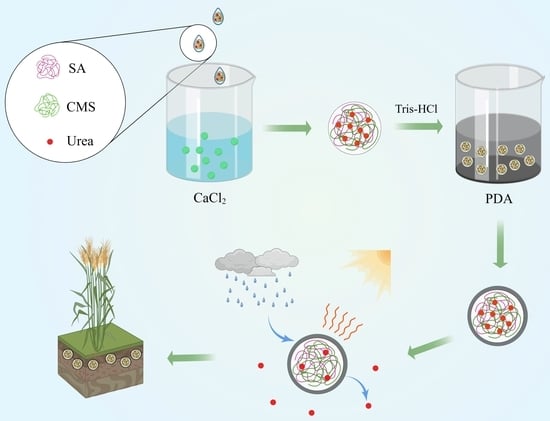
Slow-Release Urea Fertilizer with Water Retention and Photosensitivity Properties Based on Sodium Alginate/Carboxymethyl Starch Sodium/Polydopamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Sodium Alginate/Carboxymethyl Starch Sodium/Polydopamine/Urea
2.3. Characterization
2.4. Water Absorption of SCPU
2.5. Water-Holding Capacity and Water-Retention Behavior of SCPU
2.6. Slow-Release Behavior of SCPU
2.7. Release Kinetics of SCPU
2.8. Photothermal Conversion Performance of SCPU
2.9. Plant Growth Experiment
2.10. Statistical Analysis
3. Results and Discussion
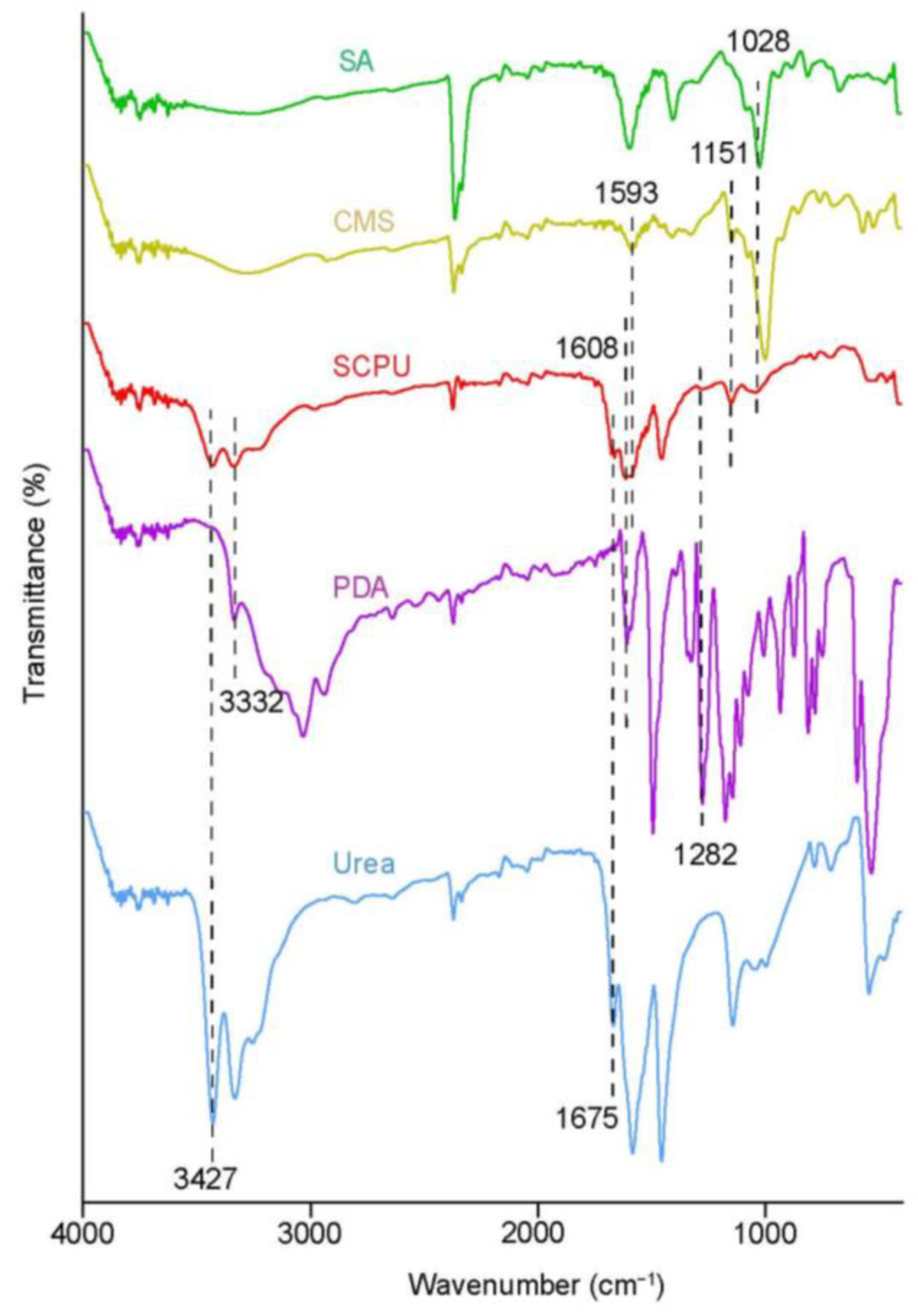
3.1. FTIR Analysis
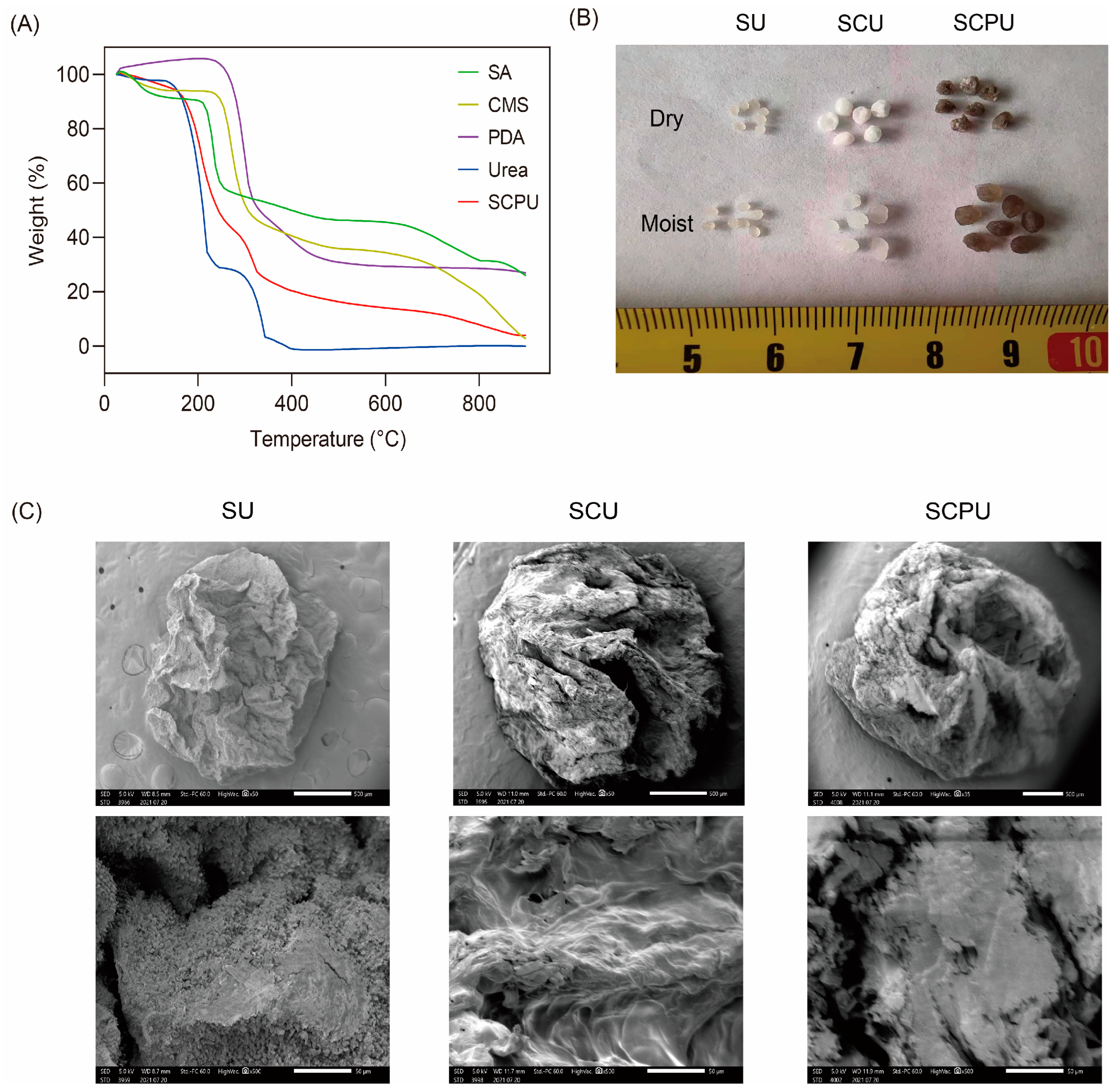
3.2. Thermogravimetric Analysis
3.3. SEM Analysis
3.4. Water Absorption, Retention, and Water-Holding Capacity of SCPU
3.5. Slow-Release Behavior and Mechanism of SCPU in Soil
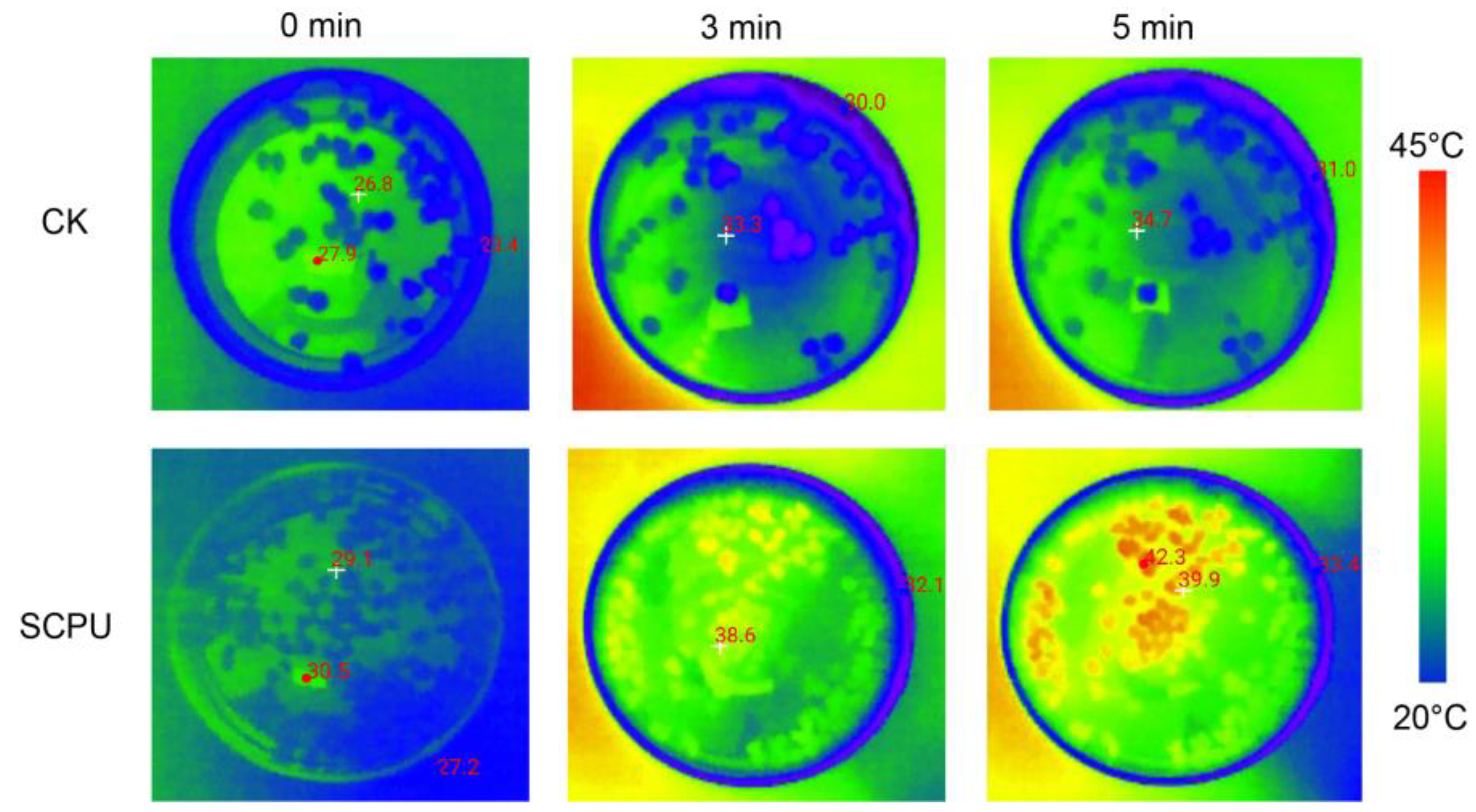
3.6. Photothermal Conversion Effect
3.7. Effects of SCPU on the Growth of Winter Wheat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, H.; Mei, P.; Wang, W.; Yin, Y.; Li, H.; Zheng, M.; Ou, X.; Cui, Z. Effects of super absorbent polymer on crop yield, water productivity and soil properties: A global meta-analysis. Agric. Water Manag. 2023, 282, 108290. [Google Scholar] [CrossRef]
- Popova, Z.; Kercheva, M. CERES model application for increasing preparedness to climate variability in agricultural planning-risk analyses. Phys. Chem. Earth. 2005, 30, 117–124. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef] [PubMed]
- Nooeaid, P.; Chuysinuan, P.; Pitakdantham, W.; Aryuwananon, D.; Techasakul, S.; Dechtrirat, D. Eco-Friendly polyvinyl alcohol/polylactic acid core/shell structured fibers as controlled-release fertilizers for sustainable agriculture. J. Polym. Environ. 2021, 29, 552–564. [Google Scholar] [CrossRef]
- Yahaya, S.M.; Mahmud, A.A.; Abdullahi, M.; Haruna, A. Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizer in soil: A review. Pedosphere 2023, 33, 385–406. [Google Scholar] [CrossRef]
- Wen, P.; Han, Y.; Wu, Z.; He, Y.; Ye, B.C.; Wang, J. Rapid synthesis of a corncob-based semi-interpenetrating polymer network slow-release nitrogen fertilizer by microwave irradiation to control water and nutrient losses. Arab. J. Chem. 2017, 10, 922–934. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, X.; Wei, X.; Kai, Z.; Xu, Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021, 11, 23015. [Google Scholar] [CrossRef] [PubMed]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control Release 2014, 181, 11–21. [Google Scholar] [CrossRef]
- Santos, A.C.S.; Henrique, H.M.; Cardoso, V.L.; Reis, M.H.M. Slow release fertilizer prepared with lignin and poly(vinylacetate) bioblend. Int. J. Biol. Macromol. 2021, 185, 543–550. [Google Scholar] [CrossRef]
- Cong, J.; Lu, P.; Zhang, M. Preparation and Characterization of Environmentally Friendly Controlled Release Fertilizers Coated by Leftovers-Based Polymer. Processes 2020, 8, 417. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Huang, M.; Liu, M. Urea intercalated halloysite/sodium alginate composite hydrogels for slow-release fertilizers. Appl. Clay Sci. 2023, 242, 107041. [Google Scholar] [CrossRef]
- Alharbi, K.; Ghoneim, A.; Ebid, A.; El-Hamshary, H.; El-Newehy, M.H. Controlled release of phosphorous fertilizer bound to carboxymethyl starch-g-polyacrylamide and maintaining a hydration level for the plant. Int. J. Biol. Macromol. 2018, 116, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, D.; Chen, X.; Yao, W.; Wang, Y.; Zheng, Z.; Tan, H.; Zhang, Y. Mussel-inspired polydopamine-modified cellulose nanocrystal fillers for the preparation of reinforced and UV-shielding poly (lactic acid) films. J. Mater. Res. Technol. 2022, 19, 4350–4359. [Google Scholar] [CrossRef]
- Du, P.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Cao, Y.; Han, R.; Shi, C.; Liang, B.; Xu, J. Melatonin and dopamine mediate the regulation of nitrogen uptake and metabolism at low ammonium levels in Malus hupehensis. Plant Physiol. Bioch. 2022, 171, 182–190. [Google Scholar] [CrossRef]
- Suppanucroa, N.; Nimpaiboon, A.; Boonchuay, K.; Khamkeaw, A.; Phisalaphong, M. Green composite sponge of natural rubber reinforced with cellulose filler using alginate as a dispersing agent. J. Mater. Res. Technol. 2023, 27, 3119–3130. [Google Scholar] [CrossRef]
- Aydınoğlu, D.; Karaca, N.; Ceylan, Ö. Natural Carrageenan/Psyllium Composite Hydrogels Embedded Montmorillonite and Investigation of Their Use in Agricultural Water Management. J. Polym. Environ. 2021, 29, 785–798. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mat. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef]
- Zheng, T.; Liang, Y.; Ye, S.; He, Z. Superabsorbent hydrogels as carriers for the controlled-release of urea: Experiments and a mathematical model describing the release rate. Biosyst. Eng. 2009, 102, 44–50. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; Mattoso, L.H.C.; Ribeiro, C. Nanocomposite PAAm/methyl cellulose/montmorillonite hydrogel: Evidence of synergistic effects for the slow release of fertilizers. J. Agric. Food Chem. 2013, 61, 7431–7439. [Google Scholar] [CrossRef]
- Olad, A.; Gharekhani, H.; Mirmohseni, A.; Bybordi, A. Study on the synergistic effect of clinoptilolite on the swelling kinetic and slow release behavior of maize bran-based superabsorbent nanocomposite. J. Polym. Res. 2016, 23, 241. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Z.; Yue, Q.; Gao, B.; Li, W.; Xu, X. Synthesis, characterization and swelling behavior of superabsorbent wheat straw graft copolymers. Bioresour. Technol. 2012, 118, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.S.; Moura, M.R.; Glenn, G.M.; Aouada, F.A. Thermal, microstructural, and spectroscopic analysis of Ca2+ alginate/clay nanocomposite hydrogel beads. J. Mol. Liq. 2018, 265, 327–336. [Google Scholar] [CrossRef]
- Dankar, I.; Haddarah, A.; Omar, F.E.L.; Pujola, M.; Sepulcre, F. Characterization of food additive-potato starch complexes by FTIR and X-ray diffraction. Food Chem. 2018, 260, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarska, K.; Grabowska, B.; Spychaj, T.; Zdanowicz, M.; Sitarz, M.; Bobrowski, A.; Cukrowicz, S. Effect of microwave treatment on structure of binders based on sodium carboxymethyl starch: FT-IR, FT-Raman and XRD investigations. Spectrochim. Acta A 2018, 199, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, Y.; Zhao, X.; He, X.; Zhang, R.; Zhao, J.; Fan, X.; Jiang, Z. Antifouling, High-flux Nanofiltration Membranes Enabled by Dual Functional Polydopamine. ACS Appl. Mater. Interfaces 2014, 6, 5548–5557. [Google Scholar] [CrossRef]
- Yadav, T.; Mukherjee, V. Interpretation of IR and Raman spectra of dopamine neurotransmitter and effect of hydrogen bond in HCl. J. Mol. Struct. 2018, 1160, 256–270. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Vo, P.T.; Nguyen, H.T.; Trinh, H.T.; Nguyen, V.M.; Le, A.T.; Tran, H.Q.; Nguyen, T.T.T. The nitrogen slow-release fertilizer based on urea incorporating chitosan and poly (vinyl alcohol) blend. Environ. Technol. Innov. 2021, 22, 101528. [Google Scholar] [CrossRef]
- Cai, X.; Du, X.; Cui, D.; Wang, X.; Yang, Z.; Zhu, G. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xanthan gum combinations microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Elhassani, C.E.; Essamlali, Y.; Aqlil, M.; Nzenguet, A.M.; Ganetri, I.; Zahouily, M. Urea-impregnated HAP encapsulated by lignocellulosic biomass-extruded composites: A novel slow-release fertilizer. Environ. Technol. Innov. 2019, 15, 100403. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Li, W.; Liu, Z.; Liu, Y.; Wei, H.; Li, J. Synthesis and characterization of double-network hydrogels based on sodium alginate and halloysite for slow release fertilizers. Int. J. Biol. Macromol. 2020, 164, 557–565. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, B.; Li, X.; Yu, Y.; Tian, Y.; Xu, X.; Jin, Z. Synthesis of pH- and ionic strength-responsive microgels and their interactions with lysozyme. Int. J. Biol. Macromol. 2015, 79, 392–397. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Cho, J.H.; Iyer, P.; Baranowitz, S.; Ellison, C.J. Thermooxidative stabilization of polymers using natural and synthetic melanins. Macromolecules 2011, 44, 9499–9507. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Chu, H.; Li, J. Preparation and characterization of slow-release and water retention fertilizer based on starch and halloysite. Int. J. Biol. Macromol. 2019, 133, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Azaam, M.M.; El-nshar, E.M. Sodium alginate-g-poly(acrylic acid-co-2-hydroxyethyl methacrylate)/montmorillonite superabsorbent composite: Preparation, swelling investigation and its application as a slow-release fertilizer. Arab. J. Chem. 2019, 12, 847–856. [Google Scholar] [CrossRef]
- Ebadi, A.; Rafati, A.A.; Bavafa, S.; Mohammadi, M. Kinetic and theoretical studies of novel biodegradable thermo-sensitive xerogels based on PEG/PVP/silica for sustained release of enrofloxacin. Appl. Surf. Sci. 2017, 425, 282–290. [Google Scholar] [CrossRef]
- Babavalian, A.; Tekie, F.S.M.; Ayazi, H.; Ranjbar, S.; Varshochian, R.; Rad-Malelkshahi, M.; Akhavan, O.; Dinarvand, R. Reduced polydopamine coated graphene for delivery of Hset1 antisense as a photothermal and gene therapy of breast cancer. J. Drug Deliv. Sci. Technol. 2022, 73, 103462. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Zhou, J.; Xu, P.; Wang, C.; Shi, R.; Wang, H.; Wang, H.; Guo, Z.; Chen, Q. In situ one-pot synthesis of MOF-Polydopamine hybrid nanogels with enhanced photothermal effect for targeted cancer therapy. Adv. Sci. 2018, 5, 1800287. [Google Scholar] [CrossRef]
- Dong, G.; Mu, Z.; Liu, D.; Shang, L.; Zhang, W.; Gao, Y.; Zhao, M.; Zhang, X.; Chen, S.; Wei, M. Starch phosphate carbamate hydrogel based slow-release urea formulation with good water retentivity. Int. J. Biol. Macromol. 2021, 190, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Haque, K.M.S.; Khan, M.Z.H. A review on application of controlled released fertilizers influencing the sustainable agricultural production: A Cleaner production process. Environ. Technol. Innov. 2021, 23, 101697. [Google Scholar] [CrossRef]
- Wan, X.; Wu, W.; Li, C.; Liu, Y.; Wen, X.; Liao, Y. Soil ammonia volatilization following urea application suppresses root hair formation and reduces seed germination in six wheat varieties. Environ. Exp. Bot. 2016, 132, 130–139. [Google Scholar] [CrossRef]
- Lawes, R.A.; Oliver, Y.M.; Robertson, M.J. Integrating the effects of climate and plant available soil water holding capacity on wheat yield. Field Crop. Res. 2019, 113, 297–305. [Google Scholar] [CrossRef]
- Paradelo, R.; Basanta, R.; Barral, M.T. Water-holding capacity and plant growth in compost-based substrates modified with polyacrylamide, guar gum or bentonite. Sci. Hortic.-Amst. 2019, 243, 344–349. [Google Scholar] [CrossRef]
- Du, P.; Yin, B.; Cao, Y.; Han, R.; Ji, J.; He, X.; Liang, B.; Xu, J. Beneficial effects of exogenous melatonin and dopamine on low nitrate stress in Malus hupehensis. Front. Plant Sci. 2022, 28, 807472. [Google Scholar] [CrossRef]


| Model | Parameter | Value |
|---|---|---|
| Zero-order | R2 | 0.814 |
| K0 | 2.734 | |
| First-order | R2 | 0.982 |
| K1 | 0.168 | |
| Higuchi | R2 | 0.973 |
| KH | 15.806 | |
| Ritger–Peppas | R2 | 0.987 |
| n | 0.392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ma, Y.; Chang, F.; Zhu, H.; Tian, C.; Jia, F.; Ke, Y.; Dai, J. Slow-Release Urea Fertilizer with Water Retention and Photosensitivity Properties Based on Sodium Alginate/Carboxymethyl Starch Sodium/Polydopamine. Processes 2024, 12, 842. https://doi.org/10.3390/pr12040842
Li Y, Ma Y, Chang F, Zhu H, Tian C, Jia F, Ke Y, Dai J. Slow-Release Urea Fertilizer with Water Retention and Photosensitivity Properties Based on Sodium Alginate/Carboxymethyl Starch Sodium/Polydopamine. Processes. 2024; 12(4):842. https://doi.org/10.3390/pr12040842
Chicago/Turabian StyleLi, Yan, Yu Ma, Fan Chang, Haiyun Zhu, Chengshan Tian, Fengan Jia, Yang Ke, and Jiakun Dai. 2024. "Slow-Release Urea Fertilizer with Water Retention and Photosensitivity Properties Based on Sodium Alginate/Carboxymethyl Starch Sodium/Polydopamine" Processes 12, no. 4: 842. https://doi.org/10.3390/pr12040842