Anim. Biotechnol. 2012, 23, 101–113. [Google Scholar] [CrossRef]
- Zeni, L.; Norden, A.G.W.; Prandi, E.; Canepa, C.; Burling, K.; Simpson, K.; Felappi, B.; Plebani, A.; Cancarini, G.; Ferraro, P.M.; et al. Exploration of a panel of urine biomarkers of kidney disease in two paediatric cohorts with Type 1 diabetes mellitus of differing duration. Diabetol. Metab. Syndr. 2022, 14, 71. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]









| Comparison Group | Enriched Pathway | Pathway Genes Total | Proteins |
|---|---|---|---|
| Healthy controls (Infected herd) | Pentose phosphate pathway | 28 | GPI, ALDOA |
| Phagosome | 160 | ACTG1, FCGR1A, HLA-DRA, CTSS, TUBA4A, SFTPD | |
| Apoptosis | 140 | ACTG1, CTSC, SEPTIN4, CTSS, TUBA4A | |
| Staphylococcus aureus infection | 98 | FCGR1A, HLA-DRA, KRT25 | |
| Tuberculosis | 182 | FCGR1A, HLA-DRA, LBP, CTSS | |
| Neutrophil degranulation | 478 | GPI, CRISP3, HP, ALDOA, PGLYRP1, PRDX6, CTSS, CTSC | |
| MHC class II antigen presentation | 137 | HLA-DRA, TUBA4A, CTSS, CTSC | |
| Regulation of actin dynamics for phagocytic cup formation | 158 | IGKC, FCGR1A, IGKV2-30, ACTG1 | |
| Interferon gamma signaling | 177 | HLA-DRA, FCGR1A | |
| FCGR activation | 103 | IGKC, FCGR1A, IGKV2-30 | |
| Role of phospholipids in phagocytosis | 129 | IGKC, FCGR1A, IGKV2-30 | |
| Transfer of LPS from LBP carrier to CD14 | 3 | LBP | |
| CD22 mediated BCR regulation | 72 | IGKC, IGKV2-30 | |
| Gene and protein expression by JAK-STAT signaling after Interleukin-12 | 73 | LCP1 | |
| Butyrophilin (BTN) family interactions | 12 | PPL | |
| Classical antibody-mediated complement activation | 97 | IGKC, IGKV2-30 | |
| Antigen activates B Cell Receptor (BCR) leading to generation of second messengers | 103 | IGKC, IGKV2-30 | |
| Endosomal/Vacuolar pathway | 15 | CTSS | |
| Uninfected herd | Staphylococcus aureus infection | 98 | KRT17, KRT14, FCGR1A, HLA-DRA, MASP1, KRT10, KRT25, KRT24 |
| Phagosome | 160 | ACTG1, TUBB, FCGR1A, HLA-DRA, CTSS, TUBB1, HLA-G, TUBA4A, SFTPD | |
| Estrogen signaling pathway | 129 | ADCY6, KRT17, KRT14, KRT10, HSPA1A, KRT25, KRT24 | |
| Antigen processing and presentation | 78 | HLA-DRA, CTSS, HLA-G, HSPA1A | |
| Gap junction | 87 | ADCY6, TUBB, TUBB1, TUBA4A | |
| Viral myocarditis | 69 | ACTG1, HLA-DRA, HLA-G | |
| Gastric acid secretion | 72 | ADCY6, ACTG1, CA2 | |
| Complement and coagulation cascades | 82 | PROCR, MASP1 | |
| Apoptosis | 140 | ACTG1, CTSC, CTSS, TUBA4A | |
| Salmonella infection | 250 | TXN, ACTG1, TUBB, TUBB1, TUBA4A | |
| Neutrophil degranulation | 478 | SERPINA3, SERPINB1, TUBB5, CRISP3, COTL1, HBB, LYZ, PRDX6, CTSS, CTSC, HSPA1A | |
| MHC class II antigen presentation | 137 | TUBB5, TUBB1, HLA-DRA, TUBA4A, CTSS, CTSC | |
| Interferon gamma signaling | 177 | HLA-DRA, FCGR1A, HLA-G | |
| PKR-mediated signaling | 88 | TUBB5, TUBB1, TUBA4A, HSPA1A | |
| Endosomal/Vacuolar pathway | 15 | HLA-G, CTSS | |
| Regulation of Complement cascade | 139 | CFH, MASP1 | |
| Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation | 73 | LCP1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda, H.C.; von Toerne, C.; Korbonits, L.; Didier, A.; Scholz, A.M.; Märtlbauer, E.; Hauck, S.M.; Deeg, C.A. Cathepsin S Is More Abundant in Serum of Mycobacterium avium subsp. paratuberculosis-Infected Dairy Cows. Metabolites 2024, 14, 215. https://doi.org/10.3390/metabo14040215
Duda HC, von Toerne C, Korbonits L, Didier A, Scholz AM, Märtlbauer E, Hauck SM, Deeg CA. Cathepsin S Is More Abundant in Serum of Mycobacterium avium subsp. paratuberculosis-Infected Dairy Cows. Metabolites. 2024; 14(4):215. https://doi.org/10.3390/metabo14040215
Chicago/Turabian StyleDuda, Heidi C., Christine von Toerne, Lucia Korbonits, Andrea Didier, Armin M. Scholz, Erwin Märtlbauer, Stefanie M. Hauck, and Cornelia A. Deeg. 2024. "Cathepsin S Is More Abundant in Serum of Mycobacterium avium subsp. paratuberculosis-Infected Dairy Cows" Metabolites 14, no. 4: 215. https://doi.org/10.3390/metabo14040215
APA StyleDuda, H. C., von Toerne, C., Korbonits, L., Didier, A., Scholz, A. M., Märtlbauer, E., Hauck, S. M., & Deeg, C. A. (2024). Cathepsin S Is More Abundant in Serum of Mycobacterium avium subsp. paratuberculosis-Infected Dairy Cows. Metabolites, 14(4), 215. https://doi.org/10.3390/metabo14040215