Widely Targeted Metabolomic Analysis Provides New Insights into the Effect of Rootstocks on Citrus Fruit Quality
Abstract
:1. Introduction
2. Materials and Methods
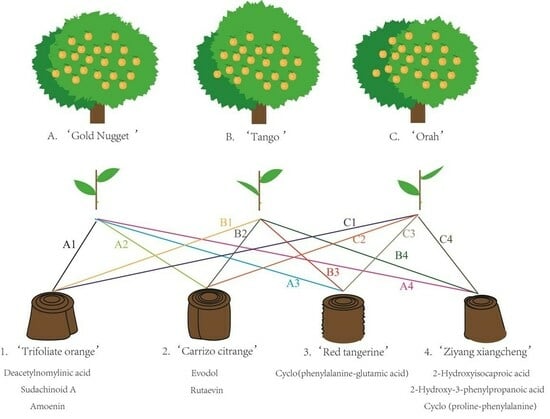
2.1. Plant Materials and Treatments
2.2. Agents and Instrument
2.3. Sample Preparation
2.4. UPLC-MS/MS Analysis
2.5. Statistical Analysis
3. Results
3.1. Fruit Quality Analysis
3.2. Metabolomic Profiling of Three Late-Maturation Mandarin Fruits with Different Rootstocks
3.3. Differential Metabolites Screening and Enrichment Analysis among Different Rootstock–Scion Combinations
3.4. Analysis of Metabolite Differences among the Three Late-Maturation Hybrid Mandarin Varieties
3.5. Rootstocks Regulate Specific Metabolites in Fruits of Three Mandarin Varieties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Full Meaning | Abbreviation |
|---|---|---|
| 1 | Ultra-performance liquid chromatography–tandem mass spectrometry | UPLC-MS/MS |
| 2 | Huanglongbing | HLB |
| 3 | Total soluble solids content | TSS |
| 4 | Titratable acidity | TA |
| 5 | Ascorbic acid | Vc |
| 6 | Curtain gas | CUR |
| 7 | Hierarchical cluster analysis | HCA |
| 8 | Unit variance scaling | UV |
| 9 | Principal component analysis | PCA |
| 10 | Kyoto Encyclopedia of Genes and Genomes | KEGG |
| 11 | Quality control sample | QC |
| 12 | Total ion current | TIC |
References
- Roose, M.L.; Williams, T.E.; Cameron, J.W.; Soost, R.K. ‘Gold Nugget’ Mandarin, A Seedless, Late-maturing Hybrid. HortScience 2000, 35, 1176–1178. [Google Scholar] [CrossRef]
- Morales, J.; Salvador, A.; Besada, C.; Navarro, P.; Bermejo, A. Physico-chemical, sensorial and nutritional quality during the harvest season of ‘Tango’ mandarins grafted onto Carrizo Citrange and Forner-Alcaide no. 5. Food Chem. 2021, 339, 127781. [Google Scholar] [CrossRef]
- Barry, G.H.; Gmitter, F.G., Jr.; Chen, C.X.; Roose, M.L.; Federici, C.T.; McCollum, G.T. Investigating the Parentage of ‘Orri’ and ‘Fortune’ Mandarin Hybrids. Acta Hortic. 2015, 1065, 449–456. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A Technique to Modify Ion Accumulation in Horticultural Crops. Front. Plant Sci. 2016, 7, 221552. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 109919. [Google Scholar] [CrossRef] [PubMed]
- Morales Alfaro, J.; Bermejo, A.; Navarro, P.; Quiñones, A.; Salvador, A. Effect of Rootstock on Citrus Fruit Quality: A Review. Food Rev. Int. 2021, 39, 2835–2853. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Zhou, X.; Pan, M.; Xu, J.; Liu, M.; Wang, M.; Liu, G.; Xu, T.; Wang, Y.; et al. Grafting with rootstocks promotes phenolic compound accumulation in grape berry skin during development based on integrative multi-omics analysis. Hortic. Res. 2022, 9, uhac055. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, Z.; Zhang, F.; Zhou, X.; Sun, X.; Li, Y.; Liu, W.; Xiao, H.; Wang, N.; Lu, H.; et al. Metabolomic and transcriptomic analyses reveal the effects of self- and hetero-grafting on anthocyanin biosynthesis in grapevine. Hortic. Res. 2022, 9, uhac103. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Shi, Y.-N.; Mou, Z.-M.; Chen, S.-Y.; Zhao, D.-K. Grafting: A potential method to reveal the differential accumulation mechanism of secondary metabolites. Hortic. Res. 2022, 9, uhac050. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Cao, J.Y.; Su, M.; Feng, G.Z.; Xu, Y.H.; Yi, H.L. Genome-wide comprehensive analysis of transcriptomes and small RNAs offers insights into the molecular mechanism of alkaline stress tolerance in a citrus rootstock. Hortic. Res. 2019, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Tietel, Z.; Srivastava, S.; Fait, A.; Tel-Zur, N.; Carmi, N.; Raveh, E. Impact of scion/rootstock reciprocal effects on metabolomics of fruit juice and phloem sap in grafted Citrus reticulata. PLoS ONE 2020, 15, e0227192. [Google Scholar] [CrossRef]
- Hussain, S.; Curk, F.; Anjum, M.A.; Pailly, O.; Tison, G. Performance evaluation of common clementine on various citrus rootstocks. Sci. Hortic. 2013, 150, 278–282. [Google Scholar] [CrossRef]
- Zhu, S.P.; Huang, T.J.; Yu, X.; Hong, Q.B.; Xiang, J.S.; Zeng, A.Z.; Gong, G.Z.; Zhao, X.C. The effects of rootstocks on performances of three late-ripening navel orange varieties. J. Integr. Agric. 2020, 19, 1802–1812. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, J.; Liu, M.M.; Yao, Q.; Chen, J.Z. Transcriptome profiling to understand the effect of citrus rootstocks on the growth of ‘Shatangju’ mandarin. PLoS ONE 2017, 12, e0169897. [Google Scholar]
- Roessner, U.; Bowne, J. What is metabolomics all about? Biotechniques 2009, 46, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xu, G. Mass-spectrometry-based metabolomics analysis for foodomics. TrAC 2013, 52, 36–46. [Google Scholar]
- Jorge, T.F.; Rodrigues, J.A.; Caldna, C.; Schmidt, R.; Dongen, J.T.V.; Thomas-Oates, J.; António, C. Mass spectrometry-based plant metabolomics: Metabolite responses to abiotic stress. Mass Spectrom. Rev. 2016, 35, 620–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.L.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.B.; Xiong, L.Z.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant. 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Gruz, J.; Novák, O.; Strnad, M. Rapid analysis of phenolic acids in beverages by UPLC-MS/MS. Food Chem. 2008, 111, 789–794. [Google Scholar] [CrossRef]
- De Brouwer, V.; Storozhenko, S.; Stove, C.P.; Van Daele, J.; Van Der Straeten, D.; Lambert, W.E. Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) for the sensitive determination of folates in rice. J. Chromatogr. B 2010, 878, 509–513. [Google Scholar] [CrossRef]
- Wang, J.; Chow, W. Applications of ultrahigh performance liquid chromatography electrospray ionization q-qrbitrap mass spectrometry and QuEChERS for fingerprinting and identification of molecular markers in orange juices. ACS Food Sci. Technol. 2023, 3, 729–737. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, X.P.; Mu, Q.E.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.X.; Yu, X.Y. Differences in total phenolics, antioxidant activity and metabolic characteristics in peach fruits at different stages of ripening. LWT-Food Sci. Technol. 2023, 178, 114586. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.L.; Liu, Z.G.; Zhao, Z.H.; Zhao, J.; Wang, Z.T.; Zhou, G.F.; Liu, P.; Liu., M.J. Transcriptome and metabolome profiling unveil the mechanisms of Ziziphus jujuba Mill. peel coloration. Food Chem. 2020, 312, 125903. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.C.; Wu, J.C.; Shahid, M.Q.; He, Y.H.; Lin, S.Q.; Liu, Z.H.; Yang, X.H. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar]
- Albrecht, U.; Fiehn, O.; Bowman, K.D. Metabolic variations in different citrus rootstock cultivars associated with different responses to Huanglongbing. Plant Physiol. Biochem. 2016, 107, 33–44. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.X.; Gmitter, F.G.; Grosser, J.W.; Wang, Y. Effects of Different Rootstocks on the Metabolites of Huanglongbing-Affected Sweet Orange Juices Using a Novel Combined Strategy of Untargeted Metabolomics and Machine Learning. J. Agric. Food Chem. 2023, 71, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U.; Tripathi, I.; Kim, H.; Bowman, K.D. Rootstock effects on metabolite composition in leaves and roots of young navel orange (Citrus sinensis L. Osbeck) and pummelo (C. grandis L. Osbeck) trees. Trees 2018, 33, 243–265. [Google Scholar]
- Cantuarias-Avilés, T.; Mourão Filho, F.A.A.; Stuchi, E.S.; Silva, S.R.d.; Espinoza-Núñez, E. Tree performance and fruit yield and quality of ‘Okitsu’ Satsuma mandarin grafted on 12 rootstocks. Sci. Hortic. 2010, 123, 318–322. [Google Scholar] [CrossRef]
- Saini, M.K.; Capalash, N.; Kaur, C.; Singh, S.P. Comprehensive metabolic profiling to decipher the influence of rootstocks on fruit juice metabolome of Kinnow (C. nobilis × C. deliciosa). Sci. Hortic. 2019, 257, 108673. [Google Scholar] [CrossRef]
- Ziogas, V.; Kokkinos, E.; Karagianni, A.; Voulgarakis, A.S.; Hussain, S.B. Effect of Rootstock Selection on Tree Growth, Yield, and Fruit Quality of Lemon Varieties Cultivated in Greece. Agronomy 2023, 13, 2265. [Google Scholar] [CrossRef]
- Camalle, M.D.; Pivonia, S.; Zurgil, U.; Fait, A.; Zur, N.T. Rootstock identity in melon-pumpkin graft combinations determines fruit metabolite profile. Front. Plant Sci. 2023, 13, 1024588. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Ghosh, S.N.; Sarkar, S.; Gantait, S. Effect of rootstocks on growth, yield, quality, and leaf mineral composition of Nagpur mandarin (Citrus reticulata Blanco.), grown in red lateritic soil of West Bengal. India. Sci. Hortic. 2018, 237, 142–147. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Chen, H.; Chen, S.; Liu, Y. Analysis of Flavonoid Metabolites in Citrus Peels (Citrus reticulata ‘Dahongpao’) Using UPLC-ESI-MS/MS. Molecules 2019, 24, 2680. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hong, Y.; Yang, D.; He, Z.; Lin, X.; Wang, G.; Yu, W. Simultaneous determination of phenolic metabolites in Chinese citrus and grape cultivars. PeerJ 2020, 8, e9083. [Google Scholar] [PubMed]
- Wang, T.; Zou, Q.J.; Guo, Q.S.; Yang, F.; Wu, L.W.; Zhang, W.Y. Widely Targeted Metabolomics Analysis Reveals the Effect of Flooding Stress on the Synthesis of Flavonoids in Chrysanthemum morifolium. Molecules 2019, 24, 203695. [Google Scholar] [CrossRef] [PubMed]
- Green, C.O.; Wheatley, A.O.; Osagie, A.U.; Morrison, E.Y.S.A.; Asemota, H.N. Determination of polymethoxylated flavones in peels of selected Jamaican and Mexican citrus (Citrus spp.) cultivars by high-performance liquid chromatography. Biomed. Chromatogr. 2007, 21, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Anacleto, S.L.; Milenkovic, D.; Kroon, P.A.; Needs, P.W.; Lajolo, F.M.; Hassimotto, N.M.A. Citrus flavanone metabolites protect pancreatic-beta cells under oxidative stress induced by cholesterol. Food Funct. 2020, 11, 8612–8624. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Tu, H.; Wan, J.; Chen, W.; Liu, X.Q.; Luo, J.; Xu, J.; Zhang, H.Y. Spatio-temporal distribution and natural variation of metabolites in citrus fruits. Food Chem. 2016, 199, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Schreiber, A.; Lacina, O.; Cajka, T.; Hajslova, J. Liquid chromatography–mass spectrometry-based metabolomics for authenticity assessment of fruit juices. Metabolomics 2011, 8, 793–803. [Google Scholar] [CrossRef]
- Xiong, B.; Li, Q.; Yao, J.F.; Ou, Y.H.; He, Y.Y.; Liao, L.; Wang, X.; Deng, H.H.; Zhang, M.F.; Sun, G.C.; et al. Transcriptome and UPLC-MS/MS reveal mechanisms of amino acid biosynthesis in sweet orange ‘Newhall’ after different rootstocks grafting. Front. Plant Sci. 2023, 14, 1216826. [Google Scholar] [CrossRef]
- Xiong, B.; Li, Q.; Yao, J.F.; Wang, C.M.; Chen, H.Z.; Ma, Q.Q.; Deng, T.M.; Liao, L.; Wang, X.; Zhang, M.F.; et al. Combined metabolomic and transcriptomic analysis reveals variation in phenolic acids and regulatory networks in the peel of sweet orange ‘Newhall’ (C. sinensis) after grafting onto two different rootstocks. Sci. Hortic. 2024, 323, 112461. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhao, Y.Y.; Shi, H.Z. Topping and grafting affect the alkaloid content and gene expression patterns of tobacco (Nicotiana tabacum L.). Plant Direct 2023, 7, e478. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yao, J.F.; Zheng, W.; Wang, J.L.; Liao, L.; Sun, G.C.; Wang, X.; Deng, H.H.; Zhang, M.F.; Wang, Z.H.; et al. Hetero-grafting affects flavonoid biosynthesis in sweet orange ‘Newhall’ (Citrus sinensis) peels: A metabolomics and transcriptomics analysis. Front. Plant Sci. 2023, 14, 1218426. [Google Scholar] [CrossRef]
- Killiny, N.; Valim, M.F.; Jones, S.E.; Hijaz, F. Effect of different rootstocks on the leaf metabolite profile of ‘Sugar Belle’ mandarin hybrid. Plant Signal. Behav. 2018, 13, e1445934. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Lian, H.S.; Li, H.X.; Dong, Y.P.; Tang, W.J.; Zhang, R.; Peng, X.M.; Li, X.M.; Tang, Y. Interspecific rootstocks improve the low-temperature resistance of bitter gourd through sucrose and nitrogen metabolism regulation. Acta Physiol. Plant. 2023, 45, 97. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [PubMed]
- Al-Badrawi, M.; Alabdulla, S.A. Effects of foliar spraying of salicylic acid with tryptophan on yield and quality parameters in wheat. Plant Arch. 2021, 21, 854–857. [Google Scholar] [CrossRef]
- Lo Piero, A.R. The State of the art in biosynthesis of anthocyanins and its regulation in pigmented sweet oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem. 2015, 63, 4031–4041. [Google Scholar] [CrossRef]
- Argamasilla, R.; Gómez-Cadenas, A.; Arbona, V. Metabolic and Regulatory Responses in Citrus Rootstocks in Response to Adverse Environmental Conditions. J. Plant Growth Regul. 2013, 33, 169–180. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Liu, Z.L.; Shu, Y.S.; Wang, M.L.; He, D.; Song, Z.Q.; Zeng, H.L.; Ning, Z.C.; Lu, C.; Lu, A.P.; et al. Chemotaxonomic Classification Applied to the Identification of Two Closely-Related Citrus TCMs Using UPLC-Q-TOF-MS-Based Metabolomics. Molecules 2017, 22, 1721. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill, E.H.; Dangles, O.A. Comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Jandrić, Z.; Islam, M.; Singh, D.K.; Cannavan, A. Authentication of Indian citrus fruit/fruit juices by untargeted and targeted metabolomics. Food Control 2017, 72, 181–188. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative Proteomic Analysis of the Graft Unions in Hickory (Carya cathayensis) Provides Insights into Response Mechanisms to Grafting Process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Wang, M.; Hou, H.; Li, S.; Guan, L.; Yang, H.; Wang, W.; Hong, L. Metabolomic and transcriptomic analyses reveal the effects of grafting on blood orange quality. Front. Plant Sci. 2023, 14, 1169220. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, X.H.; Tong, L.; Liu, M.Z.; Zhou, X.K.; Tahir, M.M.; Xing, L.B.; Ma, J.J.; An, N.; Zhao, C.P.; et al. Multi-omics analyses reveal MdMYB10 hypermethylation being responsible for a bud sport of apple fruit color. Hortic. Res. 2022, 9, uhac179. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, H.; Fan, Y.; Yan, L. Anthocyanins accumulation analysis of correlated genes by metabolome and transcriptome in green and purple peppers (Capsicum annuum). BMC Plant Biol. 2022, 22, 358. [Google Scholar] [CrossRef]







| Variety | Rootstock | Fruit Weight (g) | Fruit Height (cm) | Fruit Diameter (cm) | Fruit Index | Rind Thickness (mm) | TSS (%) | TA (‰) | TSS/TA | Ascorbic Acid (mg/100 mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gold Nugget A | 1 | 97.2 ± 10.1 a | 4.8 ± 0.2 a | 6.1 ± 0.3 a | 0.79 a | 3.5 ± 0.3 a | 15.6 ± 0.2 a | 7.2 ± 0.2 a | 21.9 ± 0.5 a | 14.9 ± 0.3 a |
| 2 | 116.9 ± 8.3 a | 5.2 ± 0.2 a | 6.5 ± 0.1 a | 0.81 a | 3.8 ± 0.2 a | 13.7 ± 0.1 b | 5.2 ± 0.2 a | 26.7 ± 0.3 a | 14.8 ± 0.2 a | |
| 3 | 118.5 ± 5.3 a | 5.2 ± 0.1 a | 6.6 ± 0.4 a | 0.80 a | 3.8 ± 0.2 a | 13.5 ± 0.2 b | 5.8 ± 0.1 a | 23.5 ± 0.4 a | 13.2 ± 0.3 a | |
| 4 | 105.9 ± 7.2 a | 4.9 ± 0.3 a | 6.4 ± 0.2 a | 0.78 a | 4.2 ± 0.3 a | 13.5 ± 0.1 b | 5.8 ± 0.1 a | 23.5 ± 0.2 a | 14.4 ± 0.6 a | |
| Tango B | 1 | 87.0 ± 5.7 a | 4.7 ± 0.2 a | 6.0 ± 0.2 a | 0.78 a | 3.1 ± 0.1 a | 12.3 ± 0.2 a | 7.9 ± 0.3 a | 15.8 ± 0.2 a | 22.3 ± 0.7 a |
| 2 | 98.3 ± 6.9 a | 4.8 ± 0.3 a | 6.2 ± 0.3 a | 0.78 a | 2.9 ± 0.1 a | 11.8 ± 0.1 a | 7.4 ± 0.2 a | 16.1 ± 0.3 a | 20.7 ± 0.5 a | |
| 3 | 90.9 ± 8.1 a | 4.7 ± 0.3 a | 6.0 ± 0.3 a | 0.78 a | 2.8 ± 0.2 a | 12.1 ± 0.0 a | 7.6 ± 0.1 a | 15.9 ± 0.5 a | 22.5 ± 0.1 a | |
| 4 | 88.17 ± 4.8 a | 4.7 ± 0.1 a | 5.9 ± 0.4 a | 0.80 a | 3.1 ± 0.3 a | 12.3 ± 0.2 a | 7.1 ± 0.2 a | 17.4 ± 0.4 a | 22.5 ± 0.3 a | |
| Orah C | 1 | 116.3 ± 8.5 a | 5.2 ± 0.2 a | 6.4 ± 0.3 a | 0.81 a | 3.8 ± 0.1 a | 14.2 ± 0.3 a | 6.1 ± 0.3 a | 23.6 ± 0.3 ab | 19.6 ± 0.2 ab |
| 2 | 127.5 ± 7.9 a | 5.4 ± 0.3 a | 6.6 ± 0.4 a | 0.82 a | 4.0 ± 0.1 a | 13.5 ± 0.3 a | 5.1 ± 0.1 a | 26.3 ± 0.5 a | 21.0 ± 0.4 ab | |
| 3 | 89.4 ± 4.0 a | 4.9 ± 0.2 a | 5.8 ± 0.1 a | 0.83 a | 3.7 ± 0.2 a | 14.0 ± 0.1 a | 7.9 ± 0.2 a | 18.5 ± 0.2 b | 23.5 ± 0.3 a | |
| 4 | 119.5 ± 6.5 a | 5.3 ± 0.5 a | 6.4 ± 0.3 a | 0.82 a | 4.3 ± 0.1 a | 13.5 ± 0.2 a | 5.4 ± 0.3 a | 25.4 ± 0.6 ab | 17.7 ± 0.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Chen, Y.; Li, S.; Yu, J.; Yang, L.; Hong, L. Widely Targeted Metabolomic Analysis Provides New Insights into the Effect of Rootstocks on Citrus Fruit Quality. Metabolites 2024, 14, 242. https://doi.org/10.3390/metabo14040242
Wang M, Chen Y, Li S, Yu J, Yang L, Hong L. Widely Targeted Metabolomic Analysis Provides New Insights into the Effect of Rootstocks on Citrus Fruit Quality. Metabolites. 2024; 14(4):242. https://doi.org/10.3390/metabo14040242
Chicago/Turabian StyleWang, Min, Yang Chen, Shuang Li, Jianjun Yu, Lei Yang, and Lin Hong. 2024. "Widely Targeted Metabolomic Analysis Provides New Insights into the Effect of Rootstocks on Citrus Fruit Quality" Metabolites 14, no. 4: 242. https://doi.org/10.3390/metabo14040242