Performance of a Solar-Driven Photocatalytic Membrane Reactor for Municipal Wastewater Treatment
Abstract
:1. Introduction
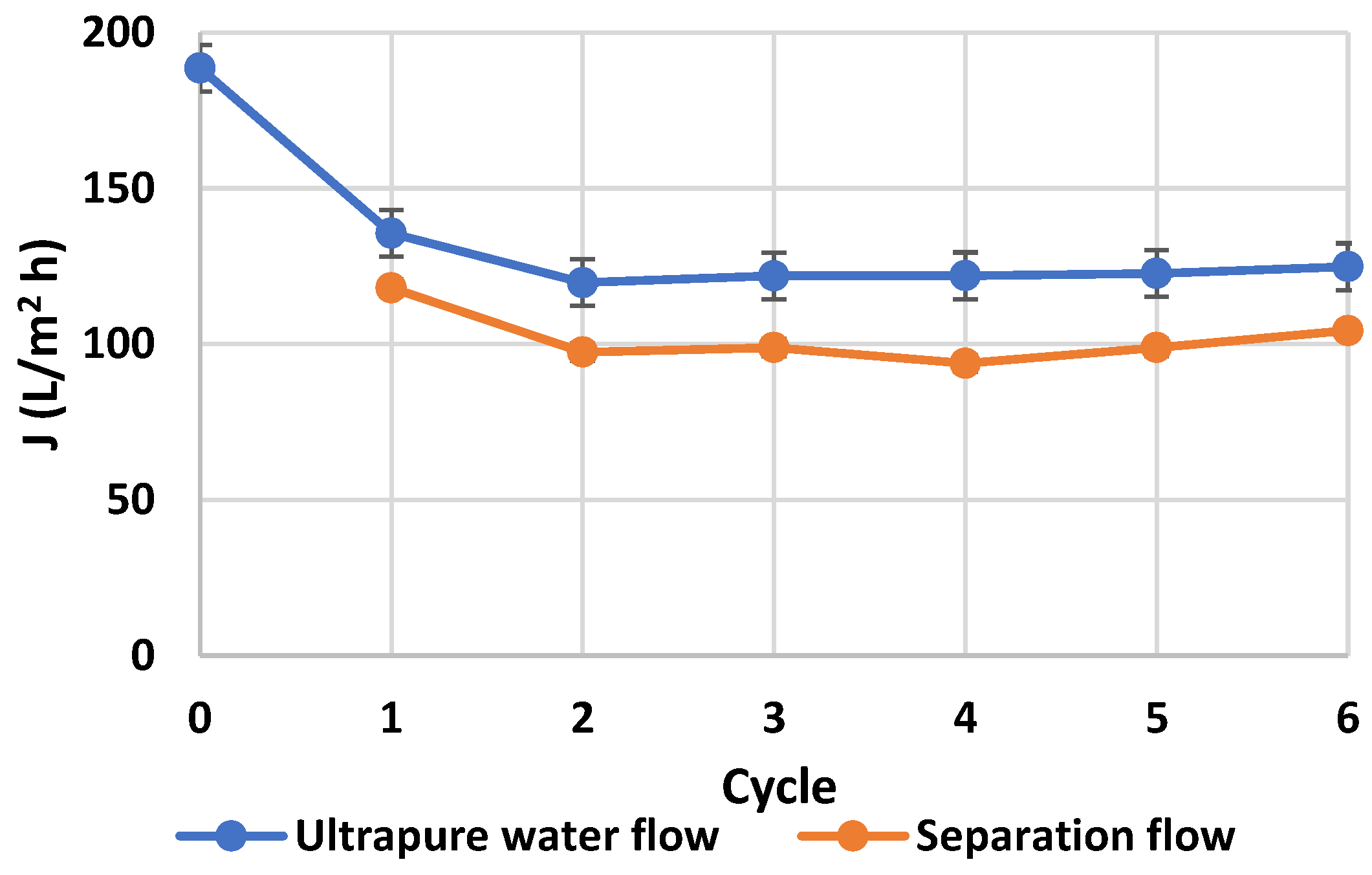
2. Materials and Methods
- Four transparent PETG (polyethylene terephthalate glycol) tubes with an outer diameter of 16 mm, an inner diameter of 12 mm, and a length of 1 m. The dimensions of the tubes were chosen to give the CPC reactor a total capacity of 2 litres.
- A submerged feed/recirculation pump (with the possibility to adjust the flow). A constant recirculation flow of approximately 1 L/min was used during the degradation tests. Recirculation flow was chosen to ensure a turbulent regime within the reactor tubes.
- A portable aeration pump with a flow of 1 L/min. Aeration was operated using a 30 min ON/30 min OFF algorithm.
- A feed/recirculation vessel with a capacity of 2 L.
3. Results and Discussion
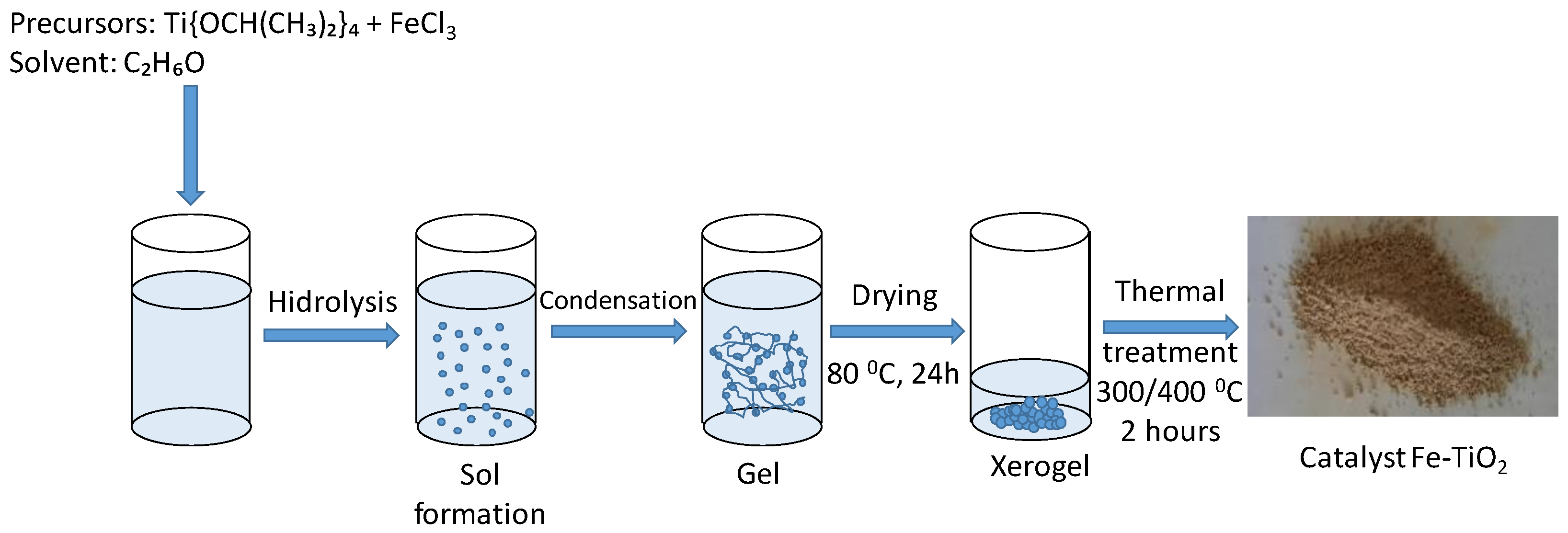
3.1. Photocatalyst Synthesis and Characterisation
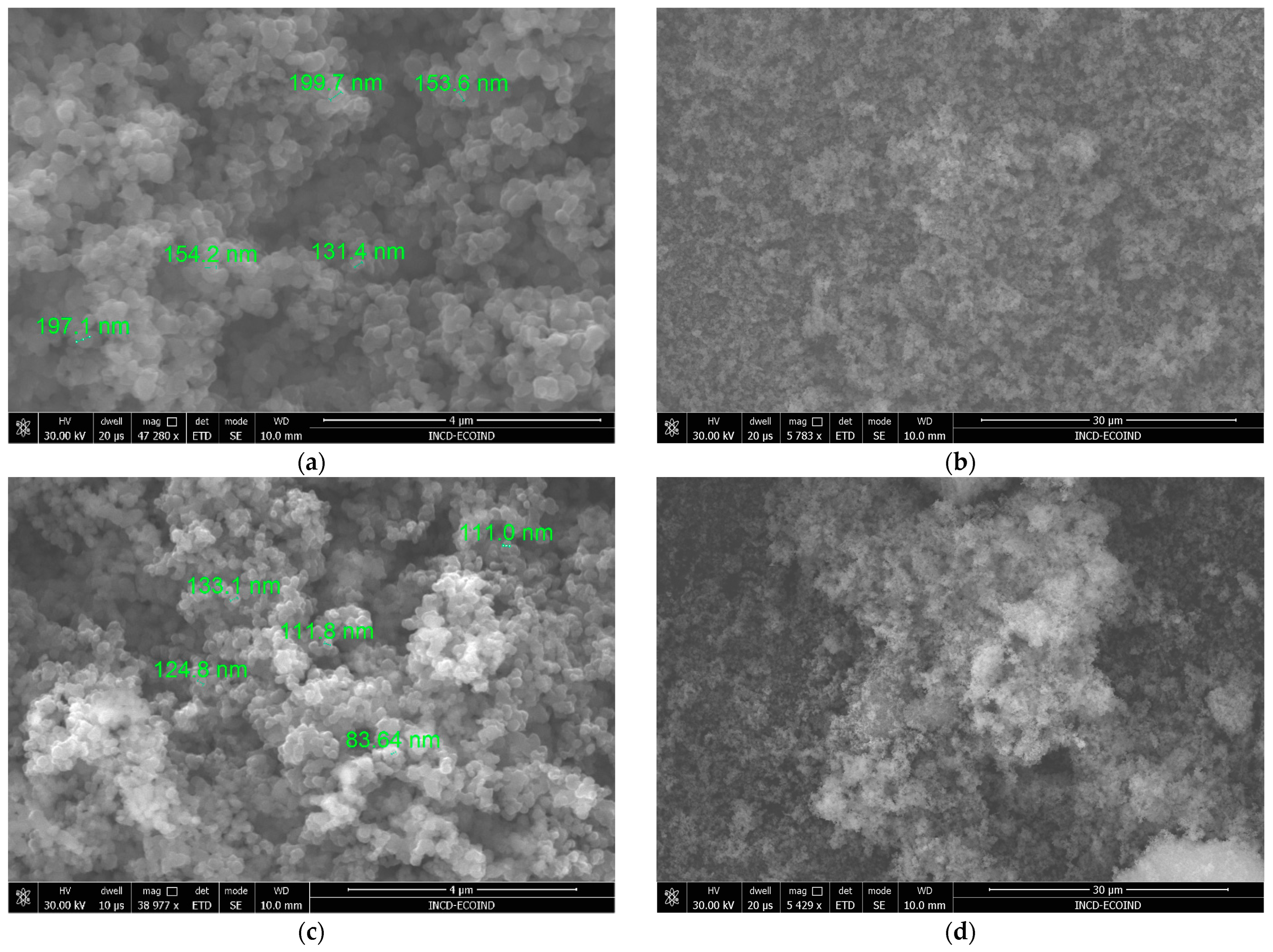
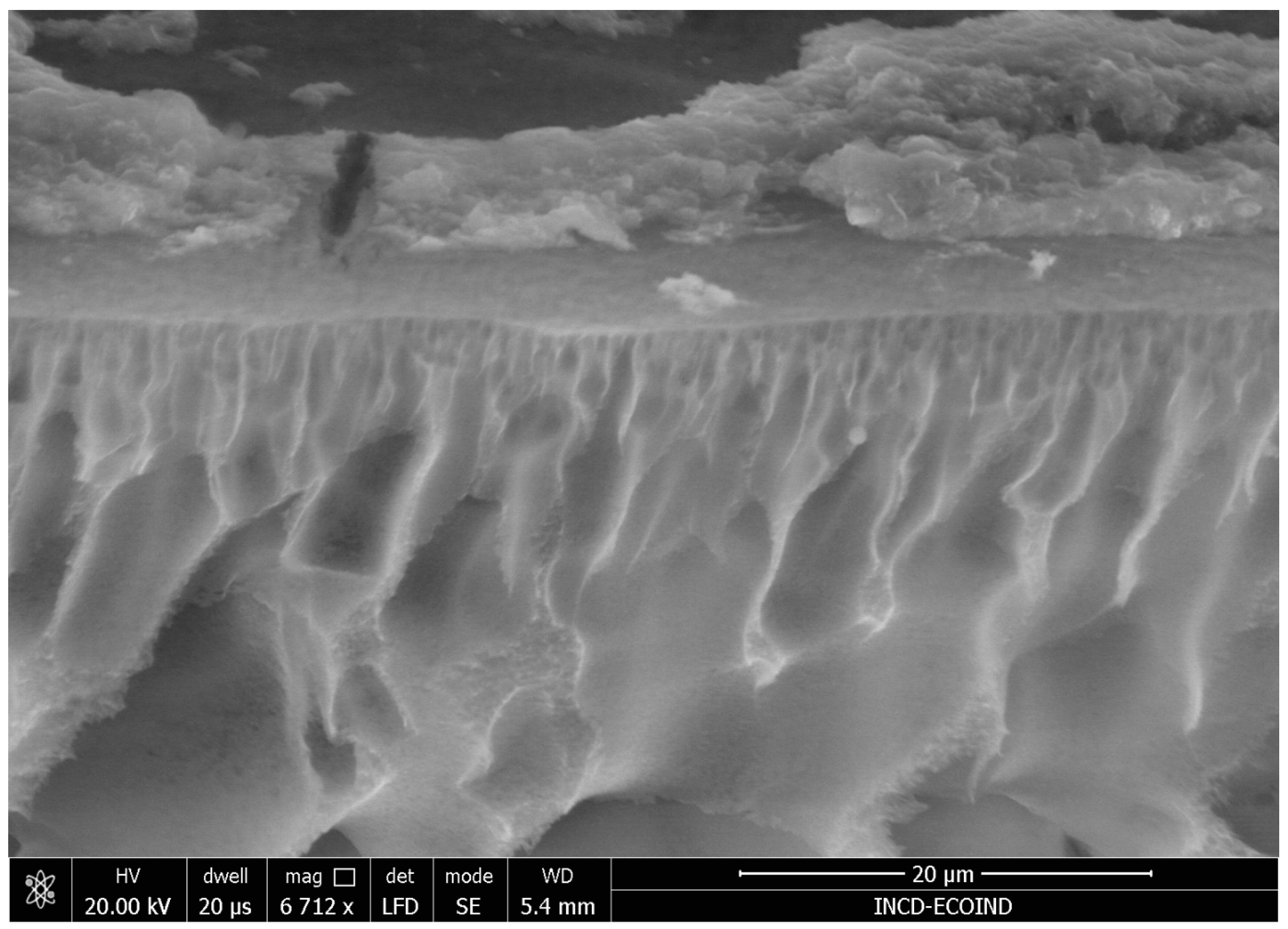
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Dimensional Analyses of Fe–TiO2 Catalysts
3.1.3. Energy-Dispersive X-ray Spectroscopy (EDX)
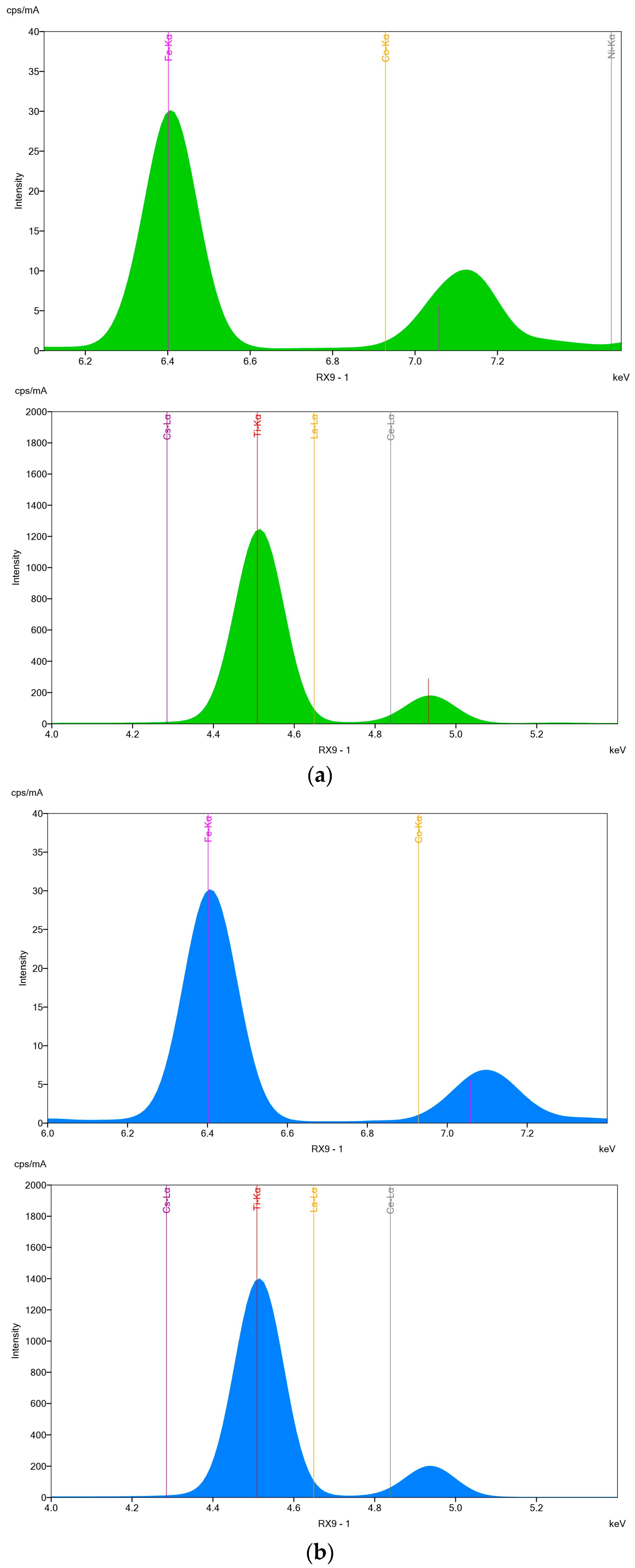
3.1.4. X-ray Fluorescence Analyses (XRF)
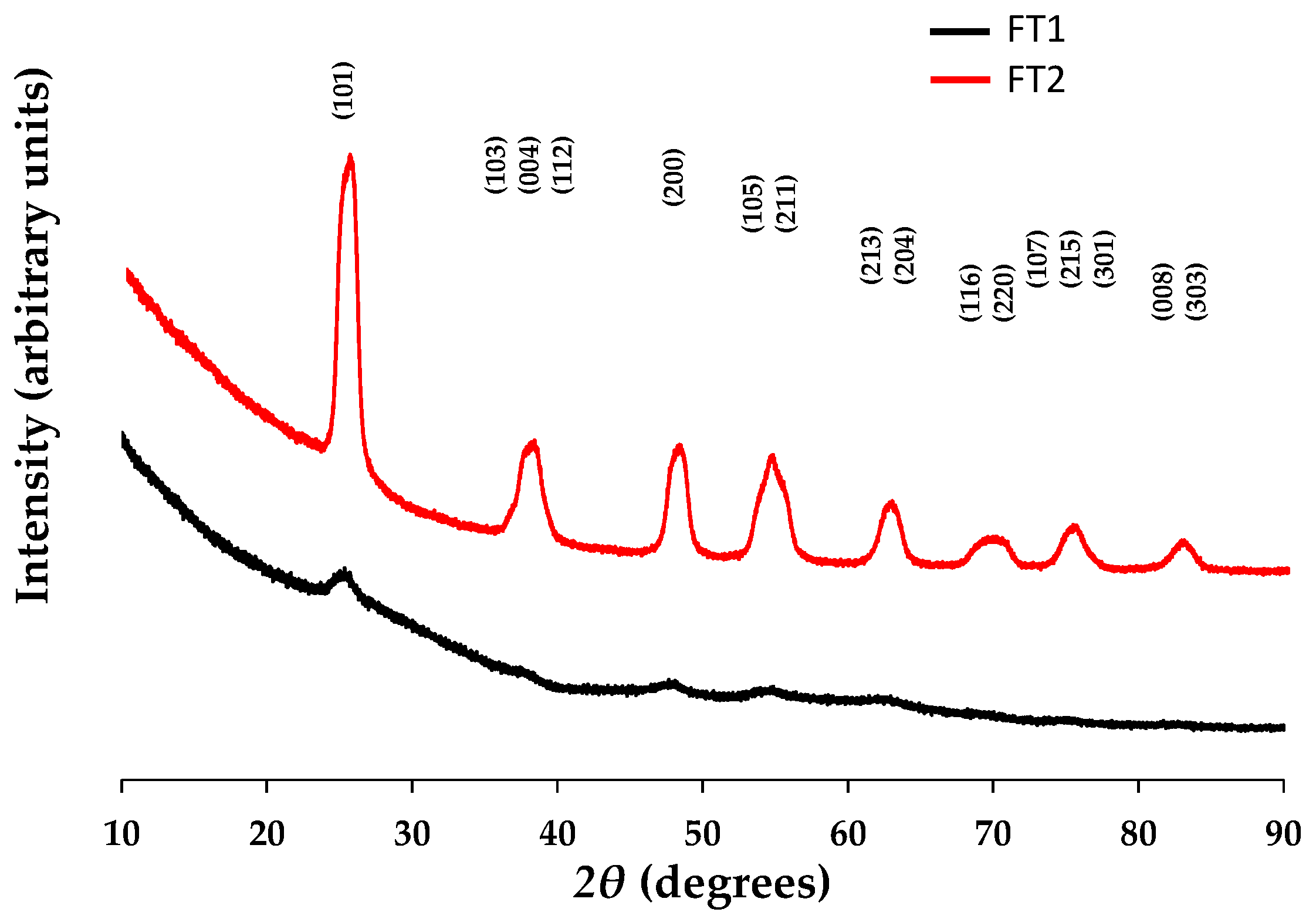
3.1.5. X-ray Diffraction (XRD)
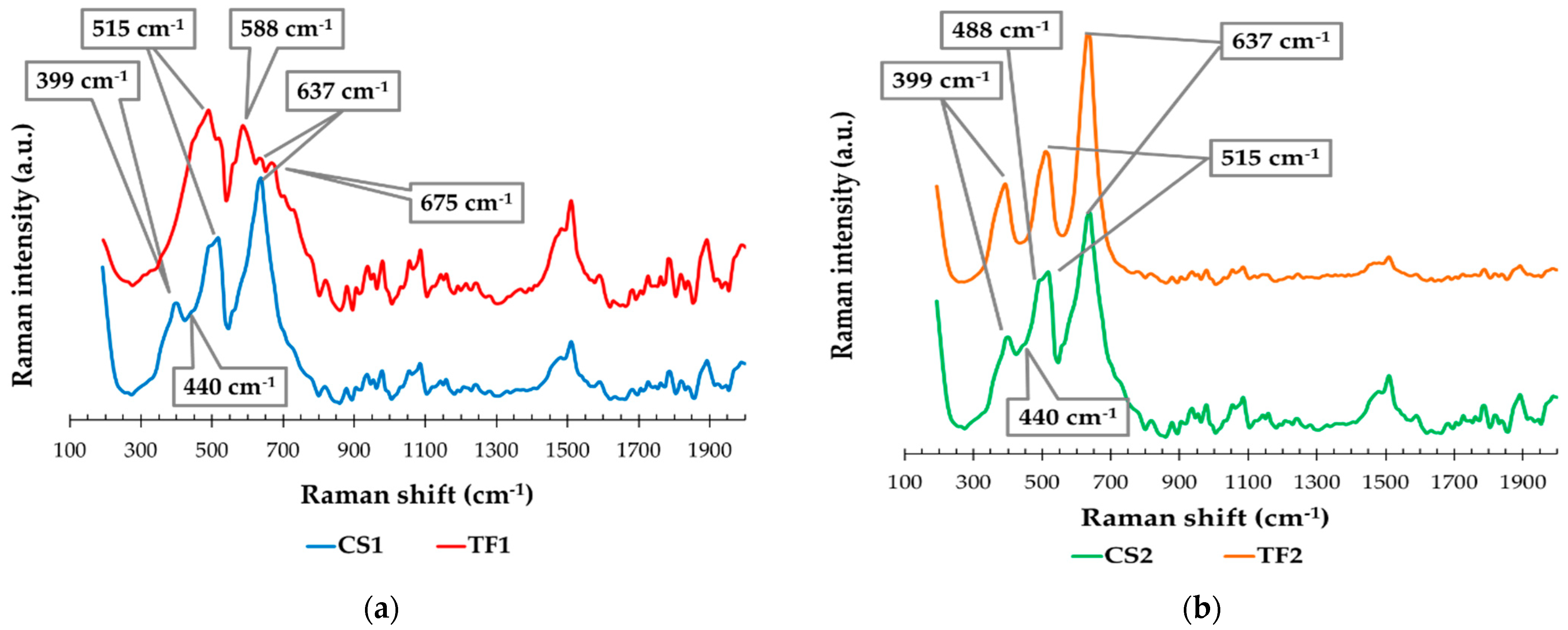
3.1.6. Raman Spectra
3.1.7. Fourier Transform Infrared Spectroscopy
3.2. Experimental Photodegradation Tests
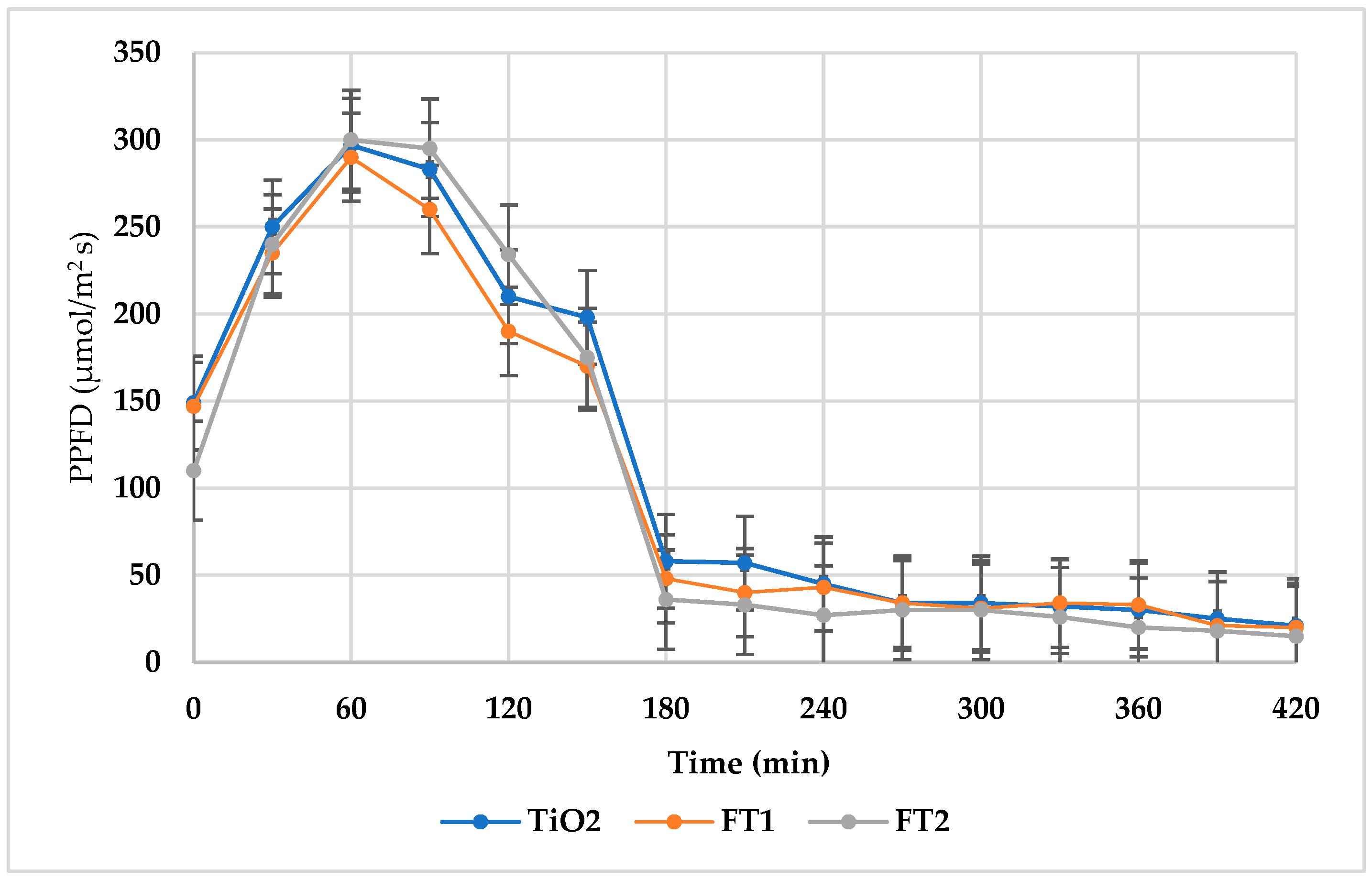
3.2.1. Experimental Photodegradation Tests Using Simulated Solar Light
3.2.2. Experimental Photodegradation Tests Using Natural Solar Light
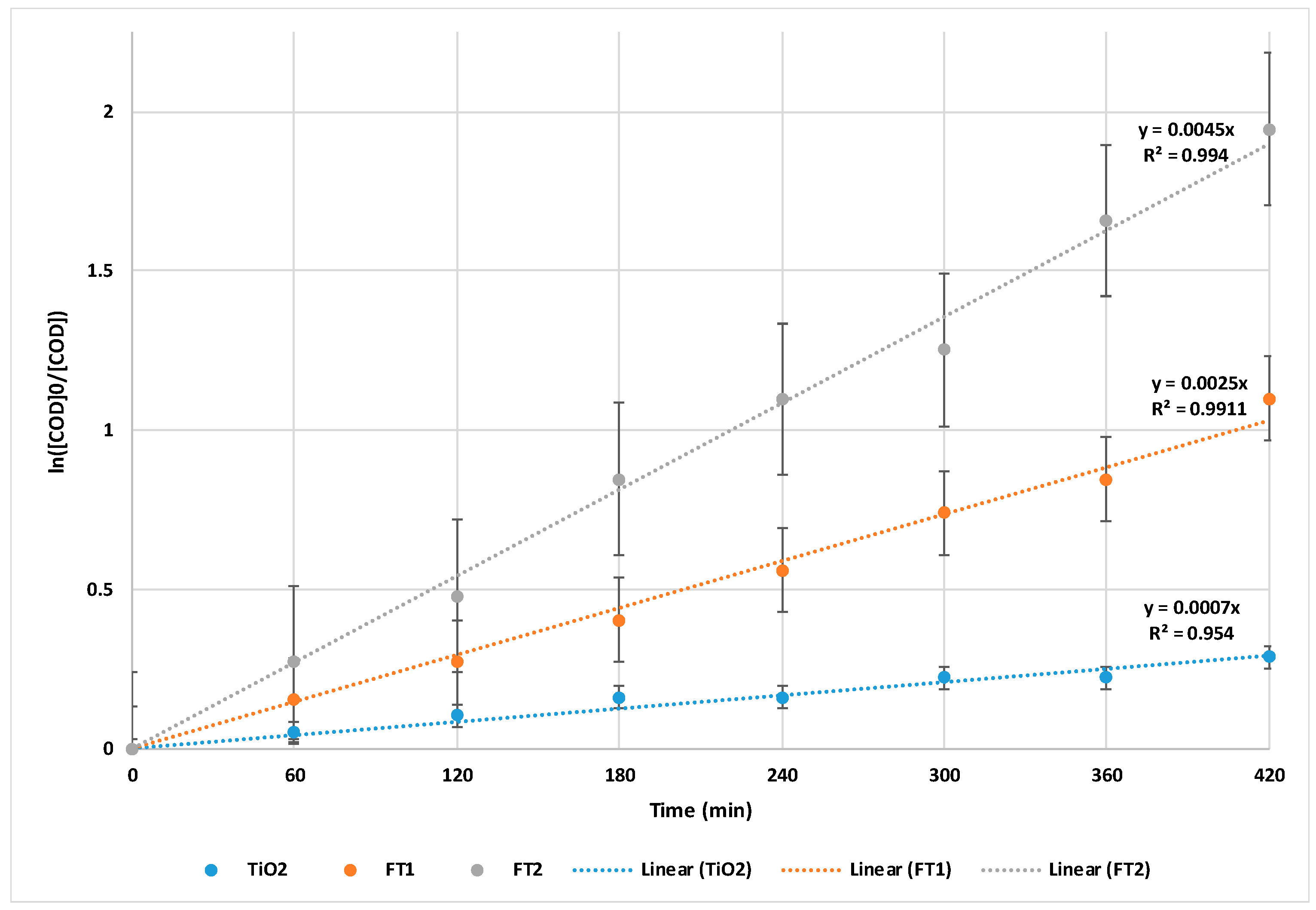
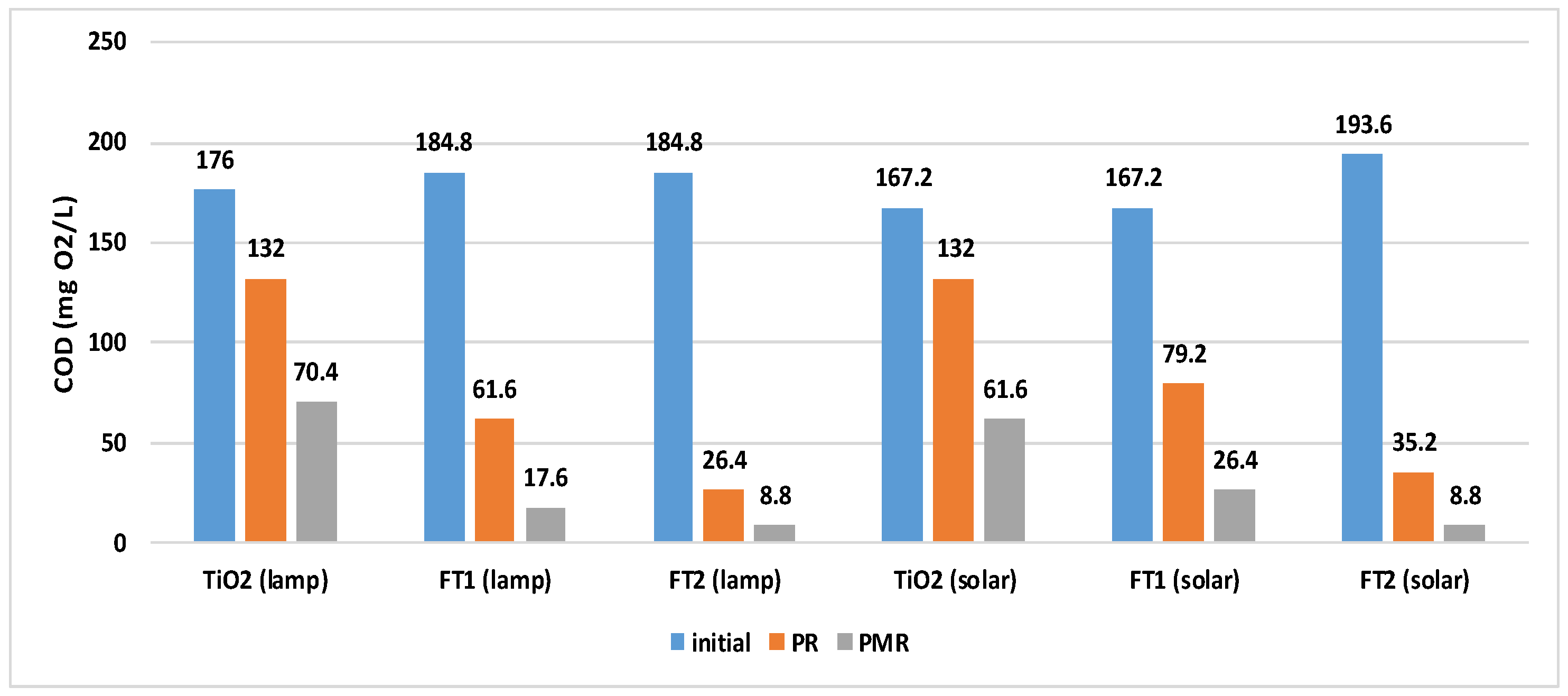
3.3. Overall COD Removal Efficiency of Solar PMR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Sharma, A.; Pandit, P.P.; Chopade, R.L.; Nagar, V.; Aseri, V.; Singh, A.; Awasthi, K.K.; Awasthi, G.; Sankhla, M.S. Eradication of microplastics in wastewater treatment: Overview. Biointerface Res. Appl. Chem. 2023, 13, 223. [Google Scholar]
- Ali, N.S.; Kalash, K.R.; Ahmed, A.N.; Albayati, T.M. Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2 and UV/H2O2 to control membrane fouling. Sci. Rep. 2022, 12, 16782. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Severino, A.; Lavorato, C.; Argurio, P. Which configuration of photocatalytic membrane reactors has a major potential to be used at an industrial level in tertiary sewage wastewater treatment? Catalysts 2023, 13, 1204. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic membranes in photocatalytic membrane reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- De Filpo, G.; Pantuso, E.; Armentano, K.; Formoso, P.; Di Profio, G.; Poerio, T.; Fontananova, E.; Meringolo, C.; Mashin, A.I.; Nicoletta, F.P. Chemical vapor deposition of photocatalyst nanoparticles on PVDF membranes for advanced oxidation processes. Membranes 2018, 8, 35. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Albayati, T.M.; Harharah, H.N.; Amari, A.; Saady, N.M.C.; Zendehboudi, S. Current trends for wastewater treatment technologies with typical configurations of photocatalytic membrane reactor hybrid systems: A review. Chem. Eng. Process 2023, 192, 109503. [Google Scholar] [CrossRef]
- Szymański, K.; Grzechulska-Damszel, J.; Mozia, S. Application of a submerged photocatalytic membrane reactor with ultrafiltration membrane for ketoprofen removal during long term process: Impact of feed matrix. J. Water Process Eng. 2024, 59, 104953. [Google Scholar] [CrossRef]
- Roso, M.; Boaretti, C.; Bonora, R.; Modesti, M.; Lorenzetti, A. Nanostructured active media for volatile organic compounds abatement: The synergy of graphene oxide and semiconductor coupling. Ind. Eng. Chem. Res. 2018, 57, 16635–16644. [Google Scholar] [CrossRef]
- Zhang, Q.; Quan, X.; Wang, H.; Chen, S.; Su, Y.; Li, Z. Constructing a visible-light-driven photocatalytic membrane by g-C3N4 quantum dots and TiO2 nanotube array for enhanced water treatment. Sci. Rep. 2017, 7, 3128. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Tran, Q.B.; Ly, Q.V.; Hai, L.T.; Le, D.T.; Tran, M.B.; Ho, T.T.T.; Nguyen, X.C.; Shokouhimehr, M.; Vo, D.-V.N.; et al. Enhanced visible photocatalytic degradation of diclofenac over N-doped TiO2 assisted with H2O2: A kinetic and pathway study. Arab. J. Chem. 2020, 13, 8361–8371. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.-S.; Chen, C.-H.; Chen, Y.-R.; Huang, P.-H.; Tung, K.-L. Phosphorus-doped g-C3N4 integrated photocatalytic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 580, 1–11. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Ashar, A.; Bhatti, I.A.; Ashraf, M.; Tahir, A.A.; Aziz, H.; Yousuf, M.; Ahmad, M.; Mohsin, M.; Bhutta, Z.A. Fe3+@ZnO/polyester based solar photocatalytic membrane reactor for abatement of RB5 dye. J. Clean. Prod. 2020, 246, 119010. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Y.; Shen, L.; Xu, Y.; Li, R.; Huang, L.; Lin, H. Magnetic field assisted arrangement of photocatalytic TiO2 particles on membrane surface to enhance membrane antifouling performance for water treatment. J. Colloid Interface Sci. 2020, 570, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.-J.; Wu, G.-P.; Darling, S.B.; Xu, Z.-K. Photocatalytic nanofiltration membranes with self-cleaning properties for wastewater. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Liu, G.; Han, K.; Zhou, Y.; Ye, H.; Zhang, X.; Hu, J.; Li, X. Facile synthesis of highly dispersed Ag doped graphene oxide/titanate nanotubes as a visible light photocatalytic membrane for water treatment. ACS Sustain. Chem. Eng. 2018, 6, 6256–6263. [Google Scholar] [CrossRef]
- Alyarnezhad, S.; Marino, T.; Parsa, J.B.; Galiano, F.; Ursino, C.; Garcìa, H.; Puche, M.; Figoli, A. Polyvinylidene fluoride-graphene oxide membranes for dye removal under visible light irradiation. Polymers 2020, 12, 1509. [Google Scholar] [CrossRef]
- Nassseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterisation of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 2017, 310, 389–398. [Google Scholar] [CrossRef]
- Kamaludin, R.; Rasdi, Z.; Othman, M.H.D.; Kadir, S.H.S.A.; Nor, N.S.M.; Khan, J.; Zain, W.N.W.M.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Visible-light active photocatalytic dual layer hollow fiber (DLHF) membrane and its potential in mitigating the detrimental effects of bisphenol A in water. Membranes 2020, 10, 32. [Google Scholar] [CrossRef]
- Nitoi, I.; Constantin, L.A.; Oancea, P.; Cristea, I.; Crisan, M. TiO2 solar light photocatalysis a promising treatment method for wastewater with trinitrotoluene content. In Proceedings of the 15th International Multidisciplinary GeoConferences SGEM, Albena, Bulgaria, 18–24 June 2015. [Google Scholar]
- Crişan, M.; Răileanu, M.; Drăgan, N.; Crişan, D.; Ianculescu, A.; Niţoi, I.; Oancea, P.; Şomăcescu, S.; Stănică, N.; Vasile, B.; et al. Sol-gel iron-doped TiO2 nanopowders with photocatalytic activity. Appl. Catal. A Gen. 2015, 504, 130–142. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Vujancevic, J.D.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.B.; Stojadinovic, S.; Brankovic, G.O.; Nikolic, M.V. Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol-gel method. R. Soc. Open Sci. 2020, 7, 20078. [Google Scholar] [CrossRef] [PubMed]
- Constantin, L.A.; Constantin, M.A.; Ionescu, I.A.; Puiu, M.D. Polysulfone and cellulose acetate–based membranes’ potential application to photocatalytic membrane reactors. Rom. J. Ecol. Environ. Chem. 2023, 5, 5–16. [Google Scholar] [CrossRef]
- Constantin, L.A.; Ionescu, I.A.; Constantin, M.A.; Stefanescu, M.; Marin, N.M. Metal–titanium dioxide doped catalysts for wastewater treatment under simulated solar light. In Proceedings of the 26th International Symposium “The Environment and the Industry” SIMI, Bucharest, Romania, 27–29 September 2023. [Google Scholar]
- Nitoi, I.; Oancea, P.; Raileanu, M.; Crisan, M.; Constantin, L.; Cristea, I. UV-VIS photocatalytic degradation of nitrobenzene from water using heavy metal doped titania. J. Ind. Eng. Chem. 2015, 21, 677–682. [Google Scholar] [CrossRef]
- Mohammed, S.; Elnoor, M.; Hamad, I. The Structural properties of iron oxides using Raman spectroscopy. J. Sci. Eng. Res. 2018, 5, 183–187. [Google Scholar]
- Perevedentseva, E.; Lin, Y.-C.; Karmenyan, A.; Wu, K.-T.; Lugovtsov, A.; Shirshin, E.; Priezzhev, A.; Cheng, C.-L. Raman spectroscopic study of TiO2 nanoparticles’ effects on the Hemoglobin state in individual red blood cells. Materials 2021, 14, 5920. [Google Scholar] [CrossRef]
- Raguram, T.; Rajni, K.S. Synthesis and analysing the structural, optical, morphological, photocatalytic and magnetic properties of TiO2 and doped (Ni and Cu) TiO2 nanoparticles by sol–gel technique. Appl. Phys. A 2019, 125, 288. [Google Scholar] [CrossRef]
- SR ISO 6060; Water Quality—Determination of the Chemical Oxygen Demand. Romanian Standards Association: Bucharest, Romania, 1996.
- Joy, V.M.; Feroz, S.; Dutta, S. Solar nanophotocatalytic pretreatment of seawater: Process optimization and performance evaluation using response surface methodology and genetic algorithm. Appl. Water Sci. 2021, 11, 18. [Google Scholar] [CrossRef]
- Aslam, T.; Masindi, V.; Ahmad, A.A.; Chatzisymeon, E. Valorization of acid mine drainage into an iron catalyst to initiate the solar photo-Fenton treatment of municipal wastewater. Environments 2023, 10, 132. [Google Scholar] [CrossRef]
- Manaserro, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilised TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Tasso Guaraldo, T.; Wenk, J.; Mattia, D. Photocatalytic ZnO foams for micropollutant degradation. Adv. Sustain. Syst. 2021, 5, 2000208. [Google Scholar] [CrossRef]


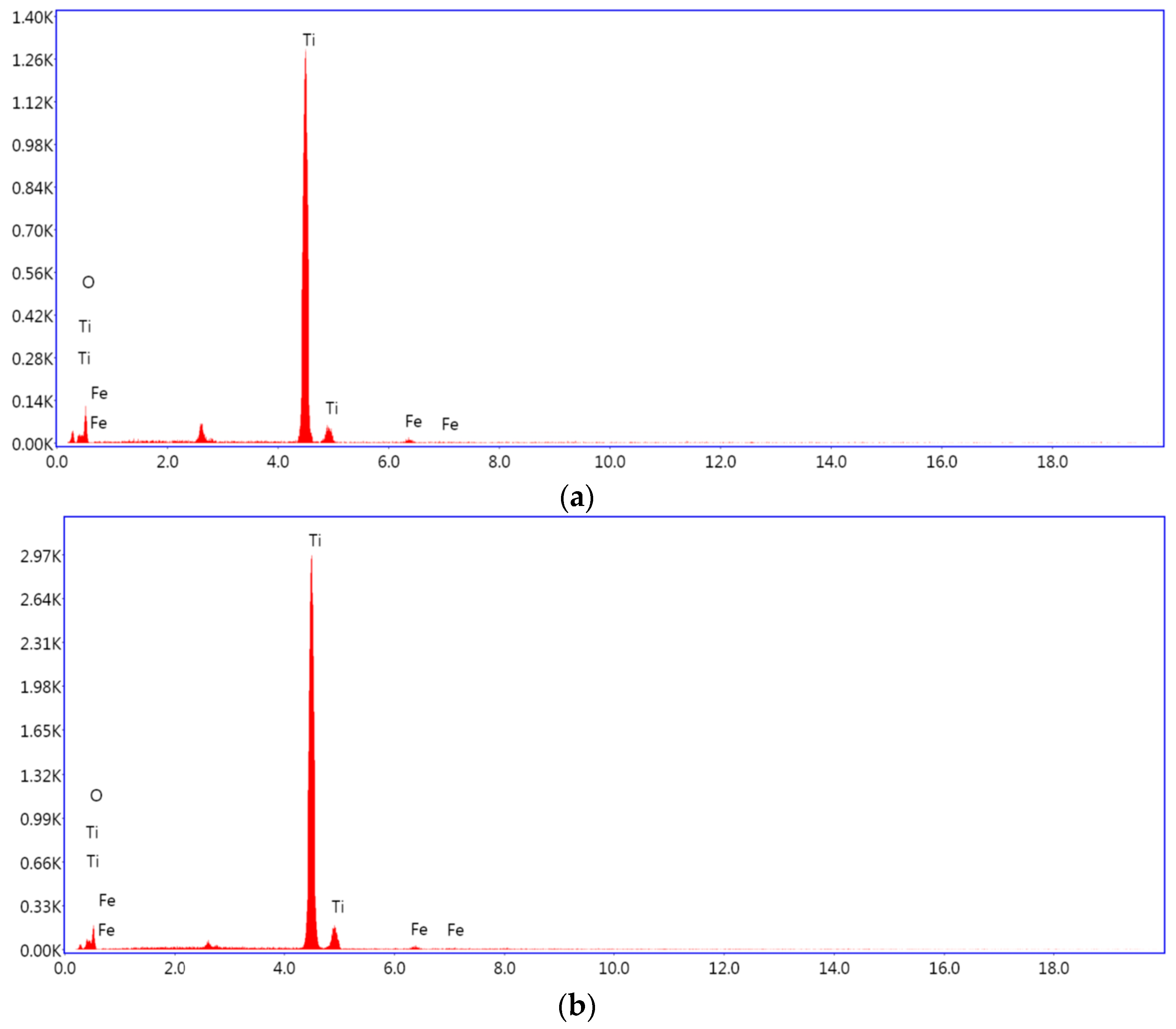



| Element | Weight % | Atomic % |
|---|---|---|
| FT1 | ||
| O K | 43.91 | 70.16 |
| Ti K | 54.90 | 29.30 |
| Fe K | 1.19 | 0.54 |
| FT2 | ||
| O K | 37.45 | 64.24 |
| Ti K | 61.68 | 35.34 |
| Fe K | 0.87 | 0.43 |
| Sample | Temp (°C) | D Spacing (Å) | Lattice Parameters | V (unit cell vol.) | FWHM (Degrees) | Crystallite Size, Williams–Hall Method | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1 0 1) | (2 0 0) | (0 0 4) | a (Å) | c (Å) | Ratio c/a | (Å3) | (1 0 1) | (2 0 0) | (0 0 4) | (nm) | ||
| TiO2 anatase (PDF 00-064-0863) | 235 | 3.51616 | 1.89268 | 2.37339 | 3.78536 (24) | 9.4936 (7) | 2.508 | 136.03 | n/a | n/a | n/a | <100 nm |
| FT1 | 300 | 3.510 (4) | 1.887 (4) | 2.842 (6) | 3.60 (8) | 11.1 (3) | 3.083 | 145 (6) | 2.68 (4) | 7.41 (12) | 5.23 (12) | 17 (4) |
| FT2 | 400 | 3.4833 (6) | 1.8934 (3) | 2.3751 (5) | 3.767 (14) | 9.42 (4) | 2.501 | 133.7 (9) | 0.912 (9) | 1.401 (6) | 1.780 (7) | 69 (15) |
| Wavenumber (cm−1) | Sample | Vibration Modes | |||
|---|---|---|---|---|---|
| CS1 | CS2 | FT1 | FT2 | ||
| 399 | Anatase—B1g mode (symmetric bending vibration of O-Ti-O) | ||||
| 440 | Rutile—Eg mode (symmetric stretching vibration of O-Ti-O) | ||||
| 515 | Anatase Ti–O A1g mode (anti-symmetric bending vibration of O-Ti-O) | ||||
| 637 | Anatase Ti–O Eg mode (symmetric stretching vibration of O-Ti-O) | ||||
| Time (h) | COD Removal Efficiency (%) | |
|---|---|---|
| FT1 | FT2 | |
| 1 | 14.29 | 23.81 |
| 2 | 23.81 | 38.10 |
| 3 | 33.33 | 57.14 |
| 4 | 42.86 | 66.67 |
| 5 | 52.38 | 71.43 |
| 6 | 57.14 | 80.95 |
| 7 | 66.67 | 85.71 |
| Time (h) | COD Removal Efficiency (%) | |
|---|---|---|
| FT1 | FT2 | |
| 1 | 15.79 | 18.18 |
| 2 | 21.05 | 36.36 |
| 3 | 31.58 | 50.00 |
| 4 | 36.84 | 54.55 |
| 5 | 36.84 | 63.64 |
| 6 | 47.37 | 77.27 |
| 7 | 52.63 | 81.82 |
| Catalyst | COD Removal Efficiency (%) | |
|---|---|---|
| After Photocatalytic Step | PMR Overall | |
| Simulated solar light | ||
| TiO2 | 25.00 | 60.00 |
| FT1 | 66.67 | 90.48 |
| FT2 | 85.71 | 95.24 |
| Natural solar light | ||
| TiO2 | 21.05 | 63.16 |
| FT1 | 52.63 | 84.21 |
| FT2 | 81.82 | 95.45 |
| Process | Results | Conditions | References |
|---|---|---|---|
| Solar photocatalysis reactor by UV, UV/H2O2 and UV/TiO2 | UV: TOC = 20% UV/H2O2: TOC = 30% UV/TiO2: TOC = 50% | Reaction time = 6 h | [3] |
| UV-VIS photocatalytic reactor with suspended TiO2 for clofibric acid degradation | k = 0.00326 min−1 | 0.5 g/L TiO2 20 mg/L clofibric acid | [4,34] |
| Photocatalytic ZnO foams for carbamazepine degradation | k = 0.0033 min−1 | 0.5 g/L ZnO 10 mg/L carbamazepine | [4,35] |
| Solar nanophotocatalytic pretreatment of seawater | TOC: 76.5% COD: 63.9% | 2.94–3.89 mg/L TOC 4.9–5.78 mgO2/L COD | [32] |
| Solar photo Fenton treatment of municipal wastewater | COD: 99% | Reaction time = 1.5 h 255 mg/L COD | [33] |
| Solar PMR for wastewater treatment | Simulated solar light: COD = 85.71%, k = 0.0045 min−1 after photocatalytic step COD = 95.24% PMR Natural solar light: COD = 81.82%, k = 0.0038 min−1 after photocatalytic step COD = 95.45% PMR | Reaction time = 7 h 100 mg/L Fe-doped TiO2 167.2–193.6 mgO2/L COD | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, M.A.; Constantin, L.A.; Ionescu, I.A.; Nicolescu, C.M.; Bumbac, M.; Tiron, O. Performance of a Solar-Driven Photocatalytic Membrane Reactor for Municipal Wastewater Treatment. Processes 2024, 12, 617. https://doi.org/10.3390/pr12030617
Constantin MA, Constantin LA, Ionescu IA, Nicolescu CM, Bumbac M, Tiron O. Performance of a Solar-Driven Photocatalytic Membrane Reactor for Municipal Wastewater Treatment. Processes. 2024; 12(3):617. https://doi.org/10.3390/pr12030617
Chicago/Turabian StyleConstantin, Mirela Alina, Lucian Alexandru Constantin, Ioana Alexandra Ionescu, Cristina Mihaela Nicolescu, Marius Bumbac, and Olga Tiron. 2024. "Performance of a Solar-Driven Photocatalytic Membrane Reactor for Municipal Wastewater Treatment" Processes 12, no. 3: 617. https://doi.org/10.3390/pr12030617





