Alterations of Photosynthetic and Oxidative Processes Influenced by the Presence of Different Zinc and Cadmium Concentrations in Maize Seedlings: Transition from Essential to Toxic Functions
Abstract
:1. Introduction
2. Results
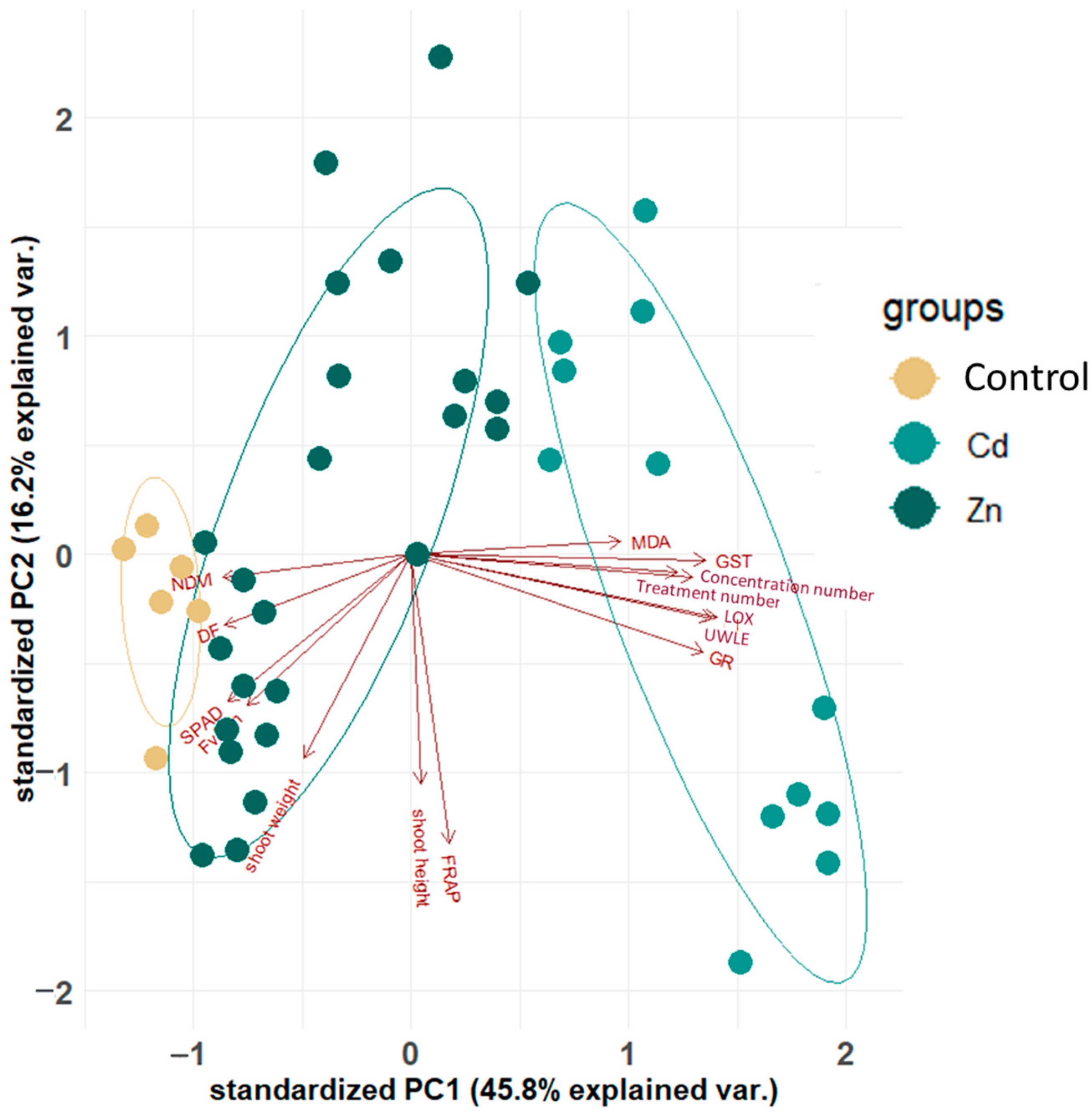
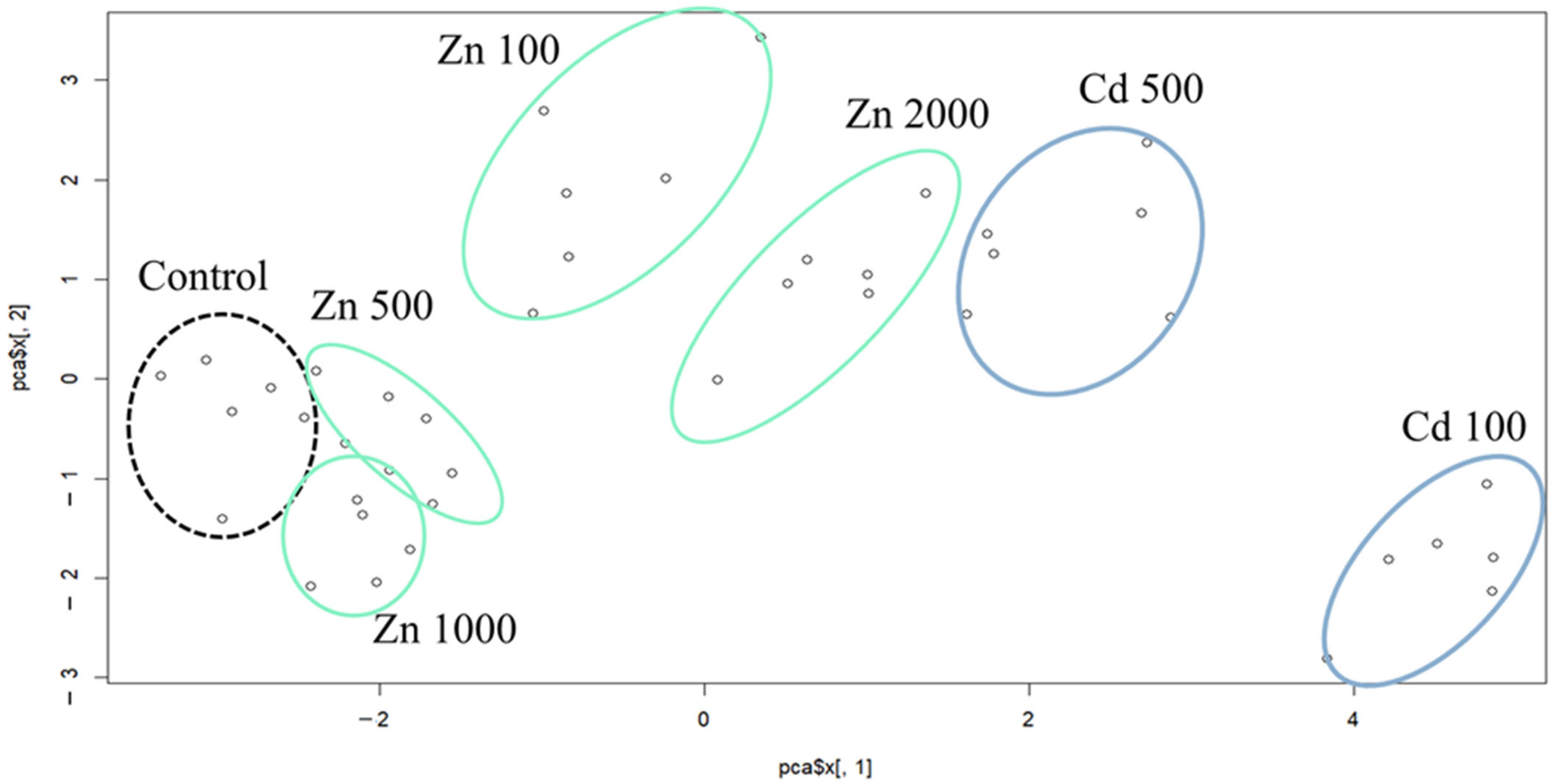
2.1. Results of the Principal Component Analysis
2.2. Element Accumulation, Shoot Height and Shoot Weight
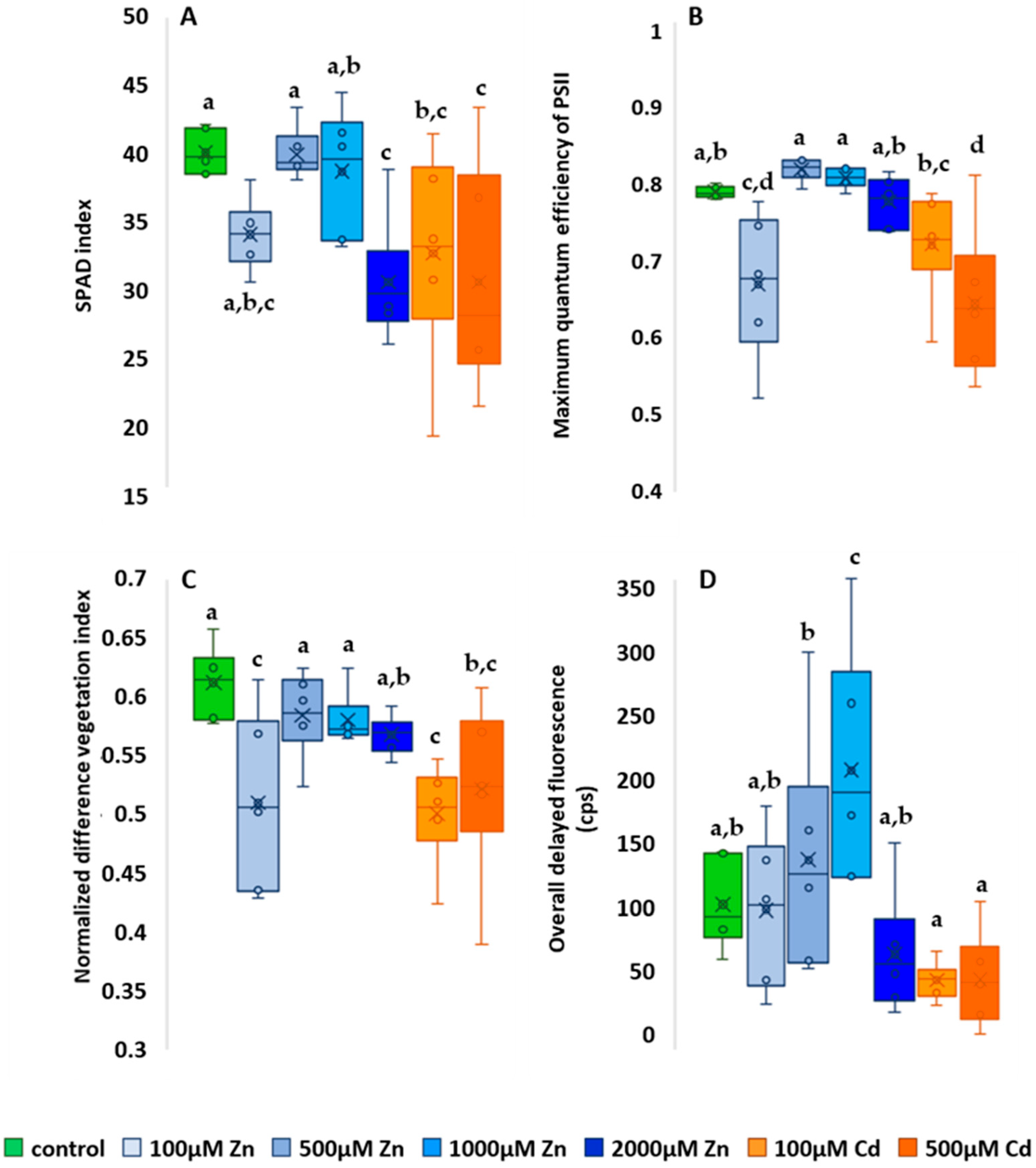
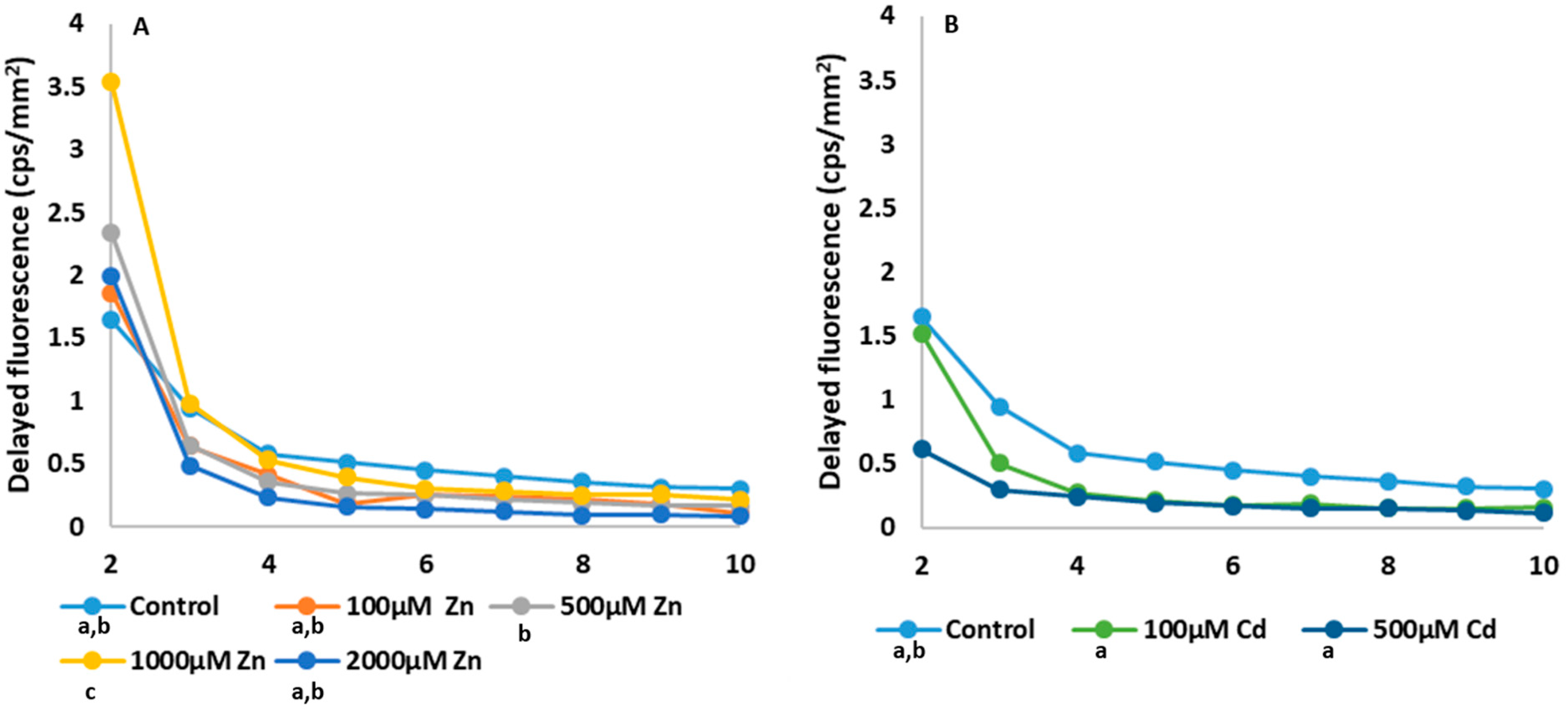
2.3. Photosynthesis-Related Studies
2.4. Oxidative Metabolism-Related Studies
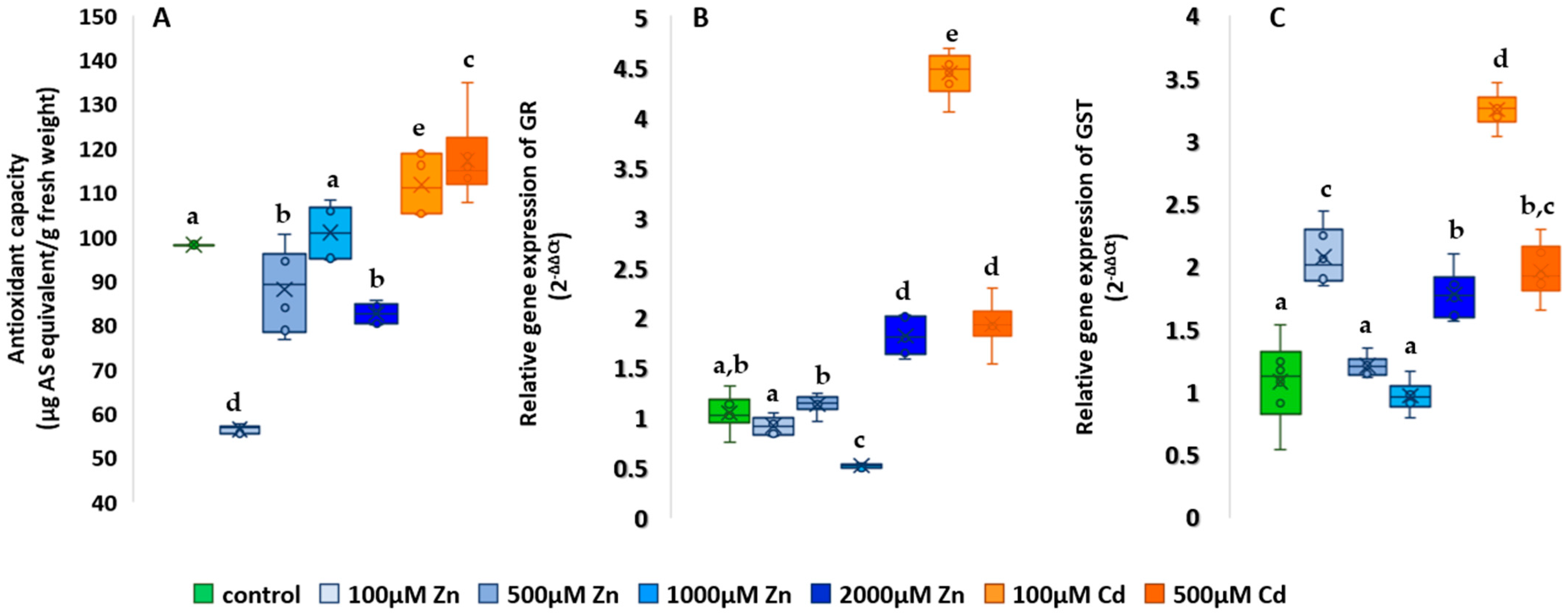
2.5. Studies on the Antioxidant System
2.6. Results of the Correlation Analysis
3. Discussion
3.1. Element Accumulation
3.2. Justification of the Investigation Methods
3.3. Analysis of the Changes in Photosynthetic Processes
3.4. Analysis of the Changes in Oxidative Metabolism-Related Processes
3.5. Summary and Future Perspectives of This Research
4. Materials and Methods
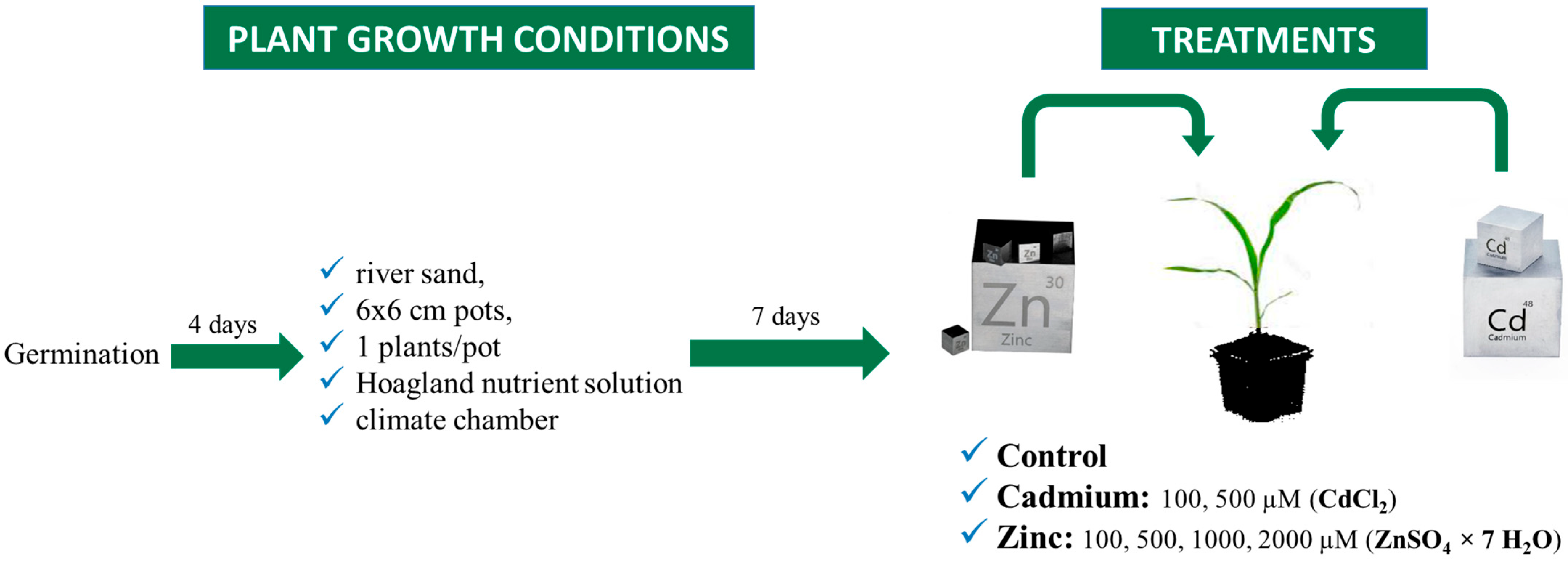
4.1. Growing Conditions
4.2. Treatments
4.3. Measurement of Physiological Parameters
4.4. Non-Invasive Measurements
4.4.1. Estimation of Relative Chlorophyll Content
4.4.2. Measurement of the Normalized Difference Vegetation Index
4.4.3. Fluorescence Induction Measurement
4.4.4. Biophoton Emission Measurement
4.5. Analytical Measurements
4.5.1. Measurement of Zn and Cd Content
4.5.2. Measurement of Ferric Reducing Antioxidant Power
4.5.3. Lipid Oxidation Measurement Based on Malondialdehyde (MDA) Determination
4.6. Gene Expression Studies
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radics, L. (Ed.) Kukorica. In Fenntartható Szemléletű Szántóföldi Növénytermesztéstan, 2nd ed.; Agroinform kiadó: Budapest, Hungary, 2012; pp. 33–65. [Google Scholar]
- Tyler, G.; Påhlsson, A.M.B.; Bengtsson, G.; Bååth, E.; Tranvik, L. Heavy-metal ecology of terrestrial plants, microorganisms and invertebrates. Water Air Soil Pollut. 1989, 47, 189–215. [Google Scholar] [CrossRef]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of Heavy Metals on Plants: An Overview. Int. J. Appl. Inn. Eng. Man. 2016, 5, 56–66. [Google Scholar]
- Greger, M. Metal Availability, Uptake, Transport and Accumulation in Plants. In Heavy Metal Stress in Plants: From Biomolecules to Ecosystems, 2nd ed.; Prasad, M.N.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Chapter 1; pp. 5–20. [Google Scholar]
- Schützendübel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Marschner, H. (Ed.) Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier Ltd.: Oxford, UK; Academic Press: Cambridge, MA, USA, 2012; Chapter 7; pp. 212–222. [Google Scholar]
- Cakmak, I.; Torun, B.; Erenoğlu, B.; Öztürk, L.; Marschner, H.; Kalayci, M.; Ekiz, H.; Yilmaz, A. Morphological and physiological differences in the response of cereals to zinc deficiency. Euphytica 1998, 100, 349–357. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Romheld, V.; Marschner, H. Function of micronutrients in plants. In Micronutrients in Agriculture, 2nd ed.; Mortvedt, J.J., Cox, F.R., Shuman, L.M., Welch, R.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1991; Chapter 9; pp. 297–328. [Google Scholar]
- Singh, B.; Kumar, S.; Natesan, A.; Singh, B.K.; Usha, K. Improving Zinc deficiency of cereals under zinc deficiency. Curr. Sci. 2005, 88, 36–44. [Google Scholar]
- Alloway, B.J. (Ed.) Fundamental Aspects of Zinc in Soils and Plants. In Zinc in Soils and Crop Nutrition, 2nd ed.; IZA and IFA: Brussels, Belgium; Paris, France, 2008; Chapter 2; pp. 14–54. [Google Scholar]
- Tsonev, T.; Cebola Lidon, F.J. Zinc in plants—An overview. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Abedi, T.; Gavanji, S.; Mojiri, A. Lead and Zinc Uptake and Toxicity in Maize and Their Management. Plants 2022, 11, 1922. [Google Scholar] [CrossRef]
- Di Baccio, D.; Tognetti, R.; Sebastiani, L.; Vitagliano, C. Responses of Populus deltoides × Populus nigra (Populus × euramericana) clone I-214 to high zinc concentrations. New Phyt. 2003, 159, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.N.; Gaur, J.P. Relationship between copper- and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 2004, 219, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Weckx, J.E.J.; Clijsters, H.M.M. Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Phys. Biochem. 1997, 35, 405–410. [Google Scholar]
- Ivanov, Y.V.; Savochkin, Y.V.; Kuznetsov, V.V. Scots pine as a model plant for studying the mechanisms of conifers adaptation to heavy metal action: 2. Functioning of antioxidant enzymes in pine seedlings under chronic zinc action. Russ. J. Plant Phys. 2012, 59, 50–58. [Google Scholar] [CrossRef]
- Pál, M.; Horváth, E.; Janda, T.; Páldi, E.; Szalai, G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J. Plant Nutr. Soil Sci. 2006, 169, 239–246. [Google Scholar] [CrossRef]
- Nriagu, J.O. (Ed.) Cadmium in the atmosphere and in precipitation. In Cadmium in the Environment; Part 1; John Wiley & Sons, Inc.: New York, NY, USA, 1980; pp. 35–114. [Google Scholar]
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martin, M.J.; Sanchez-Camazano, M. Adsorption and mobility of cadmium in natural uncultivated soils. J. Environ. Qual. 1993, 22, 737–742. [Google Scholar] [CrossRef]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on cadmium toxicity in plants: A review. Environ. Poll. 1997, 98, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Dalla Vecchia, F.; Ferretti, M.; Merlo, L.; Ghisi, R. Some effects of cadmium on maize plants. Arch. Environ. Cont. Toxic. 1993, 25, 244–249. [Google Scholar] [CrossRef]
- Seregin, I.; Kozhevnikova, A. Distribution of cadmium, lead, nickel and strontium in imbibing maize caryopses. Russ. J. Plant Phys. 2005, 52, 565–569. [Google Scholar] [CrossRef]
- Edreva, A. Generation and scavenging of reactive oxygen species in chloroplasts: A submolecular approach. Agric. Ecosys. Environ. 2005, 106, 119–133. [Google Scholar] [CrossRef]
- Khan, N.A.; Samiullah, S.S.; Nazar, R. Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress. J. Agric. Crop Sci. 2007, 193, 435–444. [Google Scholar] [CrossRef]
- Cho, U.H.; Seo, N.H. Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci. 2005, 168, 113–120. [Google Scholar] [CrossRef]
- Kleckerova, A.; Sobrova, P.; Krystofova, O.; Sochor, J.; Zitka, O.; Babula, P.; Adam, V.; Docekalova, H.; Kizek, R. Cadmium (II) and zinc (II) ions effects on maize plants revealed by spectroscopy and electrochemistry. Int. J. Electrochem. Sci. 2011, 6, 6011–6031. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F.; et al. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, G.H.; Ijaz, M.; Akhtar, J.; Anwar-Ul-Haq, M.; Jamil, M.; Ali, S.; Ahmad, R.; Khan, H.N. Profiling of Anti-Oxidative Enzymes and Lipid Peroxidation in Leaves of Salt Tolerant and Salt Sensitive Maize Hybrids under NaC1 and Cd Stress. Sains Malays. 2016, 4, 177–184. [Google Scholar]
- Prasad, A.; Sedláøová, M.; Kale, R.S.; Pospíšil, P. Lipoxygenase in singlet oxygen generation as a response to wounding: In vivo imaging in Arabidopsis thaliana. Sci. Rep. 2017, 7, 9831. [Google Scholar] [CrossRef] [PubMed]
- Oros, C.L.; Alves, F. Leaf wound induced ultraweak photon emission is suppressed under anoxic stress: Observations of under aerobic and anaerobic conditions using novel in vivo methodology. PLoS ONE 2018, 13, e0198962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Kan, X.; Chen, J.; Hua, H.; Li, Y.; Ren, J.; Feng, K.; Liu, H.; Deng, D.; Yin, Z. Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 2019, 158, 51–62. [Google Scholar] [CrossRef]
- Chen, W.; Jia, B.; Chen, J.; Feng, Y.; Li, Y.; Chen, M.; Liu, H.; Yin, Z. Effects of different planting densities on photosynthesis in maize determined via prompt fluorescence, delayed fluorescence and P700 signals. Plants 2021, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Pónya, Z.; Jócsák, I.; Keszthelyi, S. Detection of ultra-weak photon emission in sunflower (Helianthus annuus L.) infested by two spotted-spider mite, Tetranychus urticae Koch-research note. Phytoparasitica 2021, 50, 43–50. [Google Scholar] [CrossRef]
- Bodemer, U.; Gerhardt, V.; Yacobi, Y.Z.; Zohary, Z.; Friedrich, G.; Pohlmann, M. Phytoplankton abundance and composition of freshwaters systems determined by DF excitation spectroscopy and conventional methods. Arch. Hydrobiol. 2000, 55, 101–120. [Google Scholar]
- Jócsák, I.; Malgwi, I.; Rabnecz, G.; Szegő, A.; Varga-Visi, É.; Végvári, G.; Pónya, Z. Effect of cadmium stress on certain physiological parameters, antioxidative enzyme activities and biophoton emission of leaves in barley (Hordeum vulgare L.) seedlings. PLoS ONE 2020, 15, e0240470. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of chlorophyll a fluorescence in natural compound-induced stress detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef] [PubMed]
- Lukács, H.; Jócsák, I.; Somfalvi-Tóth, K.; Keszthelyi, S. Physiological Responses Manifested by Some Conventional Stress Parameters and Biophoton Emission in Winter Wheat as a Consequence of Cereal Leaf Beetle Infestation. Front. Plant Sci. 2022, 13, 839855. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, D.F. Lipoxygenases. Physiol. Plant 1989, 76, 249–253. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tamás, L.; Dudíková, J.; Ďurčeková, K. Effect of cadmium and temperature on the lipoxygenase activity in barley root tip. Protoplasm 2009, 235, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.M.; Dubbs, W.E.; Baker, R.A.; Fuller, M.A.; Stephenson, L.C.; Grimes, H.D. Protein dynamics, activity and cellular localization of soybean lipoxygenases indicate distinct functional roles for individual isoforms. Plant J. 1999, 19, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Liavonchanka, A.; Feussner, I. Lipoxygenases: Occurrence, functions and catalysis. J. Plant Phys. 2006, 163, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Ann. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Oláh, C. The Physiological and Stress-Protective Effect of a Bioactive Substance, the S-Methyl-Methionine-Salicylate (MMS) in Maize. Ph.D. Thesis, Eötvös Loránd University of Science, Újbuda, Budapest, 2020. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.P.; Skipsey, M.; Edwards, R. Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 2010, 71, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Cummins, I.; Dixon, D.P.; Freitag-Pohl, S.; Skipsey, M.; Edwards, R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metabol. Rev. 2011, 43, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in Combating Abiotic Stresses Including Arsenic Detoxification in Plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Ying, G.E. Response of glutathione and glutathione S-transferase in rice seedlings exposed to cadmium stress. Rice Sci. 2008, 15, 73–76. [Google Scholar] [CrossRef]
- Anjum, N.A.; Ahmad, I.; Mohmood, I.; Pacheco, M.; Duarte, A.C.; Pereira, E.; Umar, S.; Ahmad, A.; Khan, N.A.; Iqbal, M.; et al. Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—A review. Environ. Exp. Bot. 2012, 75, 307–324. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Wang, M.; Zou, J.; Duan, X.; Jiang, W.; Liu, D. Cadmium accumulation and its effects on metal uptake in maize (Zea mays L.). Biores. Technol. 2007, 98, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Warwick, P.; Hall, A.; Pashley, V.; Van Der Lee, J.; Maes, A. Zinc and cadmium mobility in podzol soils. Chemosphere 1999, 38, 2357–2368. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Ellis, D.R.; Grusak, M.A. Effect of zinc and manganese supply on the activities of superoxide dismutase and carbonic anhydrase in Medicago truncatula wild type and raz mutant plants. Plant Sci. 2005, 168, 1015–1022. [Google Scholar] [CrossRef]
- Bokor, B.; Vaculík, M.; Slováková, Ľ.; Masarovič, D.; Lux, A. Silicon does not always mitigate zinc toxicity in maize. Acta Phys. Plant. 2014, 36, 733–743. [Google Scholar] [CrossRef]
- Florijn, P.J.; Van Beusichem, M.L. Cadmium distribution in maize inbred lines: Effects of pH and level of Cd supply. Plant Soil 1993, 153, 79–84. [Google Scholar] [CrossRef]
- Liu, H.; Gan, W.; Rengel, Z.; Zhao, P. Effects of zinc fertilizer rate and application method on photosynthetic characteristics and grain yield of summer maize. J. Soil Sci. Plant Nutr. 2016, 16, 550–562. [Google Scholar] [CrossRef]
- Stepić, V.; Cvijanović, G.; Đurić, N.; Bajagić, M.; Marinković, J.; Cvijanović, V. Influence of zinc treatments on grain yield and grain quality of different maize genotypes. Plant Soil Environ. 2022, 68, 223–230. [Google Scholar] [CrossRef]
- Kovács, V.; Gondor, O.K.; Szalai, G.; Darkó, É.; Majláth, I.; Janda, T.; Pál, M. Synthesis and role of salicylic acid in wheat varieties with different levels of cadmium tolerance. J. Hazard. Mater. 2014, 280, 12–19. [Google Scholar] [CrossRef]
- Wiedemuth, K.; Müller, J.; Kahlau, A.; Amme, S.; Mock, H.P.; Grzam, A.; Humbeck, K. Successive maturation and senescence of individual leaves during barley whole plant ontogeny reveals temporal and spatial regulation of photosynthetic function in conjunction with C and N metabolism. J. Plant Phys. 2005, 162, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Schuerger, A.C.; Capelle, G.A.; Di Benedetto, J.A.; Mao, C.; Thai, C.N.; Evans, M.D.; Stryjewski, E.C. Comparison of two hyperspectral imaging and two laser-induced fluorescence instruments for the detection of zinc stress and chlorophyll concentration in bahia grass (Paspalum notatum Flugge.). Rem. Sens. Environ. 2003, 84, 572–588. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Li, M.; Fang, M.; Chi, W. Neural-network model for estimating leaf chlorophyll concentration in rice under stress from heavy metals using four spectral indices. Biosy. Eng. 2010, 106, 223–233. [Google Scholar] [CrossRef]
- Sridhar, B.M.; Han, F.X.; Diehl, S.V.; Monts, D.L.; Su, Y. Spectral reflectance and leaf internal structure changes of barley plants due to phytoextraction of zinc and cadmium. Int. J. Remote Sens. 2007, 28, 1041–1054. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Abdurrahman, H.A.; Abd El-Mageed, S.A. Residual acidified biochar modulates growth, physiological responses, and water relations of maize (Zea mays) under heavy metal–contaminated irrigation water. Environ. Sci. Pollut. Res. 2020, 27, 22956–22966. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Al-Zahrani, H.S.; Hakeem, K.R.; Alsamadany, H.; Desoky, E.S.M.; Rady, M.M. Silymarin-enriched biostimulant foliar application minimizes the toxicity of cadmium in maize by suppressing oxidative stress and elevating antioxidant gene expression. Biomolecules 2021, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Su, Y.; Du, Q.; Li, S. The influence mechanism of exogenous trehalose on the growth and development of maize under cadmium stress. J. Biotech Res. 2023, 15, 295–307. [Google Scholar]
- Lorenzo, F.; Marek, Ž.; Marek, K.; Andrea, C.; Simonetta, P.; Suleyman, I.A.; Marian, B. Fast chlorophyll a fluorescence induction (OJIP) phenotyping of chlorophyll-deficient wheat suggests that an enlarged acceptor pool size of Photosystem I helps compensate for a deregulated photosynthetic electron flow. J. Photochem. Photobiol. B Biol. 2022, 234, 112549. [Google Scholar]
- Jócsák, I.; Gyalog, H.; Hoffmann, R.; Somfalvi-Tóth, K. In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum eastivum L.) as Seed Priming Possibility, for Heat Stress Alleviation. Plants 2022, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, S.; Gerbovits, B.; Jócsák, I. Impact analysis of different applications of cyantraniliprole on control of horse chestnut leaf miner (Cameraria ohridella) larvae supported by biophoton emission. Biol. Futura 2023, 74, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Jócsák, I.; Lukács, H.; Varga-Visi, É.; Somfalvi-Tóth, K.; Keszthelyi, S. Identification and investigation of barley powdery mildew (Blumeria graminis f. sp. tritici) infection in winter wheat with conventional stress reactions and non-invasive biophoton emission parameters. J. Biosci. 2024, 49, 6. [Google Scholar] [CrossRef]
- Gerhardt, V.; Bodemer, U. Delayed fluorescence excitation spectroscopy: A method for determining phytoplankton composition. Erg. Limn. 2000, 55, 101–120. [Google Scholar]
- Berden-Zrimec, M.; Drinovec, L.; Zrimec, A.; Tišler, T. Delayed fluorescence in algal growth inhibition tests. Open Life Sci. 2007, 2, 169–181. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Keutgen, A.J.; Keutgen, N.; Sierka, E.; Baczewska-Dąbrowska, A.H.; Mojski, J.; Pawluśkiewicz, B.; Sieczko, L.; Kalaji, H.M. Photosynthetic efficiency of perennial ryegrass (Lolium perenne L.) seedlings in response to Ni and Cd stress. Sci. Rep. 2023, 13, 5357. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Loboda, T. Photosystem II of barley seedlings under cadmium and lead stress. Plant Soil Environ. 2007, 53, 511. [Google Scholar] [CrossRef]
- Zivcak, M.; Olsovska, K.; Brestic, M. Photosynthetic responses under harmful and changing environment: Practical aspects in crop research. Photosynth. Struct. Mech. Appl. 2017, 203–248. [Google Scholar]
- Birtic, S.; Ksas, B.; Genty, B.; Mueller, M.J.; Triantaphylidès, C.; Havaux, M. Using spontaneous photon emission to image lipid oxidation patterns in plant tissues. Plant J. 2011, 67, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Feigl, G.; Lehotai, N.; Molnár, A.; Ördög, A.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J.; Erdei, L.; Kolbert, Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2015, 116, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; AbdElgawad, H.; Hamed, B.A.; Beemster, G.T.; El-Shafey, N.M. Differences in cadmium accumulation, detoxification and antioxidant defenses between contrasting maize cultivars implicate a role of superoxide dismutase in Cd tolerance. Antioxidants 2021, 10, 1812. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Jelakovic, S.; Kopriva, S.; Suss, K.H.; Schulz, G.E. Structure and catalytic mechanism of the cytosolic D-ribulose-5-phosphate 3-epimerase from rice. J. Mol. Biol. 2003, 326, 127–135. [Google Scholar] [CrossRef]
- Kawagashira, N.; Ohtomo, Y.; Murakami, K.; Matsubara, K.; Kawai, J.; Carninci, R.; Hayashizaki, P.; Kikuchi, S.; Higo, K. Multiple zinc finger motifs with comparison of plant and insects. In Proceedings of the 12th International Conference on Genome Informatics, Tokyo, Japan, 17–19 December 2001; Matsuda, H., Miyano, S., Takagi, T., Wong, L., Eds.; The Garden Hall, Yebisu Garden Place: Tokyo, Japan, 2001; pp. 368–369. [Google Scholar]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. An effective antioxidant defense provides protection against zinc deficiency-induced oxidative stress in Zn-efficient maize plants. J. Plant Nutr. Soil Sci. 2019, 182, 701–707. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Chernov, N.N.; Novichkova, M.D. Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes. Biochemistry 2014, 79, 1562–1583. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.M. Toxicity of glutathione-binding metals: A review of targets and mechanisms. Toxics 2015, 3, 20–62. [Google Scholar] [CrossRef] [PubMed]
- Dobritzsch, D.; Grancharov, K.; Hermsen, C.; Krauss, G.J.; Schaumlöffel, D. Inhibitory effect of metals on animal and plant glutathione transferases. J. Trace Elem. Med. Biol. 2020, 57, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Moons, A. Osgstu3 and osgtu4, encoding tau class glutathione S-transferases, are heavy metal-and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett. 2003, 553, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Varjovi, M.B.; Valizadeh, M.; Bandehagh, A. Primary antioxidant enzymes and their important role in oxidative stress in plants and mammalian. Biol. Forum Int. J. 2015, 7, 148–154. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Manoli, A.; Sturaro, A.; Trevisan, S.; Quaggiotti, S.; Nonis, A. Evaluation of candidate reference genes for qPCR in maize. J. Plant Physiol. 2012, 169, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Hester, J.; Chang, W.; Bryan, J. Devtools: Tools to Make Developing R Packages Easier. 2022. Available online: https://cran.r-project.org/web/packages/devtools/devtools.pdf (accessed on 16 April 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 187–253. [Google Scholar]







| Measurement | 100 µM Zn | 500 µM Zn | 1000 µM Zn | 2000 µM Zn | 100 µM Cd | 500 µM Cd |
|---|---|---|---|---|---|---|
| Shoot height | − | = | + | − | + | − |
| Shoot weight | − | + | + | − | + | − |
| SPAD | − | − | − | − | − | − |
| NDVI | − | − | − | − | − | − |
| Fv/Fm | − | + | + | − | − | − |
| DF | − | + | + | − | − | − |
| UWLE | + | + | + | + | + | + |
| MDA | + | − | + | + | + | + |
| FRAP | − | − | + | − | + | + |
| GST | + | + | − | + | + | + |
| GR | − | + | − | + | + | + |
| LOX | + | + | − | + | + | + |
| Name | Sequence | Adhesion Temperature (°C) | Reference |
|---|---|---|---|
| MEP | F: TTCCTCATGTTCTTCGTGCC/ R: CAGTTCTCATTCCATCCGTG | 61.3 | Manoli (2012) [95] |
| GST | F: GACCATCAACTTCGCCACC/ R: ATCTACAAGTCACCATCCTGC | 62.7 | Oláh (2020) [50] |
| GR | F: GGAACCTACCAAACCAGATTA/ R: GGCAACGAAGACATCAACATC | 60.4 | Oláh (2020) [50] |
| LOX | F: CCCAACAGCATTTCCATCTG/ R: CCAATTACCACGCTTCTCATTC | 62.3 | Oláh (2020) [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jócsák, I.; Csima, F.; Somfalvi-Tóth, K. Alterations of Photosynthetic and Oxidative Processes Influenced by the Presence of Different Zinc and Cadmium Concentrations in Maize Seedlings: Transition from Essential to Toxic Functions. Plants 2024, 13, 1150. https://doi.org/10.3390/plants13081150
Jócsák I, Csima F, Somfalvi-Tóth K. Alterations of Photosynthetic and Oxidative Processes Influenced by the Presence of Different Zinc and Cadmium Concentrations in Maize Seedlings: Transition from Essential to Toxic Functions. Plants. 2024; 13(8):1150. https://doi.org/10.3390/plants13081150
Chicago/Turabian StyleJócsák, Ildikó, Ferenc Csima, and Katalin Somfalvi-Tóth. 2024. "Alterations of Photosynthetic and Oxidative Processes Influenced by the Presence of Different Zinc and Cadmium Concentrations in Maize Seedlings: Transition from Essential to Toxic Functions" Plants 13, no. 8: 1150. https://doi.org/10.3390/plants13081150






