Pediatric Palliative Care in Infants and Neonates
Abstract
:1. Introduction
2. Materials and Methods
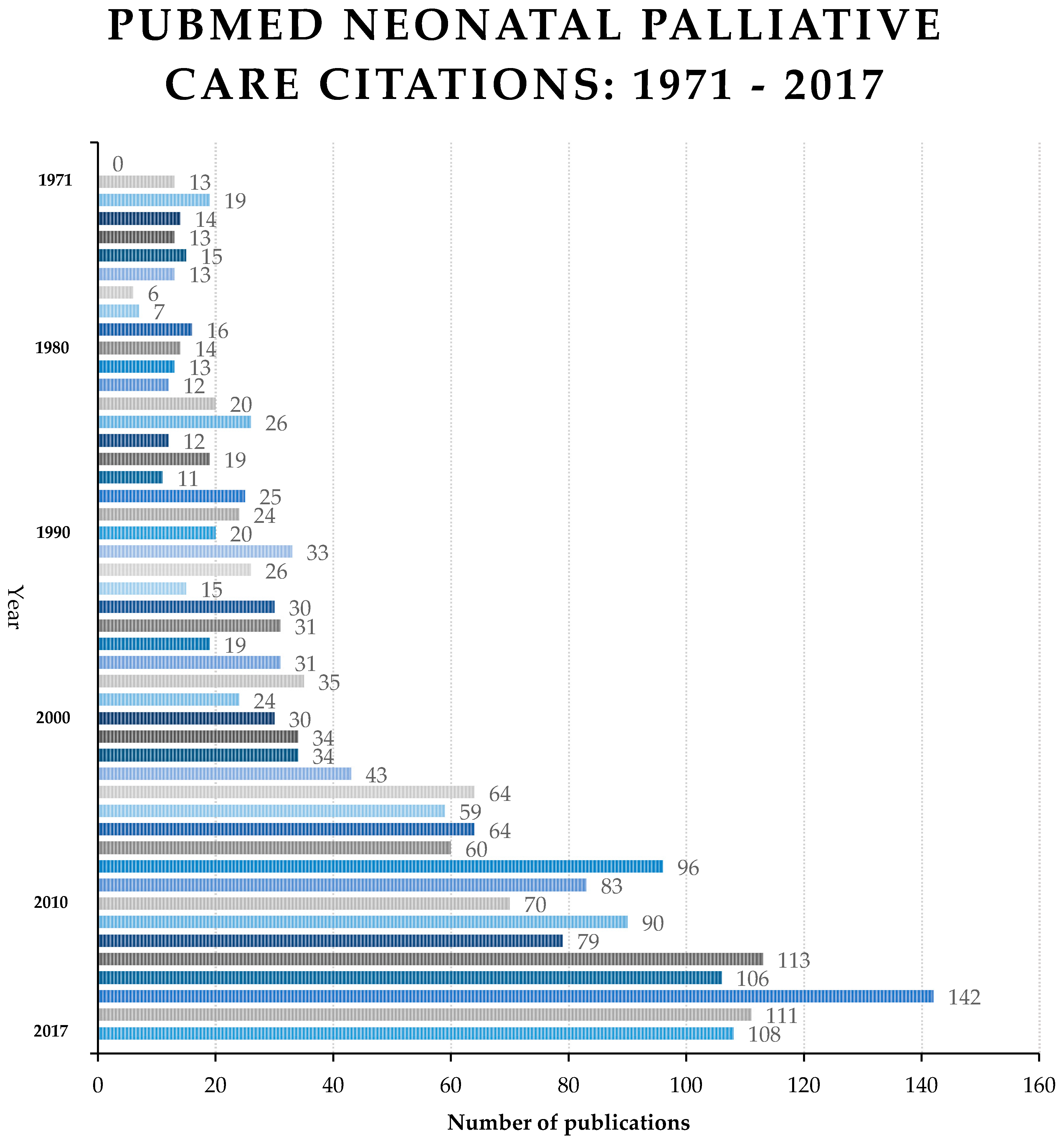
3. Results
“Palliative care for a fetus, neonate, or infant with a life-limiting condition is an active and total approach to care, from the point of diagnosis or recognition, throughout the child’s life, at the time of death and beyond. It embraces physical, emotional, social, and spiritual elements and focuses on the enhancement of quality of life for the neonatal infant and support for the family. It includes the management of distressing symptoms, the provision of short breaks, and care through death and bereavement” [21].
- Genetic/chromosomal: Chromosomal aneuploidies with complex and life-limiting prognoses; severe metabolic, storage, or mitochondrial disorders; severe forms of skeletal dysplasia.
- Organ-system problems: Severe central nervous system (CNS) malformations (neural tube defects, migrational disorders); hypoxic-ischemic encephalopathy; spinal muscular atrophy type-1 and myotonic dystrophies; epidermolysis bullosa; Potter’s syndrome, fetal oligohydramnios sequence, fetal-neonatal chronic renal failure; short-gut syndrome with parenteral nutrition dependence; multi-visceral organ transplant under consideration (e.g., liver, bowel, pancreas); biliary atresia; total aganglionosis of the bowel; severe feeding impairment with feeding tube dependence that may be permanent; complex congenital heart disease, especially if functionally univentricular; extracorporeal membrane oxygenation (ECMO) patients; severe pulmonary arterial hypertension; consideration for heart transplant; congenital diaphragmatic hernia; severe pulmonary hypoplasia; congenital central hypoventilation syndrome; asphyxiating thoracic dystrophies; multi-organ system failure.
- Infection and immune disorders: Perinatal human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS); severe combined immune deficiency (SCID); severe perinatal herpes simplex virus (HSV), cytomegalovirus (CMV), toxoplasmosis or Zika virus with meningoencephalitis or severe encephalopathy.
- Complications of prematurity: Periviable gestation; severe intraventricular hemorrhage (IVH, grade IV) or periventricular leukomalacia (PVL); refractory respiratory failure; ventilator-dependent BPD; severe necrotizing enterocolitis (NEC) with resultant short gut; liver failure.
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Whitfield, J.M.; Siegel, R.E.; Glicken, A.D.; Harmon, R.J.; Powers, L.K.; Goldson, E.J. The application of hospice concepts to neonatal care. Am. J. Dis. Child. 1982, 136, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.A. A hospice setting for humane neonatal death. Pediatrics 1982, 69, 239. [Google Scholar] [PubMed]
- Carter, B.; Howenstein, M.; Gilmer, M.J.; Throop, P.; France, D.; Whitlock, J.A. Circumstances surrounding the deaths of hospitalized children: Opportunities for pediatric palliative care. Pediatrics 2004, 114, e361–e366. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Lantos, J.; Meadow, W. End-of-life after birth: Death and dying in a neonatal intensive care unit. Pediatrics 2004, 114, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Penn, A.A.; Paris, J.J.; Moore, M.P., Jr. Decision making for seriously compromised newborns: The importance of exploring cultural differences and unintended consequences. J. Perinatol. 2013, 33, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.S.; Jones, P.J. Evidence-based comfort care for neonates toward the end of life. Semin. Fetal Neonatal Med. 2013, 18, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.S.; Brunkhorst, J. Neonatal pain management. Semin. Perinatol. 2017, 41, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.C.; Ritholz, M.D.; Burns, J.P.; Truog, R.D. Improving the quality of end-of-life care in the pediatric intensive care unit: Parents’ priorities and recommendations. Pediatrics 2006, 115, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Romesberg, T.L. Understanding grief: A component of neonatal palliative care. J. Hosp. Palliat. Nurs. 2004, 6, 161–170. [Google Scholar] [CrossRef]
- Meert, K.L.; Donaldson, A.E.; Newth, C.J.L.; Harrison, R.; Berger, J.; Zimmerman, J.; Anand, K.J.; Carcillo, J.; Dean, J.M.; Willson, D.F.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Complicated grief and associated risk factors among parents following a child’s death in the pediatric intensive care unit. Arch. Pediatr. Adolesc. Med. 2010, 164, 1045–1051. [Google Scholar] [PubMed]
- McHaffie, H.E.; Laing, I.A.; Lloyd, D.J. Follow-up care of bereaved parents after treatment withdrawal from newborns. Arch. Dis. Child. Fetal Neonatal Ed. 2001, 84, F125–F128. [Google Scholar] [CrossRef] [PubMed]
- Wool, C. State of the science on perinatal palliative care. J. Obstet. Gynecol. Neonatal Nurs. 2013, 42, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Cote-Arsenault, D.; Denney-Koelsch, E. “Have no regrets”: Parents’ experiences and developmental tasks in pregnancy with a lethal fetal diagnosis. Soc. Sci. Med. 2016, 154, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Rocha Catania, T.; Stein Bernardes, L.; Guerra Benute, G.R.; Bento Cicaroni Gibeli, M.A.; Bertolassi do Nascimento, N.; Aparecida Barbosa, T.V.; Jornada Krebs, V.L.; Francisco, R.P.V. When one knows a fetus is expected to die: Palliative care in the context of prenatal diagnosis of fetal malformations. J. Palliat. Med. 2017, 20, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.C.M.; Moldenhauer, J.S.; Jones, T.R.; Shaughnessy, E.A.; Zarrin, H.E.; Coursey, A.L.; Munson, D.A. A proposed model for perinatal palliative care. J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Tosello, B.; Haddad, G.; Gire, C.; Einaudi, M.A. Lethal fetal abnormalities: How to approach perinatal palliative care? J. Matern. Fetal Neonatal Med. 2017, 30, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Noseda, C.; Mialet-Marty, T.; Basquin, A.; Letourneur, I.; Bertorello, I.; Charlot, F.; Le Bouar, G.; Bétrémieux, P.; Collaborateurs. Severe hypoplastic left heart syndrome: Palliative care after prenatal diagnosis. Arch. Pédiatr. 2012, 19, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J.; Cantwell-Bartl, A.M. Place, age, and mode of death of infants and children with hypoplastic left heart syndrome: Implications for medical counselling, psychological counselling, and palliative care. J. Palliat. Care 2008, 24, 76–84. [Google Scholar]
- Lemmon, M.E.; Bidegain, M.; Boss, R.D. Palliative care in neonatal neurology: Robust support for infants, families and clinicians. J. Perinatol. 2013, 36, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Parravicini, E. Neonatal palliative care. Curr. Opin. Pediatr. 2017, 29, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Together for Short Lives (2009). The Neonatal Care Pathway. Available online: http://tinyurl.com/pncuwfj (accessed on 29 November 2017).
- Ferrell, B.R. Overview of the domains of variables relevant to end-of-life care. J. Palliat. Med. 2005, 8 (Suppl. 1), S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Youngblut, J.M.; Brooten, D. Perinatal and pediatric issues in palliative and end-of-life care from the 2011 Summit on the Science of Compassion. Nurs. Outlook 2012, 60, 343–350. [Google Scholar] [CrossRef] [PubMed]
- National Association of Neonatal Nurse Practitioners. Palliative care of newborns and infants. Position Statement #3051. Adv. Neonatal Care 2010, 10, 287–293. [Google Scholar]
- National Perinatal Association. Position Paper on Palliative Care. 2009. Available online: www.nationalperinatal.org/Resources/PalliativeCare2012-12-13.pdf (accessed on 1 December 2017).
- Friebert, S.; Huff, S. Standards of Practice for Pediatric Palliative Care/ Hospice; National Hospice and Palliative Care Organization: Alexandria, VA, USA, 2009. [Google Scholar]
- National Association of Neonatal Nurses (NANN). Palliative and End-of-Life Care for Newborns and Infants: Position Statement #3063; NANN: Chicago, IL, USA, 2015; Available online: http://nann.org/uploads/About/PositionPDFS/1.4.5_Palliative%20and%20End%20of%20Life%20Care%20for%20Newborns%20and%20Infants.pdf (accessed on 29 November 2017).
- Davies, B.; Sehring, S.A.; Partridge, J.C.; Cooper, B.A.; Hughes, A.; Philp, J.C.; Amidi-Nouri, A.; Kramer, R.F. Barriers to palliative care for children: Perceptions of pediatric health care providers. Pediatrics 2008, 121, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Gardner, G.; Yates, P. Neonatal palliative care attitude scale: Development of an instrument to measure the barriers to and facilitators of palliative care in neonatal nursing. Pediatrics 2009, 123, e207–e213. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.J. Palliative care delivery in the NICU: What barriers do neonatal nurses face? Neonatal Netw. 2006, 25, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wool, C. Clinician perspectives of barriers in perinatal palliative care. MCN Am. J. Matern. Child Nurs. 2015, 40, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Marc-Aurele, K.L.; English, N.K. Primary palliative care in neonatal intensive care. Semin. Perinatol. 2017, 41, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Twamley, K.; Kelly, P.; Moss, R.; Mancini, A.; Craig, F.; Koh, M.; Polonsky, R.; Bluebond-Langner, M. Palliative care education in neonatal units: Impact on knowledge and attitudes. BMJ Support Palliat. Care 2013, 3, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wool, C. Clinician confidence and comfort in providing perinatal palliative care. J. Obstet. Gynecol. Neonatal Nurs. 2013, 42, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kobler, K.; Limbo, R. Making a case: Creating a perinatal palliative care service using a perinatal bereavement program model. J. Perinat. Neonatal Nurs. 2011, 25, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, A.; Martin-Ancel, A.; Ortigoza-Escobar, D.; Escribano, J.; Argemi, J. The model of palliative care in the perinatal setting: A review of the literature. BMC Pediatr. 2012, 25. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Placencia, F.; Arnold, J.; Minard, C.; Harris, T.; Haidet, P. A structured end-of-life curriculum for neonatal-perinatal postdoctoral fellows. Am. J. Hosp. Palliat. Med. 2015, 32, 253–261. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, M.F.; Chan, M.; McAllister, M.; Hellmann, J. End-of-life care in Toronto neonatal intensive care units: Challenges for physician trainees. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F528–F533. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Kelly, P.; Bluebond-Langner, M. Training neonatal staff for the future in neonatal palliative care. Semin. Fetal Neonatal Med. 2013, 18, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Petrikin, J.E.; Willig, L.K.; Smith, L.D.; Kingsmore, S.F. Rapid whole genome sequencing and precision neonatology. Semin. Perinatol. 2015, 39, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Vanderver, A.; Simons, C.; Helman, G. Whole exome sequencing in patients with white matter abnormalities. Ann. Neurol. 2016, 79, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Bruun, T.U.J.; DesRoches, C.L.; Wilson, D.; Chau, V.; Nakagawa, T.; Yamasaki, M.; Hasegawa, S.; Fukao, T.; Marshall, C.; Mercimek-Andrews, S. Prospective cohort study for identification of underlying genetic causes in neonatal encephalopathy using whole-exome sequencing. Genet. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Khromykh, A.; Babcock, H.; Jenevein, C.; Solomon, B.D. Putting the pieces together: Clinically relevant genetic and genomic resources for hospitalists and neonatologists. Hosp. Pediatr. 2017, 7, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Uthaya, S.; Beardsley, C.; Wood, D.; Modi, N. Practical Guidance for the Management of Palliative Care on Neonatal Units; Royal College of Paediatrics and Child Health, Chelsea and Westminster Hospital, NHS Foundation Trust (UK): London, UK, 2014. [Google Scholar]
- Dean, B.; McDonald, K. Nursing Perspectives: Building an interprofessional perinatal palliative care team. NeoReviews 2014, 15, e422–e425. [Google Scholar] [CrossRef]
- Cortezzo, D.E.; Sanders, M.R.; Brownell, E.A.; Moss, K. End-of-life care in the neonatal intensive care unit: Experiences of staff and parents. Am. J. Perinatol. 2015, 32, 713–724. [Google Scholar] [PubMed]
- Boss, R.D.; Urban, A.; Barnett, M.D.; Arnold, R.M. Neonatal Critical Care Communication (NC3): Training NICU physicians and nurse practitioners. J. Perinatol. 2013, 33, 642–646. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Munson, D.; DeMauro, S.B.; Langer, J.C.; Dworetz, A.R.; Natarajan, G.; Bidegain, M.; Fortney, C.A.; Seabrook, R.; Vohr, B.R.; et al. Outcomes of Preterm Infants following discussions about withdrawal or withholding of life support. J. Pediatr. 2017, 190, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Catlin, A.; Mihara, D. End of life in the NICU: A study of ventilator withdrawal. MCN Am. J. Matern. Child Nurs. 2001, 26, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Williams, C.; Ives-Baine, L.; Shah, P.S. Withdrawal of artificial nutrition and hydration in the Neonatal Intensive Care Unit: Parental perspectives. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F21–F25. [Google Scholar] [CrossRef] [PubMed]
- Boss, R.D.; Lemmon, M.E.; Arnold, R.M.; Donohue, P.K. Communicating prognosis with parents of critically ill infants: Direct observation of clinician behaviors. J. Perinatol. 2017, 37, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Rainone, F. Infants’ best interests in end-of-life care for newborns. J. Pain Sympt. Manag. 2015, 49, 6505. [Google Scholar]
- Blumenthal-Barby, J.S.; Loftis, L.; Cummings, C.L.; Meadow, W.; Lemmon, M.; Ubel, P.A.; McCullough, L.; Rao, E.; Lantos, J.D. Should neonatologists give opinions withdrawing life-sustaining treatment? Pediatrics 2016, 138, e20162585. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, B.S. Pediatric Palliative Care in Infants and Neonates. Children 2018, 5, 21. https://doi.org/10.3390/children5020021
Carter BS. Pediatric Palliative Care in Infants and Neonates. Children. 2018; 5(2):21. https://doi.org/10.3390/children5020021
Chicago/Turabian StyleCarter, Brian S. 2018. "Pediatric Palliative Care in Infants and Neonates" Children 5, no. 2: 21. https://doi.org/10.3390/children5020021



