Production of Mannooligosaccharides from Açaí Seed by Immobilized β-Mannanase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Pretreatment
2.3. Enzymatic Immobilization
2.4. Enzymatic Activity Assay
2.5. Characterization of the Enzymatic System
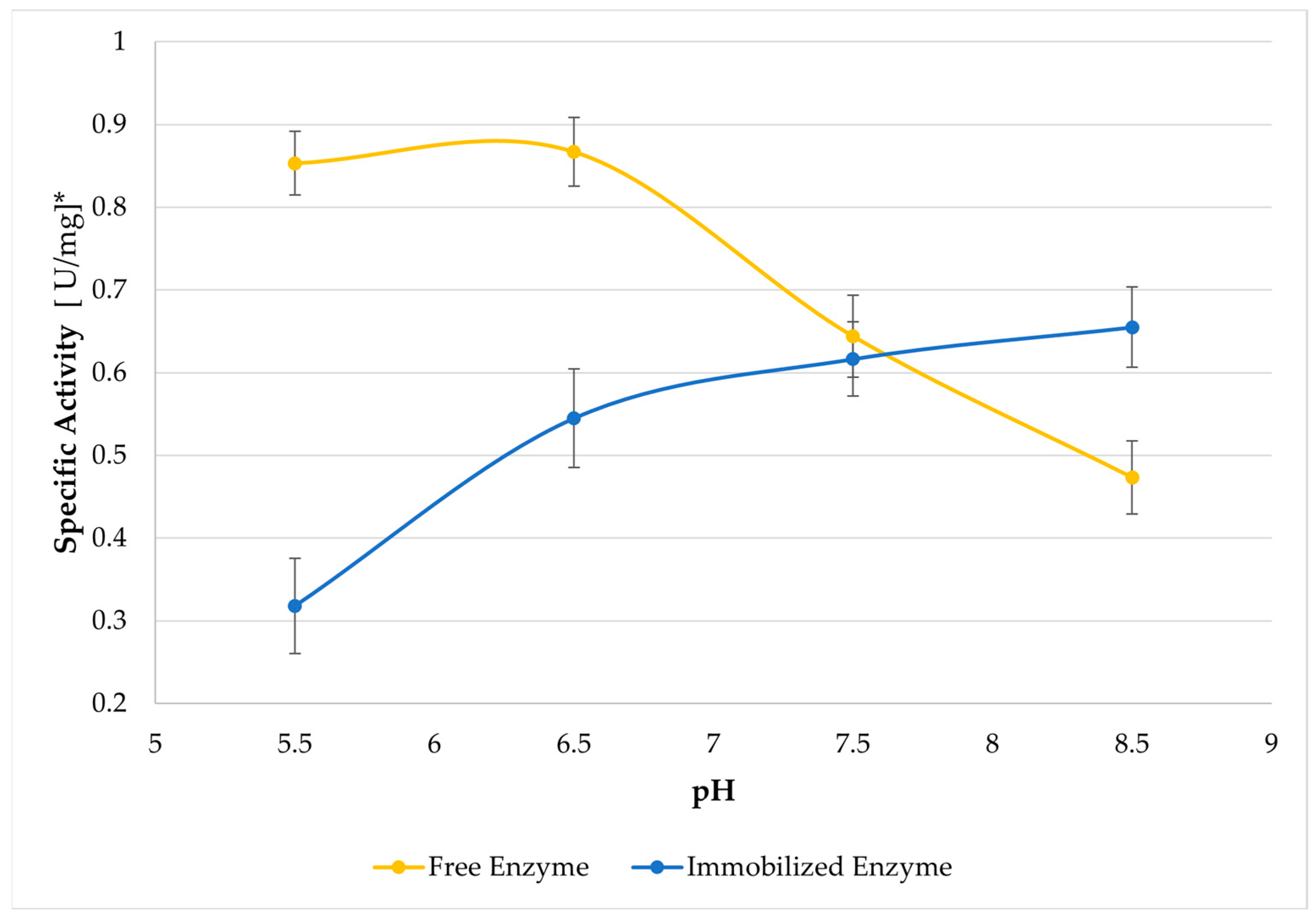
2.5.1. Effect of pH on Enzymatic Activity
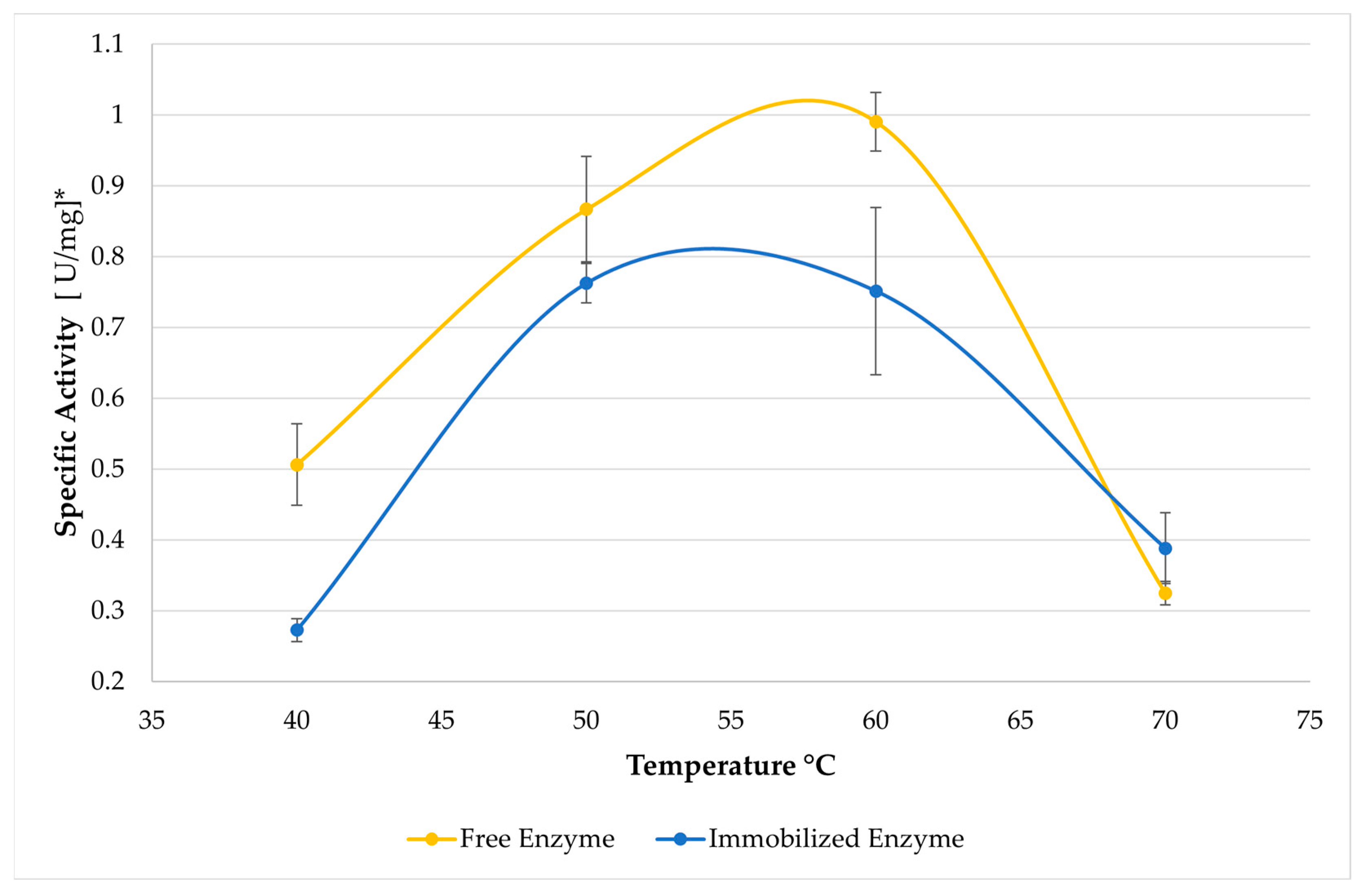
2.5.2. Effect of Temperature on Enzymatic Activity
2.5.3. Operational Stability
2.5.4. Scanning Electron Microscopy (SEM)
2.5.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.6. Measurement of Mechanical Strength
2.5.7. Heterogeneous Enzymatic Hydrolysis for MOS Production from Açaí Seeds Using Response Surface Methodology
2.5.8. Experimental Design and Procedure
2.5.9. Enzymatic Hydrolysis for MOS Production
2.5.10. Determination of MOS and Mannose
2.5.11. Statistical Analysis
3. Results and Discussion
3.1. Immobilization of the Enzymatic Cocktail in CF
3.2. Characterization of the Enzymatic System
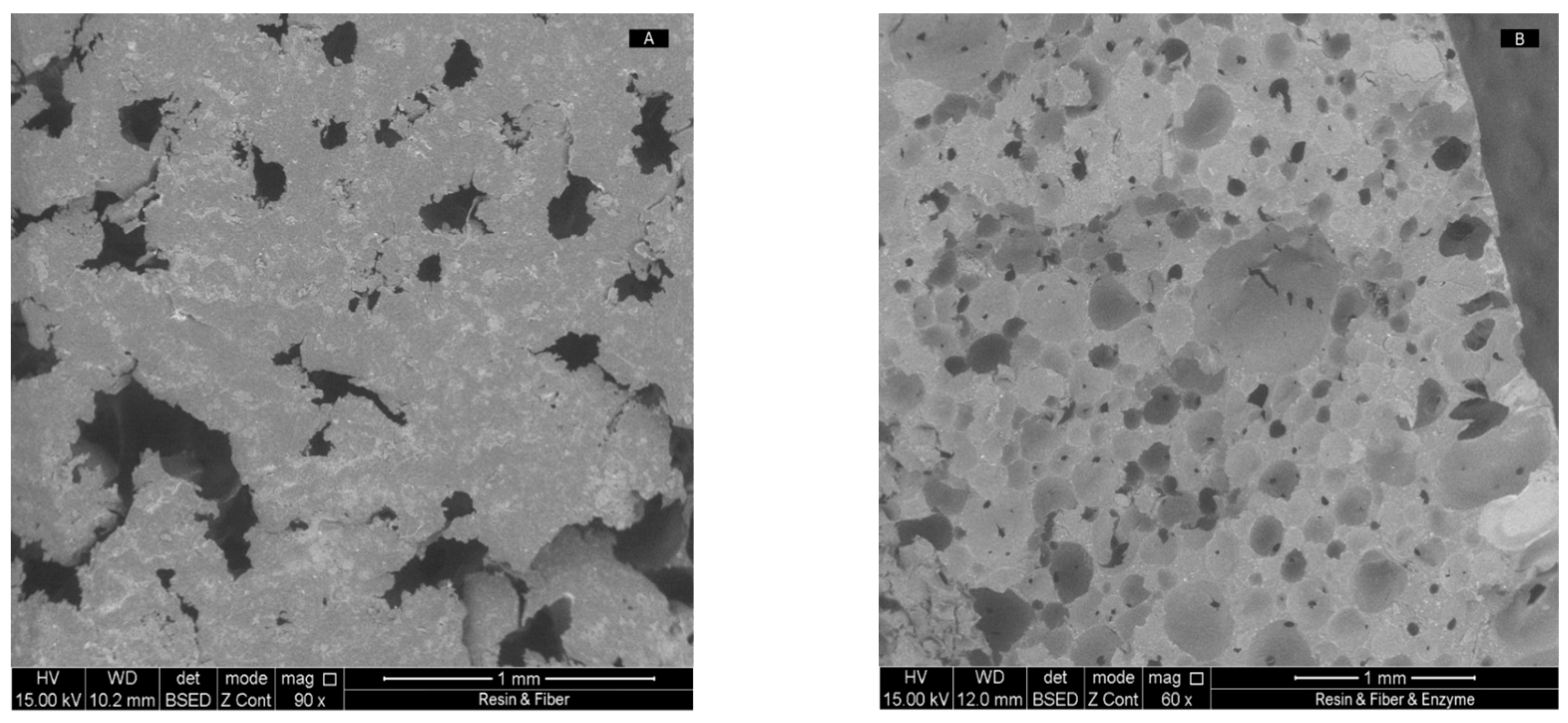
3.2.1. SEM
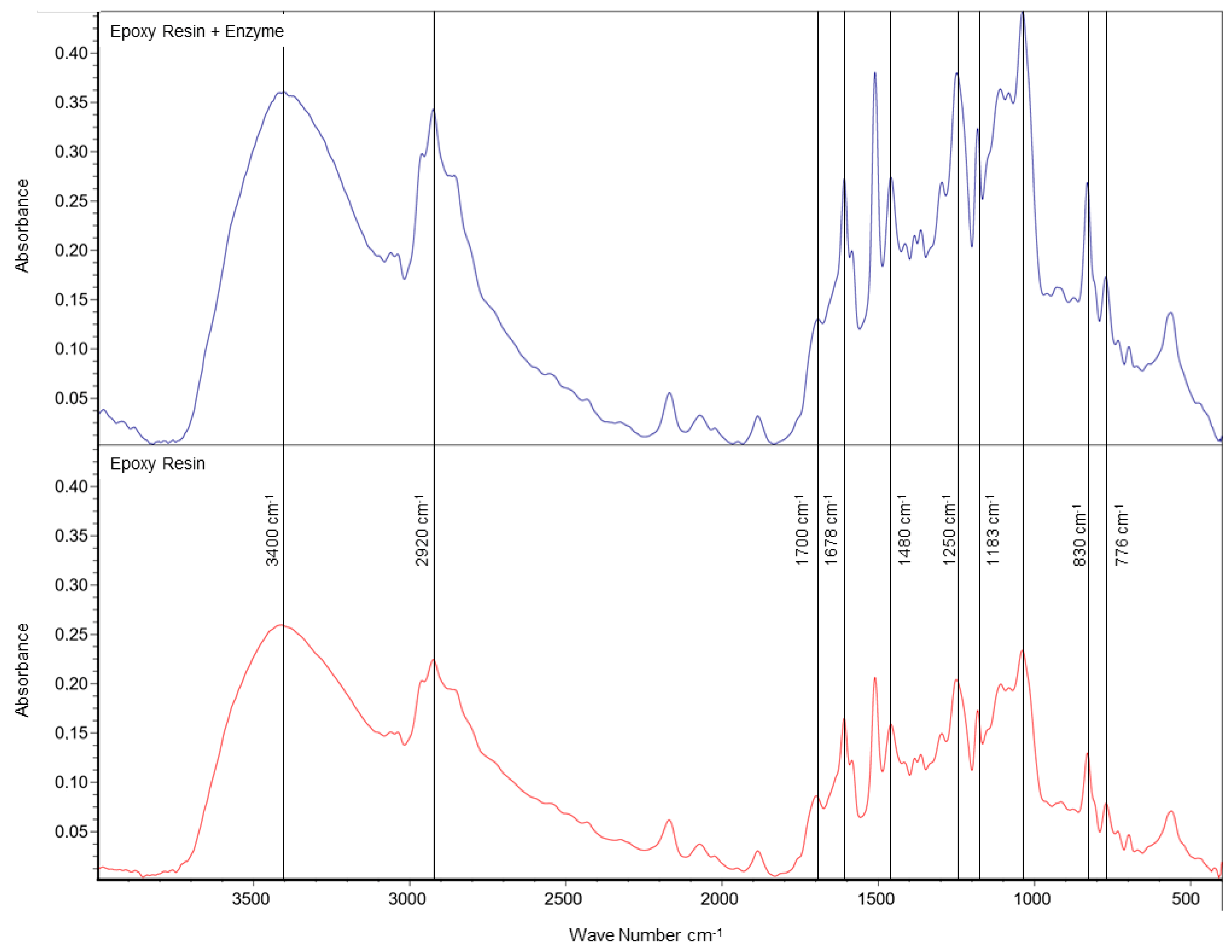
3.2.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.2.3. Mechanical Strength of the Catalyst
3.2.4. Effect of pH
3.2.5. Effect of Temperature
3.2.6. Operational Stability
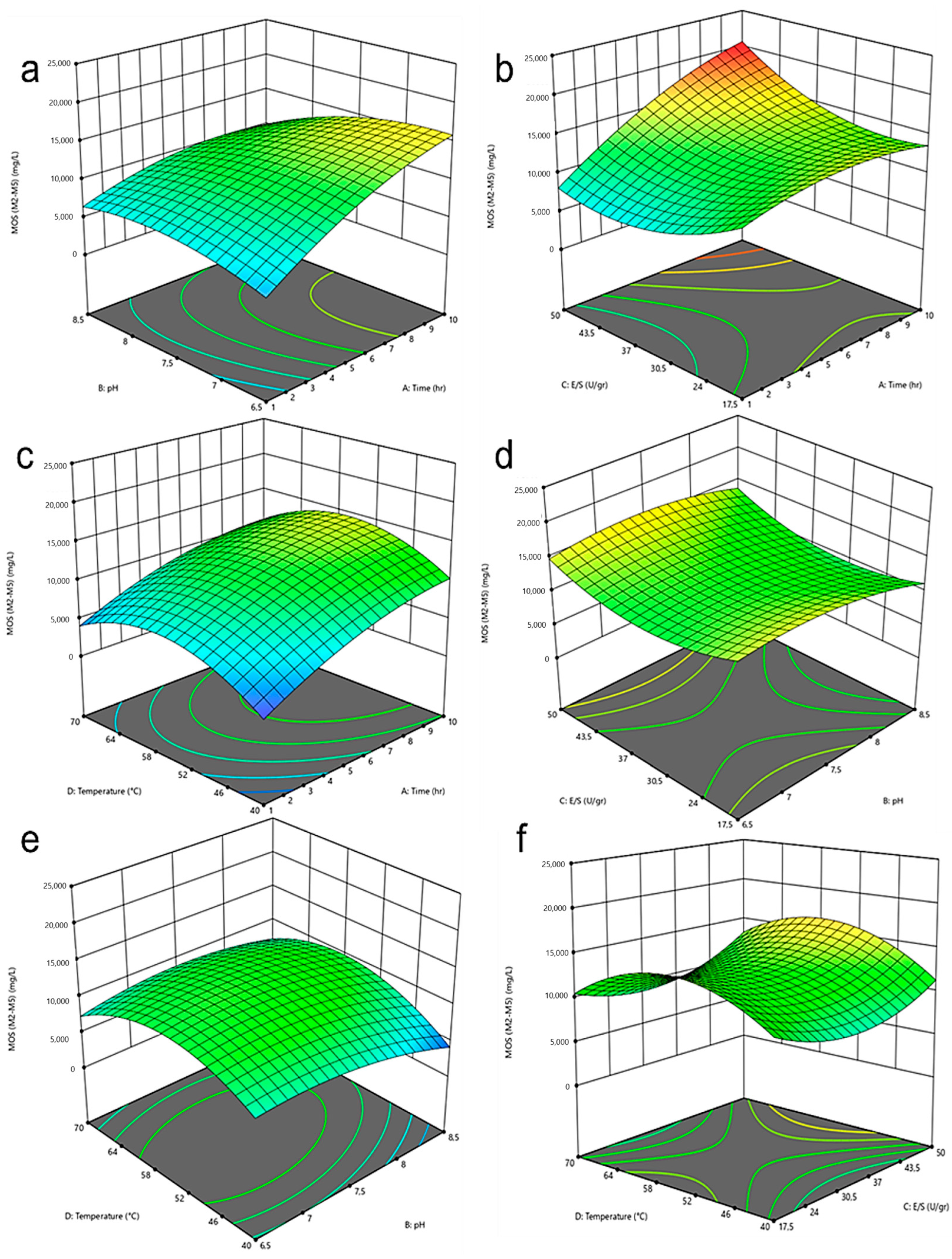
3.3. Analysis of MOS Production through Response Surface Experimental Design
3.3.1. Modeling and Influence of Design Parameters on MOS Production Using an Immobilized Enzymatic System
3.3.2. Model Verification and Optimal Reaction Conditions
4. Conclusions
5. Recommendation and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Chromatogram of Refractive Index for Mannose and MOS (M2–M5) in HPLC Analysis

Appendix A.2. Analysis of Variance (ANOVA) for Quadratic Models for Response Variables
Appendix A.2.1. Response 1: Mannose
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.54 × 107 | 14 | 1.10 × 106 | 9.22 | <0.0001 |
| A-Time | 9.90 × 105 | 1 | 9.90 × 105 | 8.31 | 0.0121 |
| B-pH | 4.56 × 106 | 1 | 4.56 × 106 | 38.25 | <0.0001 |
| C-Enzyme Load | 7.40 × 105 | 1 | 7.40 × 105 | 6.21 | 0.0259 |
| D-Temperature | 1.06 × 105 | 1 | 1.06 × 105 | 0.893 | 0.3607 |
| AB | 2.65 × 106 | 1 | 2.65 × 106 | 22.21 | 0.0003 |
| AC | 1.74 × 104 | 1 | 1.74 × 104 | 0.1464 | 0.7078 |
| AD | 8.24 × 105 | 1 | 8.24 × 105 | 6.92 | 0.0198 |
| BC | 7.49 × 105 | 1 | 7.49 × 105 | 6.29 | 0.0251 |
| BD | 7.63 × 104 | 1 | 7.63 × 104 | 0.6408 | 0.4368 |
| CD | 1.02 × 106 | 1 | 1.02 × 106 | 8.54 | 0.0111 |
| A2 | 5.73 × 102 | 1 | 5.73 × 102 | 0.0048 | 0.9457 |
| B2 | 1.80 × 106 | 1 | 1.80 × 106 | 15.1 | 0.0016 |
| C2 | 1.13 × 106 | 1 | 1.13 × 106 | 9.52 | 0.0081 |
| D2 | 2.66 × 105 | 1 | 2.66 × 105 | 2.23 | 0.1577 |
| Residual | 1.67 × 106 | 14 | 1.19 × 105 | ||
| Lack of Fit | 5.23 × 105 | 6 | 8.71 × 104 | 0.609 | 0.7189 |
| Pure Error | 1.15 × 106 | 8 | 1.43 × 105 | ||
| Cor Total | 1.71 × 107 | 28 |
Appendix A.2.2. Response 2: MOS (M2–M5)
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5.23 × 108 | 14 | 3.74 × 107 | 14.31 | <0.0001 |
| A-Time | 1.64 × 108 | 1 | 1.64 × 108 | 62.62 | <0.0001 |
| B-pH | 1.83 × 107 | 1 | 1.83 × 107 | 7 | 0.0192 |
| C-Enzyme Load | 5.74 × 106 | 1 | 5.74 × 106 | 2.2 | 0.1604 |
| D-Temperature | 4.05 × 106 | 1 | 4.05 × 106 | 1.55 | 0.2333 |
| AB | 3.24 × 107 | 1 | 3.24 × 107 | 12.39 | 0.0034 |
| AC | 6.29 × 107 | 1 | 6.29 × 107 | 24.09 | 0.0002 |
| AD | 1.02 × 106 | 1 | 1.02 × 106 | 0.391 | 0.5419 |
| BC | 1.86 × 106 | 1 | 1.86 × 106 | 0.7114 | 0.4132 |
| BD | 8.22 × 106 | 1 | 8.22 × 106 | 3.15 | 0.0977 |
| CD | 2.77 × 106 | 1 | 2.77 × 106 | 1.06 | 0.3209 |
| A2 | 1.31 × 107 | 1 | 1.31 × 107 | 5.03 | 0.0416 |
| B2 | 1.89 × 107 | 1 | 1.89 × 107 | 7.25 | 0.0175 |
| C2 | 4.04 × 107 | 1 | 4.04 × 107 | 15.49 | 0.0015 |
| D2 | 8.86 × 107 | 1 | 8.86 × 107 | 33.94 | <0.0001 |
| Residual | 3.66 × 107 | 14 | 2.61 × 106 | ||
| Lack of Fit | 2.22 × 107 | 6 | 3.70 × 106 | 2.06 | 0.1701 |
| Pure Error | 1.44 × 107 | 8 | 1.80 × 106 | ||
| Cor Total | 5.60 × 108 | 28 |
Appendix A.3. Diagnostic Plots for the Quadratic Models
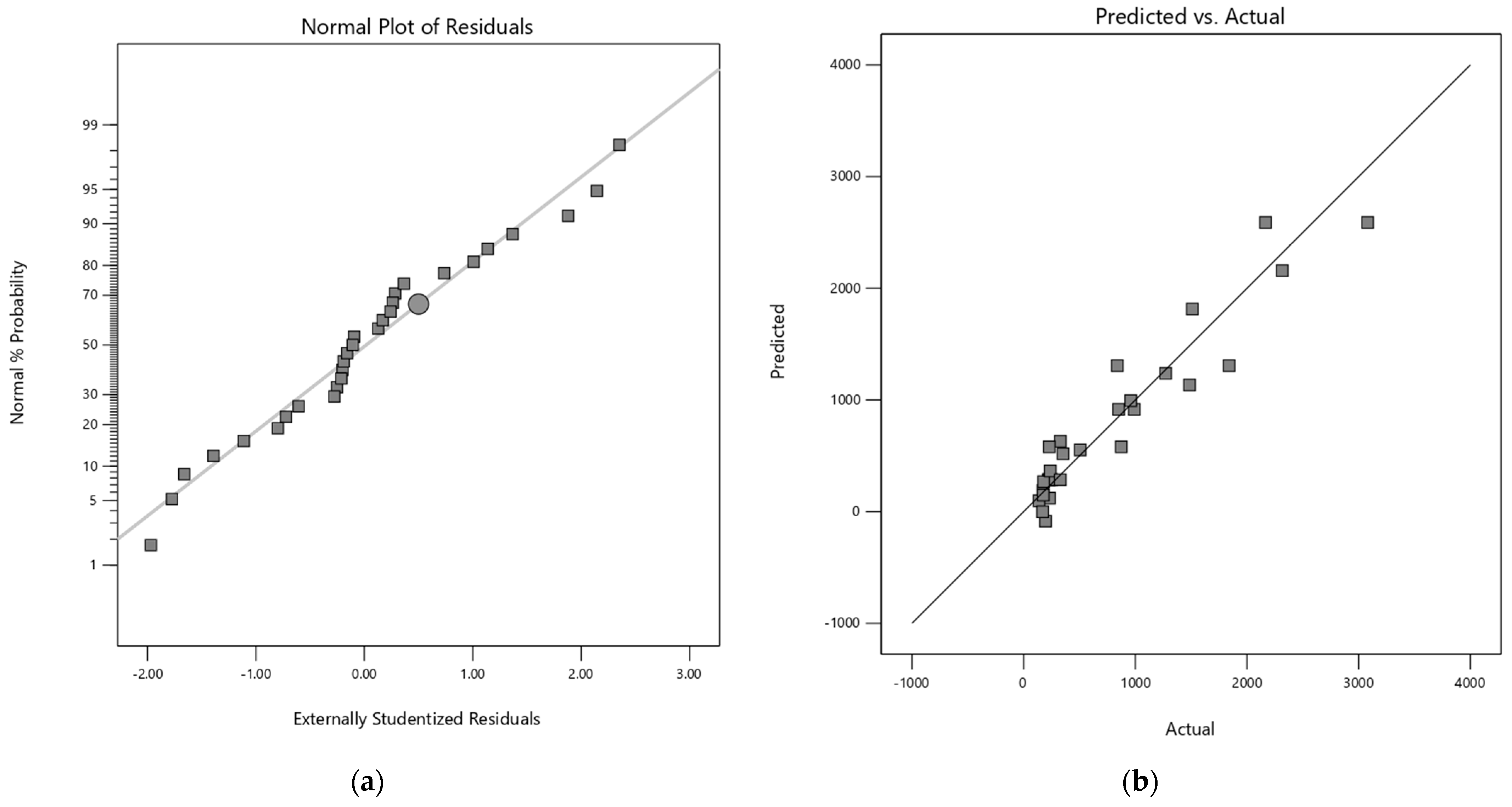
Appendix A.3.1. Response 1: Mannose
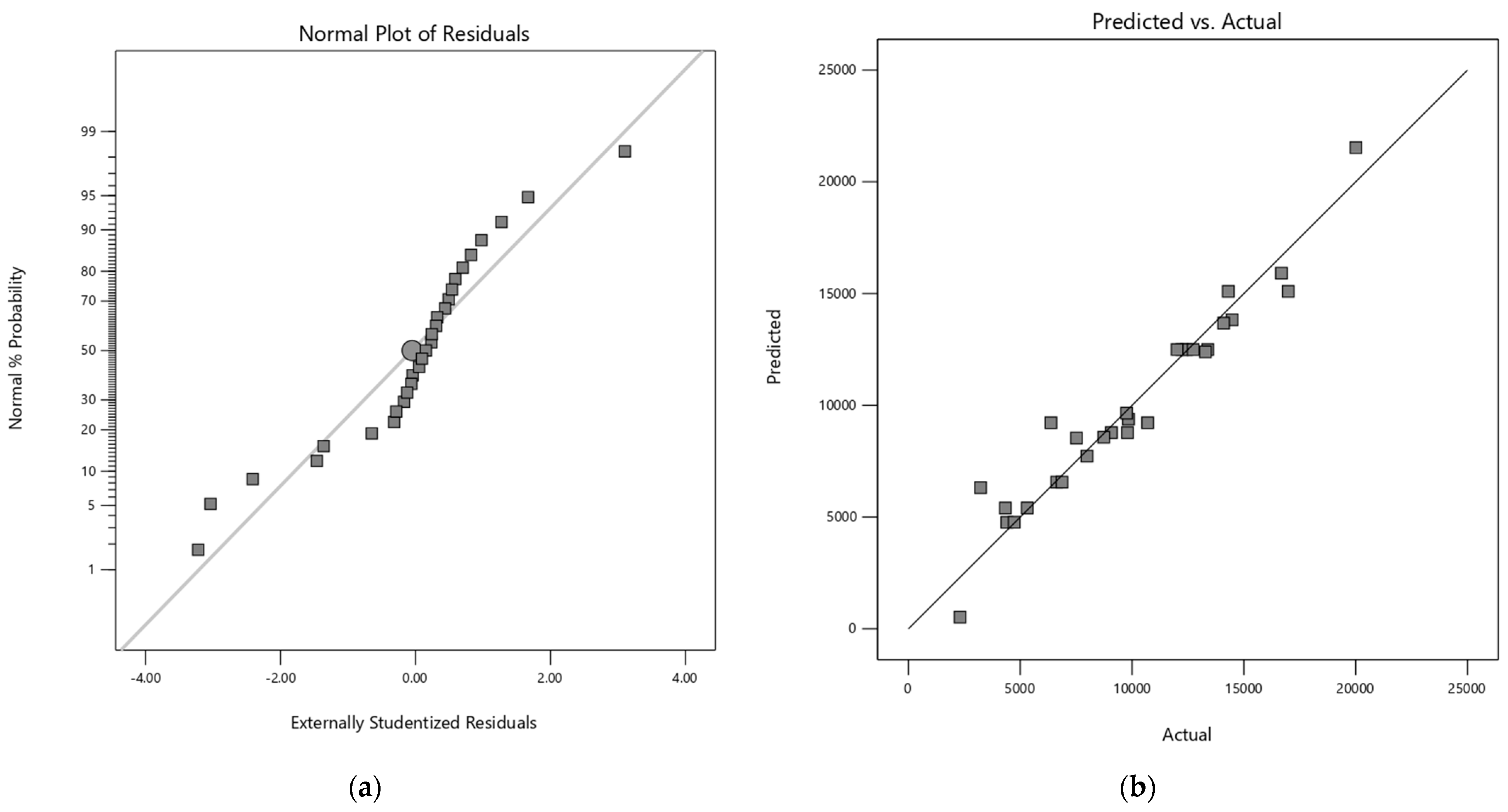
Appendix A.3.2. Response 2: MOS (M2–M5)
References
- de Lima Yamaguchi, K.K.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon Acai: Chemistry and Biological Activities: A Review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Pessoa, J.D.C.; Arduin, M.; Martins, M.A.; de Carvalho, J.E.U. Characterization of Açaí (E. oleracea) Fruits and Its Processing Residues. Braz. Arch. Biol. Technol. 2010, 53, 1451–1460. [Google Scholar] [CrossRef]
- Melo, P.S.; Selani, M.M.; Gonçalves, R.H.; de Oliveira Paulino, J.; Massarioli, A.P.; de Alencar, S.M. Açaí Seeds: An Unexplored Agro-Industrial Residue as a Potential Source of Lipids, Fibers, and Antioxidant Phenolic Compounds. Ind. Crops Prod. 2021, 161, 113204. [Google Scholar] [CrossRef]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Pedroso, A.J.S.; de Freitas Maia, C.M.B. Biochar from Acai Agroindustry Waste: Study of Pyrolysis Conditions. Waste Manag. 2019, 96, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Maciel-Silva, F.W.; Mussatto, S.I.; Forster-Carneiro, T. Integration of Subcritical Water Pretreatment and Anaerobic Digestion Technologies for Valorization of Açai Processing Industries Residues. J. Clean. Prod. 2019, 228, 1131–1142. [Google Scholar] [CrossRef]
- Murillo-Franco, S.L.; Galvis-Nieto, J.D.; Orrego, C.E. Physicochemical Characterization of Açaí Seeds (Euterpe oleracea) from Colombian Pacific and Their Potential of Mannan-Oligosaccharides and Sugar Production via Enzymatic Hydrolysis. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Buratto, R.T.; Cocero, M.J.; Martín, Á. Characterization of Industrial Açaí Pulp Residues and Valorization by Microwave-Assisted Extraction. Chem. Eng. Process.-Process Intensif. 2021, 160, 108269. [Google Scholar] [CrossRef]
- Monteiro, A.F.; Miguez, I.S.; Silva, J.P.R.B.; da Silva, A.S. High Concentration and Yield Production of Mannose from Açaí (Euterpe oleracea Mart.) Seeds via Mannanase-Catalyzed Hydrolysis. Sci. Rep. 2019, 9, 10939. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Mkabayi, L.; Malgas, S.; Kango, N.; Pletschke, B.I. Covalent Immobilisation of an Aspergillus Niger Derived Endo-1,4-β-Mannanase, Man26A, on Glutaraldehyde-Activated Chitosan Nanoparticles for the Effective Production of Prebiotic MOS from Soybean Meal. Agronomy 2022, 12, 2993. [Google Scholar] [CrossRef]
- Kango, N.; Jana, U.K.; Choukade, R.; Nath, S. Advances in Prebiotic Mannooligosaccharides. Curr. Opin. Food Sci. 2022, 47, 100883. [Google Scholar] [CrossRef]
- Jana, U.K.; Suryawanshi, R.K.; Prajapati, B.P.; Kango, N. Prebiotic Mannooligosaccharides: Synthesis, Characterization and Bioactive Properties. Food Chem. 2021, 342, 128328. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Panwar, D.; Prashanth, K.V.H.; Kapoor, M. Structural Characterization and in Vitro Fermentation of β-Mannooligosaccharides Produced from Locust Bean Gum by GH-26 Endo-β-1,4-Mannanase (ManB-1601). J. Agric. Food Chem. 2017, 65, 2827–2838. [Google Scholar] [CrossRef] [PubMed]
- Kumar Suryawanshi, R.; Kango, N. Production of Mannooligosaccharides from Various Mannans and Evaluation of Their Prebiotic Potential. Food Chem. 2021, 334, 127428. [Google Scholar] [CrossRef] [PubMed]
- Malgas, S.; van Dyk, J.S.; Pletschke, B.I. A Review of the Enzymatic Hydrolysis of Mannans and Synergistic Interactions between β-Mannanase, β-Mannosidase and α-Galactosidase. World J. Microbiol. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Blibech, M.; Chaari, F.; Bhiri, F.; Dammak, I.; Ghorbel, R.E.; Chaabouni, S.E. Production of Manno-Oligosaccharides from Locust Bean Gum Using Immobilized Penicillium Occitanis Mannanase. J. Mol. Catal. B Enzym. 2011, 73, 111–115. [Google Scholar] [CrossRef]
- Pradeep, S.A.; Rodríguez, L.J.; Kousaalya, A.B.; Farahani, S.; Orrego, C.E.; Pilla, S. Effect of Silane-Treated Pine Wood Fiber (PWF) on Thermal and Mechanical Properties of Partially Biobased Composite Foams. Compos. Part C Open Access 2022, 8, 100278. [Google Scholar] [CrossRef]
- Alzomor, A.; Rus, A.Z.M.; Wahab, H.A.; Salim, N.S.M.; Marsi, N.; Zulhakimie, M.A.; Farid, M.M. Dynamic Mechanical Analysis and Morphology of Petroleum-Based and Bio-Epoxy Foams with Wood Filler. In AIP Conference Proceedings; AIP Publishing: Arau, Malaysia, 2021; p. 020042. [Google Scholar]
- Agnihotri, S.; Shukla, S.; Pradeep, S.A.; Pilla, S. Biobased Thermosetting Cellular Blends: Exploiting the Ecological Advantage of Epoxidized Soybean Oil in Structural Foams. Polymer 2019, 177, 111–119. [Google Scholar] [CrossRef]
- Wood, I.P.; Elliston, A.; Ryden, P.; Bancroft, I.; Roberts, I.N.; Waldron, K.W. Rapid Quantification of Reducing Sugars in Biomass Hydrolysates: Improving the Speed and Precision of the Dinitrosalicylic Acid Assay. Biomass Bioenergy 2012, 44, 117–121. [Google Scholar] [CrossRef]
- ASTM D 4179; Standard Test Method for Single Pellet Crush Strength of Formed Catalyst Shapes. ASTM International: West Conshohocken, PA, USA, 2001.
- Liu, Y.-C.; Ku, Y.; Tseng, Y.-H.; Lee, H.-Y.; Kuo, Y.-L. Fabrication of Fe2O3/TiO2 Oxygen Carriers for Chemical Looping Combustion and Hydrogen Generation. Aerosol Air Qual. Res. 2016, 16, 2023–2032. [Google Scholar] [CrossRef]
- Dhiman, S.; Srivastava, B.; Singh, G.; Khatri, M.; Arya, S.K. Immobilization of Mannanase on Sodium Alginate-Grafted-β-Cyclodextrin: An Easy and Cost Effective Approach for the Improvement of Enzyme Properties. Int. J. Biol. Macromol. 2020, 156, 1347–1358. [Google Scholar] [CrossRef]
- Stefani, P.M.; Perez, C.J.; Alvarez, V.A.; Vazquez, A. Microcellulose Fibers-filled Epoxy Foams. J. Appl. Polym. Sci. 2008, 109, 1009–1013. [Google Scholar] [CrossRef]
- Bai, Y.-X.; Li, Y.-F.; Wang, M.-T. Study on Synthesis of a Hydrophilic Bead Carrier Containing Epoxy Groups and Its Properties for Glucoamylase Immobilization. Enzym. Microb. Technol. 2006, 39, 540–547. [Google Scholar] [CrossRef]
- Liu, H.; Hao, C.; Zhang, Y.; Yang, H.; Sun, R. The Interaction of Graphene Oxide-Silver Nanoparticles with Trypsin: Insights from Adsorption Behaviors, Conformational Structure and Enzymatic Activity Investigations. Colloids Surf. B Biointerfaces 2021, 202, 111688. [Google Scholar] [CrossRef]
- Mateo, C.; Grazu, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of Enzymes on Heterofunctional Epoxy Supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Klapiszewski, L.; Jedrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida Antarctica Immobilized on a Silica-Lignin Matrix as a Stable and Reusable Biocatalytic System. Catalysts 2016, 7, 14. [Google Scholar] [CrossRef]
- Dahari, R.F.; Ongkudon, C.M.; Misson, M. Effect of Crosslinkers on Immobilization of β-Galactosidase on Polymethacrylate Monolith. Trans. Sci. Technol. 2018, 5, 106–113. [Google Scholar]
- Wu, D.; Zhou, J.; Li, Y. Mechanical Strength of Solid Catalysts: Recent Developments and Future Prospects. AIChE J. 2007, 53, 2618–2629. [Google Scholar] [CrossRef]
- Bahmani, M.; Nazari, M.; Mehreshtiagh, M. A Study on the Mechanical Strength of Fe2O3/Cr2O3/CuO Catalyst for High Temperature Water Gas Shift Reaction. J. Porous Mater. 2021, 28, 683–693. [Google Scholar] [CrossRef]
- Weetall, H.H. Preparation of Immobilized Proteins Covalently Coupled through Silane Coupling Agents to Inorganic Supports. Appl. Biochem. Biotechnol. 1993, 41, 157–188. [Google Scholar] [CrossRef]
- Karim, M.R.; Hashinaga, F. Preparation and Properties of Immobilized Pummelo Limonoid Glucosyltransferase. Process Biochem. 2002, 38, 809–814. [Google Scholar] [CrossRef]
- Chen, X.; Tian, Z.; Zhou, H.; Zhou, G.; Cheng, H. Enhanced Enzymatic Performance of β-Mannanase Immobilized on Calcium Alginate Beads for the Generation of Mannan Oligosaccharides. Foods 2023, 12, 3089. [Google Scholar] [CrossRef]
- Miletić, N.; Nastasović, A.; Loos, K. Immobilization of Biocatalysts for Enzymatic Polymerizations: Possibilities, Advantages, Applications. Bioresour. Technol. 2012, 115, 126–135. [Google Scholar] [CrossRef]
- Sichina, W.J. Characterization of Polymers by TMA 2000. In Thermal Analysis Application Note; PerkinElmer Instruments TM: Norwalk, CT, USA; Available online: https://thermalsupport.com/wp-content/uploads/2018/05/PETech-28.pdf (accessed on 5 January 2024).
- Dhanalakshmi, M.; Sruthi, D.; Jinuraj, K.R.; Das, K.; Dave, S.; Andal, N.M.; Das, J. Mannose: A Potential Saccharide Candidate in Disease Management. Med. Chem. Res. 2023, 32, 391–408. [Google Scholar] [CrossRef]
- Chen, J.; Liu, D.; Shi, B.; Wang, H.; Cheng, Y.; Zhang, W. Optimization of Hydrolysis Conditions for the Production of Glucomanno-Oligosaccharides from Konjac Using β-Mannanase by Response Surface Methodology. Carbohydr. Polym. 2013, 93, 81–88. [Google Scholar] [CrossRef]
- Intaratrakul, K.; Nitisinprasert, S.; Nguyen, T.-H.; Haltrich, D.; Keawsompong, S. Manno-Oligosaccharides from Copra Meal: Optimization of Its Enzymatic Production and Evaluation Its Potential as Prebiotic. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100292. [Google Scholar] [CrossRef]
- Jian, H.-L.; Zhu, L.-W.; Zhang, W.-M.; Sun, D.-F.; Jiang, J.-X. Enzymatic Production and Characterization of Manno-Oligosaccharides from Gleditsia Sinensis Galactomannan Gum. Int. J. Biol. Macromol. 2013, 55, 282–288. [Google Scholar] [CrossRef]
- Punekar, N.S. ENZYMES: Catalysis, Kinetics and Mechanisms; Springer: Singapore, 2018; ISBN 9789811307843. [Google Scholar]
- Chato, R.J.A.; Cuevas, C.C.R.; Tangpuz, J.S.N.; Cabatingan, L.K.; Go, A.W.; Ju, Y.-H. Dilute Acid Hydrolysis as a Method of Producing Sugar-Rich Hydrolysates and Lipid-Dense Cake Residues from Copra Cake. J. Environ. Chem. Eng. 2018, 6, 5693–5705. [Google Scholar] [CrossRef]
- Dawood, A.; Ma, K. Applications of Microbial β-Mannanases. Front. Bioeng. Biotechnol. 2020, 8, 598630. [Google Scholar] [CrossRef] [PubMed]
- Rovira, C.; Males, A.; Davies, G.J.; Williams, S.J. Mannosidase Mechanism: At the Intersection of Conformation and Catalysis. Curr. Opin. Struct. Biol. 2020, 62, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Polizeli, M.; Polizeli, M.d.L.T.M.; Rai, M. (Eds.) Fungal Enzymes; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-07420-2. [Google Scholar]
- Tang, X.; Zhu, X.; Yang, Y.; Qi, Z.; Mu, Y.; Huang, Z. Research Article Product Composition Analysis and Process Research of Oligosaccharides Produced from Enzymatic Hydrolysis of High-Concentration Konjac Flour. ACS Omega 2020, 5, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yi, P.; Liu, J.; Yan, Q.; Jiang, Z. High-Level Expression of an Engineered β-Mannanase (mRmMan5A) in Pichia Pastoris for Manno-Oligosaccharide Production Using Steam Explosion Pretreated Palm Kernel Cake. Bioresour. Technol. 2018, 256, 30–37. [Google Scholar] [CrossRef]

| Independent Variable | Unit | Type | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|---|
| A-Time | Hr | Continuous | 1 | 10 | - |
| B-pH | - | Discrete | 6.5 | 7.5 | 8.5 |
| C-Enzyme Load | (U/gr substrate) | Discrete | 17 | 33.5 | 50 |
| D-Temperature | (°C) | Discrete | 40 | 50 | 70 |
| Run | Time | pH | E/S | T° | Mannose | MOS (M2–M5) |
|---|---|---|---|---|---|---|
| hr | U/gr | °C | mg/L | mg/L | ||
| 1 | 8.65 | 8.5 | 50 | 70 | 958.81 | 14,095.90 |
| 2 | 10 | 8.5 | 33.75 | 55 | 170.10 | 9757.76 |
| 3 | 10 | 7.5 | 17.5 | 70 | 850.25 | 6370.89 |
| 4 | 5.5 | 7.5 | 33.75 | 55 | 250.90 | 12,016.70 |
| 5 | 1 | 6.5 | 33.75 | 55 | 352.58 | 4400.62 |
| 6 | 8.65 | 8.5 | 17.5 | 40 | 178.35 | 4733.63 |
| 7 | 7.75 | 7.5 | 33.75 | 40 | 231.59 | 9840.03 |
| 8 | 1 | 6.5 | 50 | 40 | 237.77 | 2307.15 |
| 9 | 1 | 8.5 | 33.75 | 55 | 874.04 | 6632.93 |
| 10 | 6.94 | 6.5 | 33.75 | 70 | 838.90 | 9797.41 |
| 11 | 3.79 | 8.5 | 17.5 | 70 | 175.49 | 8739.32 |
| 12 | 1 | 8.5 | 33.75 | 55 | 229.53 | 6870.05 |
| 13 | 5.5 | 7.5 | 33.75 | 55 | 327.53 | 12,734.40 |
| 14 | 10 | 6.5 | 50 | 55 | 2315.96 | 20,011.70 |
| 15 | 1 | 7.5 | 17.5 | 40 | 1271.63 | 7985.93 |
| 16 | 10 | 6.5 | 33.75 | 40 | 1510.52 | 13,282.00 |
| 17 | 1 | 7.5 | 50 | 70 | 195.91 | 4333.87 |
| 18 | 6.94 | 6.5 | 33.75 | 70 | 1840.33 | 9076.04 |
| 19 | 4.15 | 6.5 | 33.75 | 40 | 1485.81 | 3225.43 |
| 20 | 1 | 6.5 | 17.5 | 70 | 506.53 | 5311.39 |
| 21 | 5.5 | 7.5 | 33.75 | 55 | 223.67 | 12,435.90 |
| 22 | 6.895 | 6.5 | 17.5 | 55 | 2165.95 | 14,307.10 |
| 23 | 6.4 | 8.5 | 50 | 40 | 172.89 | 7514.69 |
| 24 | 5.5 | 7.5 | 33.75 | 55 | 233.80 | 12,240.80 |
| 25 | 5.5 | 7.5 | 33.75 | 55 | 220.19 | 13,389.00 |
| 26 | 6.895 | 6.5 | 17.5 | 55 | 3080.27 | 16,988.50 |
| 27 | 10 | 7.5 | 50 | 40 | 137.58 | 16,682.30 |
| 28 | 6.175 | 7.5 | 50 | 70 | 328.52 | 14,476.60 |
| 29 | 10 | 7.5 | 17.5 | 70 | 990.43 | 10,698.70 |
| Product mg/L | Predicted | Experimental | Error |
|---|---|---|---|
| M1 | 577.02 | 565.36 | 2.06% |
| MOS (M2–M5) | 12,347.92 | 12,954.62 | 4.68% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo-Franco, S.L.; Galvis-Nieto, J.D.; Orrego, C.E. Production of Mannooligosaccharides from Açaí Seed by Immobilized β-Mannanase. Processes 2024, 12, 847. https://doi.org/10.3390/pr12050847
Murillo-Franco SL, Galvis-Nieto JD, Orrego CE. Production of Mannooligosaccharides from Açaí Seed by Immobilized β-Mannanase. Processes. 2024; 12(5):847. https://doi.org/10.3390/pr12050847
Chicago/Turabian StyleMurillo-Franco, Sarha Lucia, Juan David Galvis-Nieto, and Carlos E. Orrego. 2024. "Production of Mannooligosaccharides from Açaí Seed by Immobilized β-Mannanase" Processes 12, no. 5: 847. https://doi.org/10.3390/pr12050847





