Maximizing the Recovery of Phenolic Antioxidants from Wild Strawberry (Fragaria vesca) Leaves Using Microwave-Assisted Extraction and Accelerated Solvent Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Preparation of Phenolic Extracts
2.3.1. Microwave-Assisted Extraction (MAE)
2.3.2. Accelerated Solvent Extraction (ASE)
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of the Individual Phenolic Content
2.6. Determination of Antioxidant Properties
2.6.1. ABTS
2.6.2. DPPH
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Extraction Parameters on Yield of Phenolics
3.2. Effects of Extraction Methods on Phenolic Profile
3.3. Effects of Extraction Parameters on Antioxidant Properties
3.3.1. Antioxidant Properties Evaluated by ABTS Assay
3.3.2. Antioxidant Properties Evaluated by DPPH Assay
3.3.3. Antioxidant Properties Evaluated by FRAP Assay
3.4. Correlation between the Contents of Phenolics and Their Different Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dias, M.I.; Barros, L.; Fernandes, I.P.; Ruphuy, G.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. A bioactive formulation based on Fragaria vesca L. vegetative parts: Chemical characterisation and application in κ-carrageenan gelatin. J. Funct. Foods 2015, 16, 243–255. [Google Scholar]
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girão, H.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Fragaria vesca leaves through inflammation, proteasome and autophagy modulation. J. Ethnopharmacol. 2014, 158, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liberal, J.; Costa, G.; Carmo, A.; Vitorino, R.; Marques, C.; Domingues, M.R.; Domingues, P.; Gonçalves, A.C.; Alves, R.; Sarmento-Ribeiro, A.B.; et al. Chemical characterization and cytotoxic potential of an ellagitannin-enriched fraction from Fragaria vesca leaves. Arab. J. Chem. 2019, 12, 3652–3666. [Google Scholar] [CrossRef]
- Mudnic, I.; Modun, D.; Brizic, I.; Vukovic, J.; Generalic, I.; Katalinic, V.; Bilusic, T.; Ljubenkov, I.; Boban, M. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca L.) leaves. Phytomedicine 2009, 16, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, J.; Simões, D.M.; Antunes, P.E.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Vascular effects of Fragaria vesca L. in human arteries. Nat. Prod. Res. 2023, 37, 3851–3856. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.B.; Turker, A.U. Effects of regeneration enhancers on micropropagation of Fragaria vesca L. and phenolic content comparison of field-grown and in vitro-grown plant materials by liquid chromatography-electrospray tandem mass spectrometry (LC–ESI-MS/MS). Sci. Hortic. 2014, 169, 169–178. [Google Scholar]
- Dias, M.I.; Barros, L.; Sousa, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Enhancement of nutritional and bioactive compounds by in vitro culture of wild Fragaria vesca L. vegetative parts. Food Chem. 2017, 235, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Buricova, L.; Andjelkovic, M.; Cermakova, A.; Reblova, Z.; Jurcek, O.; Kolehmainen, E.; Verhe, R.; Kvasnicka, F. Antioxidant capacity and antioxidants of strawberry, blackberry, and raspberry leaves. Czech J. Food Sci. 2011, 29, 181–189. [Google Scholar] [CrossRef]
- Ivanov, I.G. Determination of biologically active substances with antioxidant potential in different extracts of Fragaria vesca L. leaves and flowers. J. Pharmacogn. Phytochem. 2018, 7, 2733–2737. [Google Scholar]
- Couto, J.; Figueirinha, A.; Batista, M.T.; Paranhos, A.; Nunes, C.; Gonçalves, L.M.; Marto, J.; Fitas, M.; Pinto, P.; Ribeiro, H.M.; et al. Extract: A Promising Cosmetic Ingredient with Antioxidant Properties. Antioxidants 2020, 9, 154. [Google Scholar] [CrossRef]
- D’Urso, G.; Pizza, C.; Piacente, S.; Montoro, P. Combination of LC–MS based metabolomics and antioxidant activity for evaluation of bioactive compounds in Fragaria vesca leaves from Italy. J. Pharm. Biomed. Anal. 2018, 150, 233–240. [Google Scholar] [CrossRef]
- Tripathi, M.; Diwan, D.; Shukla, A.C.; Gaffey, J.; Pathak, N.; Dashora, K.; Pandey, A.; Sharma, M.; Guleria, S.; Varjani, S.; et al. Valorization of dragon fruit waste to value-added bioproducts and formulations: A review. Crit. Rev. Biotechnol. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yeasmen, N.; Orsat, V. Green extraction and characterization of leaves phenolic compounds: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5155–5193. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting; Association of Official Analytical Chemists: Rockville, MD, USA, 1990; Volume 15. [Google Scholar]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS2 Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef]
- Katalinic, V.; Modun, D.; Music, I.; Boban, M. Gender differences in antioxidant capacity of rat tissues determined by 2,2′-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2005, 140, 47–52. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Tade, M.O.; Ali, H.A. Thermodynamics and kinetic studies for the microwave-enhanced extraction of phenolics from Phyllanthus niruri leaves. Chem. Eng. Commun. 2022, 1–9. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Kala, H.K.; Mehta, R.; Sen, K.K.; Tandey, R.; Mandal, V. Critical analysis of research trends and issues in microwave assisted extraction of phenolics: Have we really done enough. TrAC Trends Anal. Chem. 2016, 85, 140–152. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.P.; Nguyen, N.T.U.; Le, V.H.; Phan, T.H.; Nguyen, T.H.Y.; Nguyen, D.Q. Optimizing ultrasonic-assisted and microwave-assisted extraction processes to recover phenolics and flavonoids from passion fruit peels. ACS Omega 2023, 8, 33870–33882. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Ma, Q.; Zhang, Y.; Peng, Z. Phenolic compounds with antioxidant activity from strawberry leaves: A study on microwave-assisted extraction optimization. Prep. Biochem. Biotechnol. 2020, 50, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Alhallaf, W.; Bishop, K.; Perkins, L.B. Optimization of accelerated solvent extraction of phenolic compounds from chaga using Response Surface Methodology. Food Anal. Methods 2022, 15, 2777–2790. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Doi, S.; Higashino, H.; Karatsu, A.; Masuda, A.; Masuda, T. Identification of polyphenol and reductone antioxidants in the caramelization product of N-acetylglucosamine. ACS Food Sci. Technol. 2022, 2, 1135–1140. [Google Scholar] [CrossRef]
- Terpinc, P.; Polak, T.; Šegatin, N.; Hanzlowsky, A.; Ulrih, N.P.; Abramovič, H. Antioxidant properties of 4-vinyl derivatives of hydroxycinnamic acids. Food Chem. 2011, 128, 62–69. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of phenolic antioxidants extraction from Fucus vesiculosus by pressurized liquid extraction. J. Appl. Phycol. 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Luthria, D.L. Optimization of extraction of phenolic acids from a vegetable waste product using a pressurized liquid extractor. J. Funct. Foods 2012, 4, 842–850. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar]
- Dobroslavić, E.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Polyphenolic Characterization and Antioxidant Capacity of Laurus nobilis L. Leaf Extracts Obtained by Green and Conventional Extraction Techniques. Processes 2021, 9, 1840. [Google Scholar]
- Carev, I.; Maravić, A.; Ilić, N.; Čikeš Čulić, V.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS Phytochemical Analysis of Two Croatian Cistus Species and Their Biological Activity. Life 2020, 10, 112. [Google Scholar] [CrossRef]
- Malin, V.; Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction. Processes 2022, 10, 510. [Google Scholar]
- Bursać Kovačević, D.; Gajdoš Kljusurić, J.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and Characterization of Low Molecular Weight Polyphenols in Berry Leaf Extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Hanhineva, K.; Lehtonen, M.; McDougall, G.J.; Stewart, D.; Karjalainen, R.O. Non-targeted metabolite profiling highlights the potential of strawberry leaves as a resource for specific bioactive compounds. J. Sci. Food Agric. 2017, 97, 2182–2190. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Gilbert-López, B.; Mendiola, J.A.; Quirantes-Piné, R.; Segura-Carretero, A.; Ibáñez, E. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface methodology. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Punia Bangar, S.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A flavone with myriads of bioactivities and food applications. Food Biosci. 2023, 52, 102366. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Sadeer, N.B.; Hussain, M.; Mahwish, N.; Alsagaby, S.A.; Imran, M.; Mumtaz, T.; Umar, M.; Tauseef, A.; Al Abdulmonem, W.; et al. Therapeutical properties of apigenin: A review on the experimental evidence and basic mechanisms. Int. J. Food Prop. 2023, 26, 1914–1939. [Google Scholar] [CrossRef]
- Čulina, P.; Cvitković, D.; Pfeifer, D.; Zorić, Z.; Repajić, M.; Elez Garofulić, I.; Balbino, S.; Pedisić, S. Phenolic Profile and Antioxidant Capacity of Selected Medicinal and Aromatic Plants: Diversity upon Plant Species and Extraction Technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Pérez-Jiménez, J.; Torres, J.L.; Agosin, E.; Pérez-Correa, J.R. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris). J. Agric. Food Chem. 2012, 60, 10920–10929. [Google Scholar] [CrossRef]
- Badwaik, L.S.; Borah, P.K.; Deka, S.C. Optimization of microwave assisted extraction of antioxidant extract from Garcinia pedunculata Robx. Sep. Sci. Technol. 2015, 50, 1814–1822. [Google Scholar] [CrossRef]
- Cha, K.H.; Kang, S.W.; Kim, C.Y.; Um, B.H.; Na, Y.R.; Pan, C.-H. Effect of pressurized liquids on extraction of antioxidants from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 4756–4761. [Google Scholar] [CrossRef]
- Koroleva, O.; Torkova, A.; Nikolaev, I.; Khrameeva, E.; Fedorova, T.; Tsentalovich, M.; Amarowicz, R. Evaluation of the antiradical properties of phenolic acids. Int. J. Mol. Sci. 2014, 15, 16351–16380. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Dragović-Uzelac, V.; Režek Jambrak, A.; Jukić, M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). J. Food Eng. 2013, 117, 437–442. [Google Scholar] [CrossRef]
- Tang, Y.-Z.; Liu, Z.-Q. Free-radical-scavenging effect of carbazole derivatives on DPPH and ABTS Radicals. J. Am. Oil Chem. Soc. 2007, 84, 1095–1100. [Google Scholar] [CrossRef]
- Marković, S.; Tošović, J. Comparative study of the antioxidative activities of caffeoylquinic and caffeic acids. Food Chem. 2016, 210, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the conditions for determination of antioxidant activity by ABTS and DPPH assays— A practical approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Anouar, E.; Marzouk, M.; Taie, H.A.A.; Ahudhaif, A.; Al-Salahi, R. DFT study and radical scavenging activity of 2-phenoxypyridotriazolo pyrimidines by DPPH, ABTS, FRAP and reducing power capacity. Chem. Pap. 2020, 74, 2893–2899. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Maximising recovery of phenolic compounds and antioxidant properties from banana peel using microwave assisted extraction and water. J. Food Sci. Technol. 2019, 56, 1360–1370. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids—Ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]


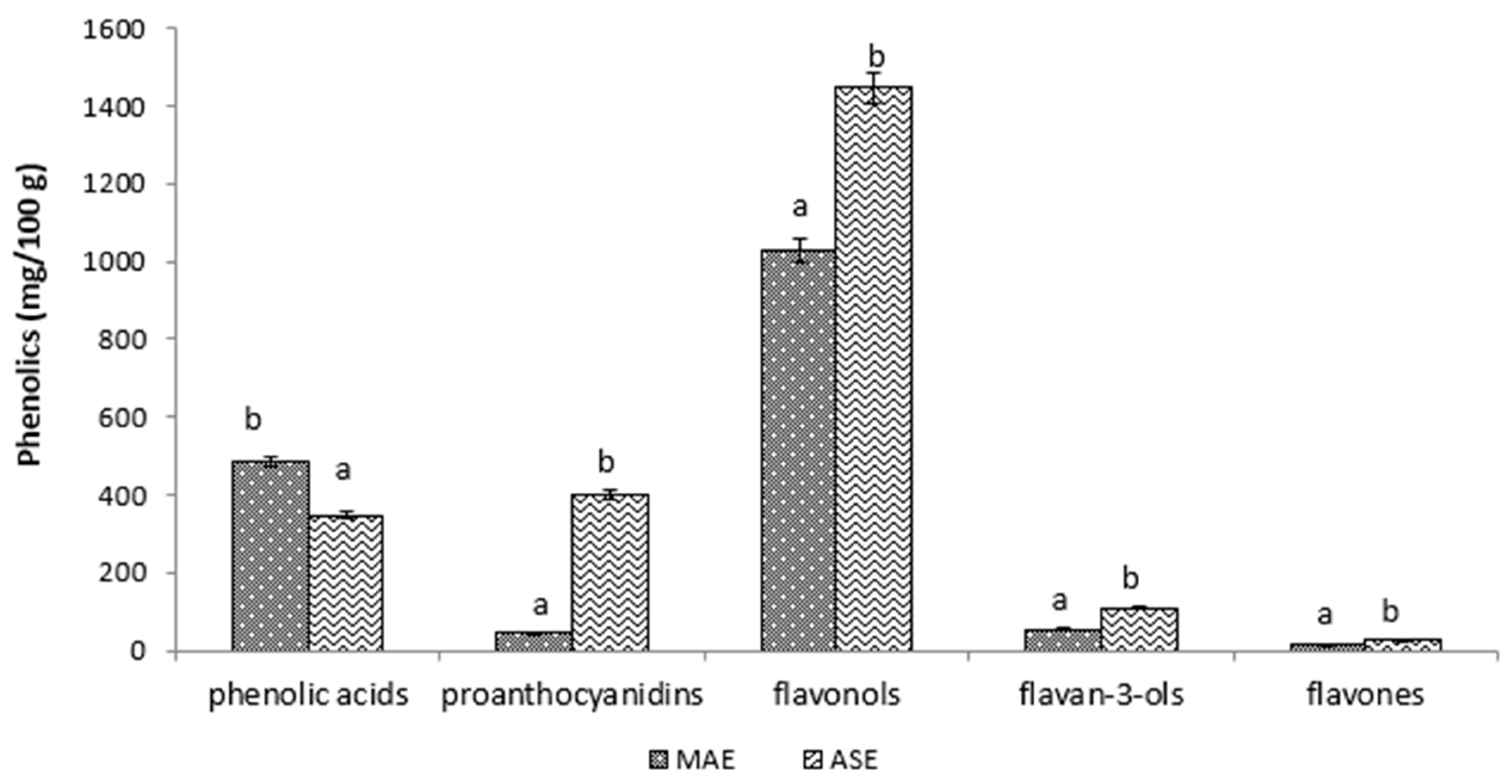
| No. | Compound Name | Retention Time (min) | m/z | m/z (Prod.) | MAE mg/100 g | ASE mg/100 g |
|---|---|---|---|---|---|---|
| phenolic acids | ||||||
| 1 | ferulic acid * | 1.937 | 193 | 178 | 5.13 ± 015 a | 6.07 ± 0.17 b |
| 2 | 3-p-caffeoylquinic acid | 2.906 | 337 | 163 | 3.64 ± 0.10 b | 2.90 ± 0.08 a |
| 3 | rosmarinic acid * | 3.138 | 359.1 | 161 | 16.74 ± 0.47 a | 16.82± 0.48 a |
| 5 | chlorogenic acid * | 4.615 | 353 | 191 | 43.57 ± 1.23 a | 40.06 ± 1.13 a |
| 8 | 3,5-di-caffeoylquinic acid | 5.573 | 515 | 173 | 0.83 ± 0.02 b | 0.76 ± 0.02 a |
| 9 | 4,5-di-caffeoylquinic acid | 5.573 | 515 | 353 | 1.12 ± 0.03 a | 1.13± 0.03 a |
| 12 | syringic acid * | 6.354 | 197 | 182 | 7.39 ± 0.21 a | 19.42± 0.55 b |
| 13 | caffeic acid * | 6.368 | 179 | 135 | 8.44 ± 0.24 b | 6.91± 0.20 a |
| 17 | 4-O-caffeoylquinic acid | 7.821 | 324 | 173 | 0.15 ± 0.00 b | 0.07± 0.00 a |
| 25 | 5-O-galloylquinic acid | 9.775 | 343 | 191 | 61.38 ± 1.74 a | 62.22± 1.76 a |
| 28 | 3-O-ferruylquinic acid | 11.238 | 367 | 193 | 1.77 ± 0.05 a | 1.42± 0.04 a |
| 39 | gallic acid * | 11.528 | 169 | 125 | 156.96 ± 4.44 b | 39.84± 1.13 a |
| 40 | p-hydroxybenzoic acid | 11.538 | 137 | 93 | 172.41 ± 4.88 b | 141.78 ± 4.01 a |
| 53 | 3,5-Digalloylquinic acid | 11.968 | 495 | 343 | 5.19 ± 0.15 a | 6.50 ± 0.18 b |
| proanthocyanidins | ||||||
| 4 | procyanidin trimer | 3.438 | 865 | 575 | 1.84 ± 0.05 a | 43.71± 1.24 b |
| 10 | procyanidin B2 * | 5.815 | 577 | 289 | 0.92 ± 0.03 a | 21.84± 0.62 b |
| 30 | procyanidin B1 | 11.351 | 579 | 291 | 44.73 ± 1.27 a | 332.26 ± 9.40 b |
| flavonols | ||||||
| 6 | isorhamnetin-3-rhamnoside | 5.178 | 625 | 317 | 1.13 ± 0.03 a | 1.72 ± 0.05 b |
| 7 | isorhamnetin-3-hexoside | 5.232 | 479 | 317 | 7.84 ± 0.22 b | 2.06 ± 0.06 a |
| 11 | kaempferol-3-O-hexoside | 6.252 | 449 | 287 | 5.36 ± 0.15 a | 5.69 ± 0.16 a |
| 15 | myricetin * | 7.258 | 319 | 273 | 48.33 ± 1.37 b | 33.19 ± 0.94 a |
| 16 | quercetin-3-glucuronide | 7.442 | 479 | 303 | 129.31 ± 3.66 a | 268.97 ± 7.61 b |
| 18 | kaempferol-3-glucuronide | 8.192 | 463 | 287 | 39.79 ± 1.13 a | 70.47 ± 1.99 b |
| 19 | quercetin-3-rhamnoside | 8.213 | 449 | 303 | 2.30 ± 0.06 a | 3.97 ± 0.11 b |
| 21 | kaempferol-3-O-deoxyhexoside | 8.475 | 433 | 286 | 1.40 ± 0.04 a | 1.93 ± 0.05 a |
| 23 | quercetin-3-pentoside | 9.700 | 435 | 303 | 1.68 ± 0.05 a | 11.81 ± 0.33 b |
| 26 | myricetin-3-O-rhamnoside | 9.905 | 465 | 319 | 6.13 ± 0.17 a | 43.71 ± 1.24 b |
| 27 | kaempferol-3-O-pentoside | 10.689 | 419 | 287 | 3.08 ± 0.09 b | 0.91 ± 0.03 a |
| 29 | kaempferol-pentosyl-hexoside | 11.344 | 581 | 287 | 0.25 ± 0.01 a | 0.77 ± 0.02 b |
| 31 | quercetin-acetyl-hexoside | 11.357 | 507 | 303 | 1.41 ± 0.04 a | 2.55 ± 0.07 b |
| 32 | kaempferol-acetyl-hexoside | 11.361 | 491 | 287 | 1.22 ± 0.03 b | 0.34 ± 0.01 a |
| 33 | isorhamnetin-3-O-glucoside | 11.364 | 483 | 317 | 1.41 ± 0.04 b | 0.83 ± 0.02 a |
| 34 | myricetin-3-O-galactoside | 11.368 | 481 | 319 | 68.99 ± 1.95 b | 52.33 ± 1.48 a |
| 35 | quercetin-3-glucoside * | 11.381 | 465 | 303.1 | 11.45 ± 0.32 a | 37.48 ± 1.06 b |
| 37 | myricetin-3-O-arabinoside | 11.395 | 451 | 319 | 8.37 ± 0.24 b | 4.36 ± 0.12 a |
| 41 | quercetin-acetyl-rutinoside | 11.552 | 653 | 303 | 0.28 ± 0.01 a | 1.29 ± 0.04 b |
| 42 | kaempferol-acetyl-rutinoside | 11.556 | 637 | 287 | 0.12 ± 0.00 a | 0.54 ± 0.01 b |
| 43 | quercetin-3-O-dihexoside | 11.559 | 627 | 303 | 2.74 ± 0.08 b | 1.97 ± 0.06 a |
| 44 | rutin * | 11.566 | 611 | 303 | 44.31 ± 1.25 a | 116.04 ± 3.28 b |
| 45 | isorhamnetin-pentosylhexoside | 11.566 | 611 | 317 | 1.23 ± 0.03 b | 0.43 ± 0.01 a |
| 46 | quercetin-3-O-vicianoside | 11.576 | 597 | 434 | 2.77 ± 0.08 b | 1.82 ± 0.05 a |
| 47 | kaempferol-3-rutinoside * | 11.586 | 595 | 287 | 3.56 ± 0.10 a | 7.54 ± 0.21 b |
| 49 | quercetin | 11.681 | 303 | 303 | 336.35 ± 9.51 a | 472.63 ± 13.34 b |
| 50 | kaempferol | 11.698 | 287 | 287 | 296.57 ± 8.39 a | 298.96 ± 8.46 a |
| 51 | quercetin-pentosylhexoside | 11.825 | 597 | 303 | 1.42 ± 0.04 a | 2.67 ± 0.08 b |
| flavan-3-ols | ||||||
| 24 | epicatechin | 9.727 | 291 | 139 | 45.42 ± 1.28 a | 100.29 ± 2.84 b |
| 36 | epigallocatechin gallate * | 11.388 | 459 | 289 | 5.41 ± 0.15 a | 5.48 ± 0.15 a |
| 54 | epicatechin gallate * | 12.149 | 442.9 | 273 | 3.53 ± 0.10 b | 2.26 ± 0.06 a |
| flavones | ||||||
| 14 | luteolin-6-C-glucoside | 6.978 | 449 | 359 | 2.73 ± 0.08 b | 0.80 ± 0.02 a |
| 20 | luteolin * | 8.264 | 287 | 153 | 9.82 ± 0.28 a | 17.75 ± 0.50 b |
| 22 | apigenin * | 8.758 | 271 | 153 | 0.34 ± 0.01 a | 0.56 ± 0.02 b |
| 38 | apigenin pentoside | 11.429 | 403 | 271 | 0.399 ± 0.01 a | 0.55 ± 0.02 b |
| 48 | apigenin-6-C-(O-deoxyhexosyl)-hexoside | 11.593 | 579 | 459 | 0.24 ± 0.01 a | 0.57 ± 0.02 b |
| 52 | luteolin-7-O-rutinoside | 11.828 | 595 | 287 | 3.45 ± 0.109 a | 7.51 ± 0.21 b |
| total phenolics | 1632.32 ± 26.17 a | 2326.42 ± 65.80 b | ||||
| MAE | T (°C) | t (min) | R (mL/g) | ABTS (μmol TE/g) | DPPH (μmol TE/g) | FRAP (μmol TE/g) |
|---|---|---|---|---|---|---|
| T60-t5-r20 | 60 | 5 | 20 | 350 ± 12 ab | 444 ± 5 ab | 458 ± 29 abcd |
| T60-t5-r30 | 60 | 5 | 30 | 368 ± 51 abc | 539 ± 33 abc | 536 ± 63 abcd |
| T60-t5-r40 | 60 | 5 | 40 | 433 ± 12 abc | 661 ± 32 bc | 604 ± 79 abcd |
| T60-t10-r20 | 60 | 10 | 20 | 312 ± 8 a | 434 ± 14 ab | 429 ± 12 abc |
| T60-t10-r30 | 60 | 10 | 30 | 466 ± 5 abc | 557 ± 20 abc | 515 ± 49 abcd |
| T60-t10-r40 | 60 | 10 | 40 | 466 ± 42 abc | 669 ± 14 bc | 541 ± 25 abcd |
| T70-t5-r20 | 70 | 5 | 20 | 392 ± 77 abc | 477 ± 28 abc | 427 ± 31 ab |
| T70-t5-r30 | 70 | 5 | 30 | 371 ± 24 abc | 545 ± 1 abc | 360 ± 20 a |
| T70-t5-r40 | 70 | 5 | 40 | 442 ± 47 abc | 711 ± 20 c | 510 ± 73 abcd |
| T70-t10-r20 | 70 | 10 | 20 | 347 ± 19 ab | 470 ± 12 abc | 402 ± 36 ab |
| T70-t10-r30 | 70 | 10 | 30 | 444 ± 43 abc | 617 ± 8 abc | 515 ± 86 abcd |
| T70-t10-r40 | 70 | 10 | 40 | 440 ± 12 abc | 697 ± 3 c | 442 ± 12 abcd |
| T80-t5-r20 | 80 | 5 | 20 | 471 ±31 abc | 352 ± 2 a | 1834 ± 76 abcd |
| T80-t5-r30 | 80 | 5 | 30 | 517 ± 28 bc | 475 ± 7 abc | 2118 ± 34 bcd |
| T80-t5-r40 | 80 | 5 | 40 | 681 ± 11 c | 583 ± 45 abc | 2389 ± 175 cd |
| T80-t10-r20 | 80 | 10 | 20 | 413 ± 29 abc | 345 ± 23 a | 1666 ± 55 abcd |
| T80-t10-r30 | 80 | 10 | 30 | 584 ± 32 bc | 457 ± 1 abc | 2082 ± 143 bcd |
| T80-t10-r40 | 80 | 10 | 40 | 683 ± 73 c | 580 ± 3 abc | 2461 ± 89 d |
| ASE | T (°C) | t (min) | R (mL/g) | ABTS (μmol TE/g) | DPPH (μmol TE/g) | FRAP (μmol TE/g) |
|---|---|---|---|---|---|---|
| T100-t5-r20 | 100 | 5 | 20 | 503 ± 13 abcd | 514 ± 3 ab | 758 ± 26 a |
| T100-t5-r30 | 100 | 5 | 30 | 483 ± 16 abc | 688 ± 2 abcd | 809 ± 5 abc |
| T100-t5-r40 | 100 | 5 | 40 | 509 ± 35 abcd | 717 ± 8 abcd | 742 ± 69 a |
| T100-t10-r20 | 100 | 10 | 20 | 508 ± 7 abcd | 515 ± 3 abc | 757 ± 8 a |
| T100-t10-r30 | 100 | 10 | 30 | 575 ± 8 abcd | 734 ± 1 abcd | 953 ± 21 abc |
| T100-t10-r40 | 100 | 10 | 40 | 496 ± 10 abcd | 757 ± 4 abcd | 1057 ± 37 c |
| T125-t5-r20 | 125 | 5 | 20 | 581 ± 4 abcd | 514 ± 4 ab | 860 ± 14 abc |
| T125-t5-r30 | 125 | 5 | 30 | 602 ± 23 bcd | 742 ± 4 abcd | 1045 ± 10 bc |
| T125-t5-r40 | 125 | 5 | 40 | 627 ± 14 cd | 839 ± 5 abcd | 786 ± 19 ab |
| T125-t10-r20 | 125 | 10 | 20 | 581 ± 5 abcd | 514 ± 0 ab | 966 ± 3 abc |
| T125-t10-r30 | 125 | 10 | 30 | 595 ± 8 bcd | 756 ± 4 abcd | 853 ± 18 abc |
| T125-t10-r40 | 125 | 10 | 40 | 605 ± 13 cd | 843 ± 2 bcd | 896 ± 23 abc |
| T150-t5-r20 | 150 | 5 | 20 | 540 ± 6 abcd | 512 ± 2 a | 865 ± 19 abc |
| T150-t5-r30 | 150 | 5 | 30 | 595 ± 7 bcd | 754 ± 3 abcd | 959 ± 15 abc |
| T150-t5-r40 | 150 | 5 | 40 | 595 ± 4 bcd | 903 ± 3 d | 1086 ± 15 c |
| T150-t10-r20 | 150 | 10 | 20 | 450 ± 5 ab | 514 ± 1 abc | 893 ± 9 abc |
| T150-t10-r30 | 150 | 10 | 30 | 442 ± 7 a | 706 ± 3 abcd | 928 ± 13 abc |
| T150-t10-r40 | 150 | 10 | 40 | 504 ± 9 abcd | 889 ± 3 cd | 911 ± 14 abc |
| TPC | ABTS | DPPH | FRAP | |
|---|---|---|---|---|
| TPC | 0.894 ** | 0.261 * | 0.790 ** | |
| ABTS | 0.627 ** | 0.21 | 0.798 ** | |
| DPPH | 0.311 ** | 0.340 ** | −0.297 * | |
| FRAP | 0.524 ** | 0.21 | 0.373 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpinc, P.; Dobroslavić, E.; Garofulić, I.E.; Repajić, M.; Cegledi, E.; Dobrinčić, A.; Pedisić, S.; Levaj, B. Maximizing the Recovery of Phenolic Antioxidants from Wild Strawberry (Fragaria vesca) Leaves Using Microwave-Assisted Extraction and Accelerated Solvent Extraction. Processes 2023, 11, 3378. https://doi.org/10.3390/pr11123378
Terpinc P, Dobroslavić E, Garofulić IE, Repajić M, Cegledi E, Dobrinčić A, Pedisić S, Levaj B. Maximizing the Recovery of Phenolic Antioxidants from Wild Strawberry (Fragaria vesca) Leaves Using Microwave-Assisted Extraction and Accelerated Solvent Extraction. Processes. 2023; 11(12):3378. https://doi.org/10.3390/pr11123378
Chicago/Turabian StyleTerpinc, Petra, Erika Dobroslavić, Ivona Elez Garofulić, Maja Repajić, Ena Cegledi, Ana Dobrinčić, Sandra Pedisić, and Branka Levaj. 2023. "Maximizing the Recovery of Phenolic Antioxidants from Wild Strawberry (Fragaria vesca) Leaves Using Microwave-Assisted Extraction and Accelerated Solvent Extraction" Processes 11, no. 12: 3378. https://doi.org/10.3390/pr11123378











