Stability Analysis of Plankton–Fish Dynamics with Cannibalism Effect and Proportionate Harvesting on Fish
Abstract
:1. Introduction
2. Formulation of the Model
2.1. Model Variables and Parameters
2.2. Assumptions and Model Formulation
- (i)
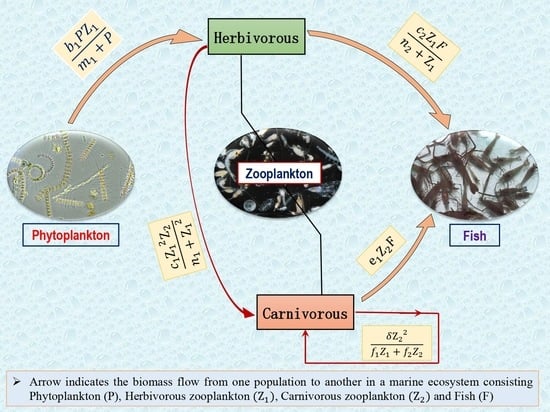
- It is assumed in our proposed model that zooplankton species are divided into two subclasses as herbivorous zooplankton and carnivorous zooplankton , because the zooplankton community at any trophic level is represented by herbivorous and carnivorous according to their distinctive food habits.
- (ii)
- In the absence of zooplankton, the phytoplankton population growth obeys the logistic law with intrinsic growth rate r and the carrying capacity of the environment K.
- (iii)
- It is assumed that only herbivorous zooplankton consumes phytoplankton by following the Holling type II response function with consumption rate and a half saturation constant . This response reflects the predator’s ability to handle and process prey at a maximum rate. Many planktonic predators exhibit type II functional responses, including zooplankton such as rotifers and small crustaceans. These organisms can actively search for and capture prey, and their consumption rate typically increases with prey density until a point of satiation is reached.
- (iv)
- The fish species consumes both herbivorous zooplankton as well as carnivorous zooplankton. The consumption of herbivorous zooplankton by fish is modeled as Holling type II functional response with half saturation constant (). On the other hand, the functional response for predation to carnivorous zooplankton by fish species is assumed to be taken as the Holling type I response function. In the type I response, the predator’s feeding rate increases linearly with the prey density until it reaches a saturation point. However, the type II response shows an initial rapid increase in the predator’s feeding rate, which eventually levels off as prey density continues to increase. Predators (fish) typically respond to a decreasing density of prey by removing a constant (type I functional response) or increasing (type II functional response) fraction of the remaining prey. Switching permits a predator, when given a choice of alternate prey, to alter its functional response to prey in low abundance in order to feed more effectively on prey in greater abundance. Under certain conditions, this behavior can stabilize fluctuations in populations of prey by providing a refuge from predation for rare prey.
- (v)
- The consumption of carnivorous zooplankton to herbivorous zooplankton is modeled as Holling type III response function with consumption rate and half-saturation constant . The representation of this type of functional response is more appropriate for situations in which the predation rate per capita prey tends to become smaller as the density of prey decreases, which may often occur if there are refuges for prey or there is another predator for the same prey. A type III functional response is characterized by a sigmoidal curve, where the predator’s consumption rate is initially slow at low prey densities, then accelerates rapidly as prey density increases, and finally plateaus or slows down again at high prey densities. Type III functional responses are commonly observed in predators that exhibit prey-switching behavior or have complex foraging strategies. In plankton ecosystems, some larger zooplankton species may display type III functional responses.
- (vi)
- Cannibalism is a biological phenomenon used by some species due to limited food availability. Here cannibalism is considered on carnivorous zooplankton species depending on both the herbivorous and carnivorous zooplankton. Thus, the food available for carnivorous zooplankton is with cannibalism rate . Due to the act of cannibalism, there is a clear gain for the cannibalistic predator. This gain results in an increase in reproduction in carnivorous zooplankton with reproduction rate . This, in turn, leads to a gain in the carnivorous zooplankton population which is expressed by the term .

3. Positivity and Boundedness of Solutions
3.1. Positivity
3.2. Boundedness
4. Equilibria and Stability Analysis
4.1. Equilibrium Points
- (i)
- The trivial equilibrium always exists.
- (ii)
- The zooplankton and fish free axial equilibrium always exists on the boundary of the first octant.
- (iii)
- The carnivorous zooplankton and fish free planar equilibrium , whereandNow, the planar equilibrium exists if , which implies . Therefore, the carnivorous zooplankton and fish free equilibrium exists if the natural mortality rate of the herbivorous zooplankton is less than a threshold value, which is determined by other biological parameters.
- (iv)
- The fish free equilibrium , where , and .Now, the equilibrium exists if , , and . Therefore, the fish free equilibrium exists if the consumption rate of herbivorous zooplankton to phytoplankton and the half-saturation constant is greater than their respective threshold value, which is determined by other biological parameters of the system (1).
- (v)
- The carnivorous zooplankton free equilibrium , where, , andNow, the equilibrium exists if and . Therefore, the carnivorous zooplankton free equilibrium exists if the intrinsic growth rate of phytoplankton and conversion efficiency of herbivorous zooplankton to fish population through predation is greater than their respective threshold value, which is determined by other biological parameters.
- (vi)
- The interior equilibrium , where , , and . It is noted that, . Now, the interior equilibrium exists if , , and . Therefore, the interior equilibrium exists if the conversion to the newly juvenile carnivorous zooplankton is directly produced through cannibalism and the natural mortality rate of fish are greater than their respective threshold values. These threshold values are determined by other biological parameters associated with the system (1). Now, from the existence condition of the interior equilibrium it is noted that the critical threshold of is obtained and is denoted by
4.2. Stability Analysis
4.3. Global Stability
- (i)
- The zooplankton and fish free axial equilibrium is globally asymptotically stable if and .
- (ii)
- The carnivorous zooplankton and fish free planar equilibrium is globally asymptotically stable if and and .
- (iii)
- The fish free equilibrium is globally asymptotically stable if , and .
- (iv)
- The carnivorous zooplankton free equilibrium is globally asymptotically stable if and .
5. Hopf Bifurcation Analysis
- (i)
- (ii)
- ,
- .
- ,
- ,
- ,
- .
5.1. Stability and Direction of Hopf Bifurcation
6. Optimal Harvesting Policy
7. Numerical Simulation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
- The algebraic expression for , , , used in Theorem 6:
- The algebraic expression for , , , used in Theorem 7:
- The algebraic expression for , , , used in Theorem 8:
- Expression of the functions , , and used in Section 5.1:
References
- Wilken, S.; Soares, M.; Urrutia-Cordero, P.; Ratcovich, J.; Ekvall, M.K.; Van Donk, E.; Hansson, L.A. Primary producers or consumers? Increasing phytoplankton bacterivory along a gradient of lake warming and browning. Limnol. Oceanogr. 2018, 63, S142–S155. [Google Scholar] [CrossRef] [Green Version]
- Malone, T.C. The relative importance of nannoplankton and netplankton as primary producers in tropical oceanic and neritic phytoplankton communities 1. Limnol. Oceanogr. 1971, 16, 633–639. [Google Scholar] [CrossRef]
- Falkowski, P.; Knoll, A.H. Evolution of Primary Producers in the Sea; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Dai, Y.; Wu, J.; Ma, X.; Zhong, F.; Cui, N.; Cheng, S. Increasing phytoplankton-available phosphorus and inhibition of macrophyte on phytoplankton bloom. Sci. Total Environ. 2017, 579, 871–880. [Google Scholar] [CrossRef]
- Chang, C.W.; Shiah, F.K.; Wu, J.T.; Miki, T.; Hsieh, C.h. The role of food availability and phytoplankton community dynamics in the seasonal succession of zooplankton community in a subtropical reservoir. Limnologica 2014, 46, 131–138. [Google Scholar] [CrossRef]
- Rehim, M.; Imran, M. Dynamical analysis of a delay model of phytoplankton–zooplankton interaction. Appl. Math. Model. 2012, 36, 638–647. [Google Scholar] [CrossRef]
- Petzoldt, T.; Rudolf, L.; Rinke, K.; Benndorf, J. Effects of zooplankton diel vertical migration on a phytoplankton community: A scenario analysis of the underlying mechanisms. Ecol. Model. 2009, 220, 1358–1368. [Google Scholar] [CrossRef]
- Truscott, J.; Brindley, J. Ocean plankton populations as excitable media. Bull. Math. Biol. 1994, 56, 981–998. [Google Scholar] [CrossRef]
- Castellani, M.; Rosland, R.; Urtizberea, A.; Fiksen, Ø. A mass-balanced pelagic ecosystem model with size-structured behaviourally adaptive zooplankton and fish. Ecol. Model. 2013, 251, 54–63. [Google Scholar] [CrossRef]
- Chakraborty, K.; Das, K. Modeling and analysis of a two-zooplankton one-phytoplankton system in the presence of toxicity. Appl. Math. Model. 2015, 39, 1241–1265. [Google Scholar] [CrossRef]
- Mehner, T.; Padisak, J.; Kasprzak, P.; Koschel, R.; Krienitz, L. A test of food web hypotheses by exploring time series of fish, zooplankton and phytoplankton in an oligo-mesotrophic lake. Limnologica 2008, 38, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Panja, P.; Mondal, S.K. Stability analysis of coexistence of three species prey–predator model. Nonlinear Dyn. 2015, 81, 373–382. [Google Scholar] [CrossRef]
- Walters, C.; Christensen, V.; Fulton, B.; Smith, A.D.; Hilborn, R. Predictions from simple predator-prey theory about impacts of harvesting forage fishes. Ecol. Model. 2016, 337, 272–280. [Google Scholar] [CrossRef]
- Panja, P.; Mondal, S.K.; Jana, D.K. Effects of toxicants on Phytoplankton-Zooplankton-Fish dynamics and harvesting. Chaos Solitons Fractals 2017, 104, 389–399. [Google Scholar] [CrossRef]
- Li, M.; Chen, B.; Ye, H. A bioeconomic differential algebraic predator–prey model with nonlinear prey harvesting. Appl. Math. Model. 2017, 42, 17–28. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L. Bifurcation analysis in a prey–predator model with nonlinear predator harvesting. J. Frankl. Inst. 2016, 353, 4701–4714. [Google Scholar] [CrossRef]
- Banerjee, M.; Venturino, E. A phytoplankton–toxic phytoplankton–zooplankton model. Ecol. Complex. 2011, 8, 239–248. [Google Scholar] [CrossRef]
- Turner, E.L.; Bruesewitz, D.A.; Mooney, R.F.; Montagna, P.A.; McClelland, J.W.; Sadovski, A.; Buskey, E.J. Comparing performance of five nutrient phytoplankton zooplankton (NPZ) models in coastal lagoons. Ecol. Model. 2014, 277, 13–26. [Google Scholar] [CrossRef]
- Malthus, T.R. An Essay on the Principle of Population, as it Affects the Future Improvement of Society with Remarks on the Speculations of Mr. Godwin, M. Condorcet, and Other Writers; John Murray: London, UK, 1817; Volume 3. [Google Scholar]
- Verhulst, P.F. Notice sur la loi que la population suit dans son accroissement. Corresp. Math. Phys. 1838, 10, 113–126. [Google Scholar]
- Lotka, A.J. Elements of Physical Biology; Williams & Wilkins: Ambler, PA, USA, 1925. [Google Scholar]
- Volterra, V. Variazioni e Fluttuazioni del Numero d’Individui in Specie Animali Conviventi (Reprinted in English); McGraw-Hill: New York, NY, USA, 1926. [Google Scholar]
- Levin, S.A. A more functional response to predator-prey stability. Am. Nat. 1977, 111, 381–383. [Google Scholar] [CrossRef]
- Kazarinoff, N.; Van Den Driessche, P. A model predator-prey system with functional response. Math. Biosci. 1978, 39, 125–134. [Google Scholar] [CrossRef]
- Ma, Z.; Li, W.; Zhao, Y.; Wang, W.; Zhang, H.; Li, Z. Effects of prey refuges on a predator–prey model with a class of functional responses: The role of refuges. Math. Biosci. 2009, 218, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Mortoja, S.G.; Panja, P.; Paul, A.; Bhattacharya, S.; Mondal, S.K. Is the intermediate predator a key regulator of a tri-trophic food chain model? An illustration through a new functional response. Chaos Solitons Fractals 2020, 132, 109613. [Google Scholar] [CrossRef]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European Pine Sawfly1. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism1. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Cabello, T.; Gámez, M.; Varga, Z. An improvement of the Holling type III functional response in entomophagous species model. J. Biol. Syst. 2007, 15, 515–524. [Google Scholar] [CrossRef]
- Andrews, J.F. A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol. Bioeng. 1968, 10, 707–723. [Google Scholar] [CrossRef]
- Song, J.; Xia, Y.; Bai, Y.; Cai, Y.; O’Regan, D. A non-autonomous Leslie–Gower model with Holling type IV functional response and harvesting complexity. Adv. Differ. Equ. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Upadhyay, R.K.; Datta, J. Bifurcation analysis of a modified Leslie–Gower model with Holling type-IV functional response and nonlinear prey harvesting. Adv. Differ. Equ. 2018, 2018, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Huang, Q. The dynamics of a harvested predator–prey system with Holling type IV functional response. Biosystems 2018, 169, 26–39. [Google Scholar] [CrossRef]
- Mortoja, S.G.; Panja, P.; Mondal, S.K. Dynamics of a predator-prey model with stage-structure on both species and anti-predator behavior. Inform. Med. Unlocked 2018, 10, 50–57. [Google Scholar] [CrossRef]
- Dawes, J.; Souza, M. A derivation of Holling’s type I, II and III functional responses in predator–prey systems. J. Theor. Biol. 2013, 327, 11–22. [Google Scholar] [CrossRef]
- Armstrong, R.A. The Effects of Predator Functional Response and Prey Productivity on Predator-Prey Stabillity: A Graphical Approach. Ecology 1976, 57, 609–612. [Google Scholar] [CrossRef]
- Jun-Ping, C.; Hong-De, Z. The qualitative analysis of two species predator-prey model with Holling’s type III functional response. Appl. Math. Mech. 1986, 7, 77–86. [Google Scholar] [CrossRef]
- Magnússon, K.G. Destabilizing effect of cannibalism on a structured predator–prey system. Math. Biosci. 1999, 155, 61–75. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Y.; Wu, J. Effect of predator cannibalism and prey growth on the dynamic behavior for a predator-stage structured population model with diffusion. J. Math. Anal. Appl. 2017, 449, 1479–1501. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, K.; Das, K.; Kar, T.K. Combined harvesting of a stage structured prey–predator model incorporating cannibalism in competitive environment. Comptes Rendus Biol. 2013, 336, 34–45. [Google Scholar] [CrossRef]
- Kaewmanee, C.; Tang, I. Cannibalism in an age-structured predator–prey system. Ecol. Model. 2003, 167, 213–220. [Google Scholar] [CrossRef]
- Polis, G.A. The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Diekmann, O.; Nisbet, R.; Gurney, W.; Van den Bosch, F. Simple mathematical models for cannibalism: A critique and a new approach. Math. Biosci. 1986, 78, 21–46. [Google Scholar] [CrossRef] [Green Version]
- Van den Bosch, F.; De Roos, A.; Gabriel, W. Cannibalism as a life boat mechanism. J. Math. Biol. 1988, 26, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Hastings, A.; Costantino, R. Cannibalistic egg-larva interactions in Tribolium: An explanation for the oscillations in population numbers. Am. Nat. 1987, 130, 36–52. [Google Scholar] [CrossRef]
- Persson, L.; Byström, P.; Wahlström, E. Cannibalism and competition in Eurasian perch: Population dynamics of an ontogenetic omnivore. Ecology 2000, 81, 1058–1071. [Google Scholar] [CrossRef]
- Botsford, L.W. The effects of increased individual growth rates on depressed population size. Am. Nat. 1981, 117, 38–63. [Google Scholar] [CrossRef]
- Claessen, D.; de Roos, A.M. Bistability in a size-structured population model of cannibalistic fish—A continuation study. Theor. Popul. Biol. 2003, 64, 49–65. [Google Scholar]
- Costantino, R.F.; Desharnais, R.; Cushing, J.M.; Dennis, B. Chaotic dynamics in an insect population. Science 1997, 275, 389–391. [Google Scholar] [CrossRef]
- Lehtinen, S.O.; Geritz, S.A. Cyclic prey evolution with cannibalistic predators. J. Theor. Biol. 2019, 479, 1–13. [Google Scholar] [CrossRef]
- Chen, M.; Wu, R. Dynamics of a harvested predator–prey model with predator-taxis. Bull. Malays. Math. Sci. Soc. 2023, 46, 76. [Google Scholar]
- Singh, M.K.; Bhadauria, B.; Singh, B.K. Qualitative analysis of a Leslie-Gower predator-prey system with nonlinear harvesting in predator. Int. J. Eng. Math. 2016, 2016, 2741891. [Google Scholar] [CrossRef] [Green Version]
- Heggerud, C.M.; Lan, K. Local stability analysis of ratio-dependent predator–prey models with predator harvesting rates. Appl. Math. Comput. 2015, 270, 349–357. [Google Scholar] [CrossRef]
- Das, T.; Mukherjee, R.; Chaudhuri, K. Bioeconomic harvesting of a prey–predator fishery. J. Biol. Dyn. 2009, 3, 447–462. [Google Scholar] [CrossRef] [Green Version]
- Pal, D.; Mahaptra, G.; Samanta, G. Optimal harvesting of prey–predator system with interval biological parameters: A bioeconomic model. Math. Biosci. 2013, 241, 181–187. [Google Scholar] [CrossRef]
- Hale, J.K. Ordinary Differential Equations; Courier Corporation: North Chelmsford, MA, USA, 2009. [Google Scholar]
- La Salle, J.P. The Stability of Dynamical Systems; SIAM: Philadelphia, PA, USA, 1976. [Google Scholar]
- Hassard, B.D.; Kazarinoff, N.D.; Wan, Y.H. Theory and Applications of Hopf Bifurcation; CUP Archive: Cambridge, UK, 1981. [Google Scholar]
- Basheer, A.; Quansah, E.; Bhowmick, S.; Parshad, R.D. Prey cannibalism alters the dynamics of Holling–Tanner-type predator–prey models. Nonlinear Dyn. 2016, 85, 2549–2567. [Google Scholar] [CrossRef] [Green Version]
- Santra, N.; Mondal, S.; Samanta, G. Complex Dynamics of a Predator–Prey Interaction with Fear Effect in Deterministic and Fluctuating Environments. Mathematics 2022, 10, 3795. [Google Scholar] [CrossRef]
- Colucci, R.; Diz-Pita, É.; Otero-Espinar, M.V. Dynamics of a two prey and one predator system with indirect effect. Mathematics 2021, 9, 436. [Google Scholar] [CrossRef]
- Abbas, Z.S.; Naji, R.K. Modeling and Analysis of the Influence of Fear on a Harvested Food Web System. Mathematics 2022, 10, 3300. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, J.; Yang, R. Stability Analysis and Hopf Bifurcation of a Delayed Diffusive Predator–Prey Model with a Strong Allee Effect on the Prey and the Effect of Fear on the Predator. Mathematics 2023, 11, 1996. [Google Scholar] [CrossRef]























| Parameter | Ecological/Biological Description |
|---|---|
| P | Total phytoplankton population. |
| Total herbivorous zooplankton population. | |
| Total carnivorous zooplankton population. | |
| F | Total fish population. |
| r | Intrinsic growth rate of phytoplankton. |
| K | Environmental carrying capacity of phytoplankton. |
| Capture rate. | |
| (Holling Type II) Half saturation constant. | |
| Conversion rate of herbivorous zooplankton. | |
| Capture rate. | |
| (Holling Type III) Half saturation constant. | |
| Death rate of herbivorous zooplankton. | |
| Conversion rate of carnivorous zooplankton. | |
| Efficiency of predation. | |
| Capture rate. | |
| (Holling Type II) Half saturation constant. | |
| Death rate of carnivorous zooplankton. | |
| Growth rate of carnivorous zooplankton due to cannibalism. | |
| Rate of cannibalism. | |
| Efficiency of conversion of herbivorous zooplankton to fish population. | |
| Efficiency of conversion of carnivorous zooplankton to fish population. | |
| Death rate of fish. | |
| q | Catchability coefficient. |
| E | Effort applied to harvest the fish species. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortoja, S.G.; Panja, P.; Mondal, S.K. Stability Analysis of Plankton–Fish Dynamics with Cannibalism Effect and Proportionate Harvesting on Fish. Mathematics 2023, 11, 3011. https://doi.org/10.3390/math11133011
Mortoja SG, Panja P, Mondal SK. Stability Analysis of Plankton–Fish Dynamics with Cannibalism Effect and Proportionate Harvesting on Fish. Mathematics. 2023; 11(13):3011. https://doi.org/10.3390/math11133011
Chicago/Turabian StyleMortoja, Sk Golam, Prabir Panja, and Shyamal Kumar Mondal. 2023. "Stability Analysis of Plankton–Fish Dynamics with Cannibalism Effect and Proportionate Harvesting on Fish" Mathematics 11, no. 13: 3011. https://doi.org/10.3390/math11133011