Effects of Alignment of Weak Interaction Sites in Molecular Shape Recognition High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Amino Acid Derivatives-Bonded Stationary Phases
2.2. Instrumentations
2.3. Liquid Chromatography
3. Results and Discussions
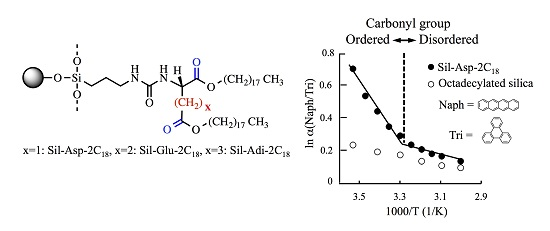
3.1. Evaluation of the Molecular Orientation of Organic Phases
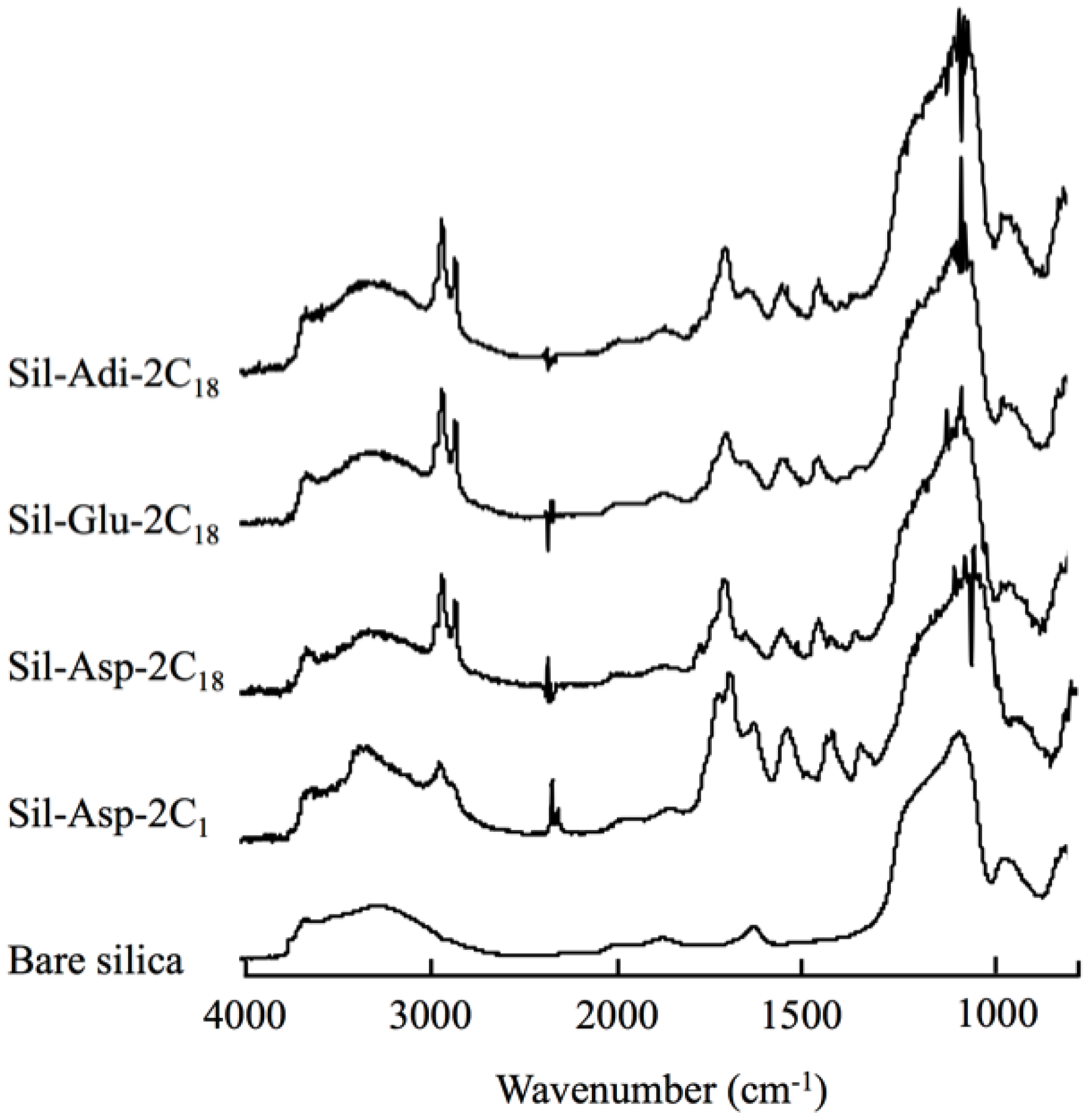
3.2. Bonding Density of Amino Acid Derivatives on Silica Surface
3.3. Reversed Phase Liquid Chromatography
3.4. Hydrophilic Interaction Chromatography
3.5. Separation of Tocopherol Isomers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, M.; Feng, J.; Chen, W.; Li, L.; Duan, H.; Luo, C. Improvement of the chromatographic separation performance of an imidazolium ionic liquid functionalized silica column by in situ anion-exchange with dodecyl sulfonate and dodecylbenzene sulfonate anions. J. Sep. Sci. 2014, 37, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Mallik, A.K.; Sawada, T.; Takafuji, M.; Ihara, H. New surface-confined ionic liquid stationary phases with enhanced chromatographic selectivity and stability by co-immobilization of polymerizable anion and cation pairs. Chem. Commun. 2012, 48, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mai, W.; Zhao, L.; Guo, Y.; Qiu, H. A polar-embedded C30 stationary phase: Preparation and evaluation. J. Chromatogr. A 2015, 1388, 133–140. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Scully, N.M.; Glennon, J.D. Polar-Embedded and Polar-Endcapped Stationary Phases for LC. Anal. Lett. 2010, 43, 1609–1629. [Google Scholar] [CrossRef]
- Noguchi, H.; Charoenraks, T.; Takafuji, M.; Ihara, H. Effects of substitution groups of glutamide-derived molecular gels on molecular shape recognition. J. Chromatogr. A 2015, 1392, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.K.; Qiu, H.; Takafuji, M.; Ihara, H. High molecular-shape selectivity by molecular gel-forming compounds: Bioactive and shape-constrained isomers through the integration and orientation of weak interaction sites. Chem. Commun. 2011, 47, 10341–10343. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Takafuji, M.; Ansarian, H.R.; Ihara, H. Molecular Shape Selectivity through Multiple Carbonyl-π Interactions with Noncrystalline Solid Phase for RP-HPLC. Anal. Chem. 2005, 77, 6671–6681. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Takafuji, M.; Ihara, H. Chromatographic evaluation of a newly designed peptide-silica stationary phase in reverse phase liquid chromatography and hydrophilic interaction liquid chromatography: Mixed mode behavior. J. Chromatogr. A 2012, 1266, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Inoue, Y.; Kishikawa, N.; Kuroda, N. Preparation and characterization of surfactin-modified silica stationary phase for reversed-phase and hydrophilic interaction liquid chromatography. J. Chromatogr. A 2014, 1371, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.C.; Wise, S.A. Synthesis and characterization of polymeric C18 stationary phases for liquid chromatography. Anal. Chem. 1984, 56, 504–510. [Google Scholar] [CrossRef]
- Jinno, K.; Nagoshi, T.; Tanaka, N.; Okamoto, M.; Fetzer, J.C.; Biggs, W.R. Eluation behaviour of planar and non-planar polycyclic aromatic hydrocarbons on various chemically bonded stationary phases in liquid chromatography. J. Chromatogr. A 1987, 392, 75–82. [Google Scholar] [CrossRef]
- Silva, C.R.; Airoldi, C.; Collins, K.E.; Collins, C.H. Preparation and characterization of a new C18 urea phase based on titanized silica. J. Chromatogr. A 2005, 1087, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Layne, J. Characterization and comparison of the chromatographic performance of conventional, polar-embedded, and polar-endcapped reversed-phase liquid chromatography stationary phases. J. Chromatogr. A 2002, 957, 149–164. [Google Scholar] [CrossRef]
- Kučerová, G.; Kalíková, K.; Procházková, H.; Popr, M.; Jindřich, J.; Coufal, P.; Tesařová, E. Chromatographic Characterization of a New Cationic β-CD Based Stationary Phase Prepared by Dynamic Coating. Chromatographia 2016, 79, 529–536. [Google Scholar] [CrossRef]
- Kakhki, R.M. Application of crown ethers as stationary phase in the chromatographic methods. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 11–22. [Google Scholar] [CrossRef]
- Noguchi, H.; Takafuji, M.; Maurizot, V.; Huc, I. Chiral separation by a terminal chirality triggered P-helical quinoline oligoamide foldamer. J. Chromatogr. A 2016, 1437, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, R.; Wang, H.; Sakai, R.; Satho, T.; Kakuchi, T.; Liu, L.; Okamoto, Y. Influence of Helical Structure on Chiral Recognition of Poly(phenylacetylene)s Bearing Phenylcarbamate Residues of l-Phenylglycinol and Amide Linage as Pendants. Chirality 2015, 27, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Ihara, H.; Sakaki, S.; Shosenji, H.; Hirayama, C. Chromatographic separation of geometrical isomers using highly oriented polymer-immobilized silica gels. J. Chromatogr. A 1994, 672, 237–241. [Google Scholar] [CrossRef]
- Goto, Y.; Nakashima, K.; Mitsuishi, K.; Takafuji, M.; Sakaki, S.; Ihara, H. Selectivity enhancement of diastereomer separation in RPLC using crystalline-organic phase-bonded silica. Chromatographia 2002, 56, 19–23. [Google Scholar] [CrossRef]
- Fowler, L.S.; Thomas, L.H.; Ellis, D.; Sutherland, A. A one-pot, reductive amination/6-endo-trig cyclisation for the stereoselective synthesis of 6-substituted-4-oxopipecolic acids. Chem. Commun. 2011, 47, 6569–6571. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, G.E.; Pikaart, K.A.; Galan, L. Preparation of various bonded phases for HPLC using monochlorosilanes. J. Chromatogr. 1980, 3, 1437–1464. [Google Scholar] [CrossRef]
- Sander, L.C.; Pursch, M.; Wise, S.A. Shape Selectivity for Constrained Solutes in Reversed-Phase Liquid Chromatography. Anal. Chem. 1999, 71, 4821–4830. [Google Scholar] [CrossRef] [PubMed]
- Jinno, K.; Kawasaki, K. Correlation between the Retention Data of Polycyclic Aromatic Hydrocarbons. Chromatograhia 1983, 17, 445–449. [Google Scholar] [CrossRef]
- Wise, S.A.; Bonnett, W.J.; Guenther, F.R.; May, W.E. A Relationship between Reversed-Phase C18 Liquid Chromatographic Retention and the Shape of Polycyclic Aromatic Hydrocarbons. J. Chromatogr. Sci. 1981, 19, 457–465. [Google Scholar] [CrossRef]
- Hemström, P.; Irgum, K. Hydrophilic interaction chromatography. J. Sep. Sci. 2006, 29, 1784–1821. [Google Scholar] [CrossRef] [PubMed]
- Kahsay, G.; Song, H.; Schepdael, A.V.; Cabooter, D.; Adams, E. Hydrophilic interaction chromatography (HILIC) in the analysis of antibiotics. J. Pharm. Biomed. Anal. 2014, 87, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Bicker, W.; Linder, W. Separation properties of novel and commercial polar stationary phases in hydrophilic interaction and reversed-phase liquid chromatography mode. J. Sep. Sci. 2008, 31, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Verdolino, V.; Cammi, R.; Munk, B.H.; Schlegel, B. Calculation of pKa Values of Nucleobases and the Guanine Oxidation Products Guanidinohydantoin and Spiroiminodihydantoin using Density Functional Theory and a Polarizable Continuum Model. J. Phys. Chem. B 2008, 112, 16860–16873. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Hou, G.; Xue, Z.; Zhang, L.; Zhou, Y.; Liu, C.; Liu, Y.; Li, Z. Vitamin E isomer δ-tocopherol enhances the efficiency of neural stem cell differentiation via l-type calcium channel. Neurosci. Lett. 2015, 585, 166–170. [Google Scholar] [CrossRef] [PubMed]






| %C | %N | %H | C/N | Bonded Amount | ||||
|---|---|---|---|---|---|---|---|---|
| Found | calcd. | μmol·m−2 | wt % | TG % | ||||
| Sil-Asp-2C18 | 17.05 | 0.9 | 2.86 | 18.94 | 18.86 | 0.98 | 23.32 | 23.00 |
| Sil-Glu-2C18 | 13.71 | 0.7 | 2.37 | 19.59 | 19.29 | 0.77 | 18.69 | 18.20 |
| Sil-Adi-2C18 | 15.36 | 0.8 | 2.66 | 19.20 | 19.71 | 0.84 | 20.88 | 22.06 |
| Sil-Asp-2C1 | 14.59 | 3.5 | 2.28 | 4.17 | 4.29 | 3.68 | 33.23 | 26.19 |
| Analyte | Sil-Asp-2C18 | Sil-Glu-2C18 | Sil-Adi-2C18 | ODS | |||||
|---|---|---|---|---|---|---|---|---|---|
| k | α | k | α | k | α | k | α | ||
| cis-Stilbene |  | 1.01 | - | 0.99 | - | 1.03 | - | 1.74 | - |
| trans-Stilbene |  | 1.50 | 1.48 | 1.39 | 1.40 | 1.43 | 1.38 | 1.84 | 1.06 |
| o-Terphenyl |  | 1.32 | - | 0.88 | - | 1.23 | - | 2.27 | - |
| m-Terphenyl | 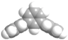 | 2.45 | 1.86 | 1.88 | 2.14 | 2.12 | 1.72 | 3.22 | 1.42 |
| p-Terphenyl |  | 3.14 | 2.39 | 2.16 | 2.46 | 2.50 | 2.03 | 3.26 | 1.43 |
| Triphenylene |  | 5.02 | 3.81 | 2.38 | 2.72 | 3.67 | 2.99 | 3.51 | 1.54 |
| Triphenylene |  | 5.02 | - | 2.38 | - | 3.67 | - | 3.51 | - |
| Benz[a]anthracene |  | 5.66 | 1.13 | 2.56 | 1.07 | 4.05 | 1.10 | 3.73 | 1.06 |
| Chrysene |  | 5.84 | 1.16 | 2.58 | 1.08 | 4.11 | 1.12 | 3.69 | 1.05 |
| Naphthacene |  | 7.90 | 1.57 | 3.11 | 1.31 | 5.08 | 1.38 | 4.26 | 1.21 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, H.; Liu, T.; Takafuji, M.; Ihara, H. Effects of Alignment of Weak Interaction Sites in Molecular Shape Recognition High-Performance Liquid Chromatography. Separations 2016, 3, 25. https://doi.org/10.3390/separations3030025
Noguchi H, Liu T, Takafuji M, Ihara H. Effects of Alignment of Weak Interaction Sites in Molecular Shape Recognition High-Performance Liquid Chromatography. Separations. 2016; 3(3):25. https://doi.org/10.3390/separations3030025
Chicago/Turabian StyleNoguchi, Hiroki, Tianhang Liu, Makoto Takafuji, and Hirotaka Ihara. 2016. "Effects of Alignment of Weak Interaction Sites in Molecular Shape Recognition High-Performance Liquid Chromatography" Separations 3, no. 3: 25. https://doi.org/10.3390/separations3030025