Agrocybe aegerita Polysaccharide Combined with Bifidobacterium lactis Bb-12 Attenuates Aging-Related Oxidative Stress and Restores Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fly Strains and Diet
2.3. Lifespan Assay
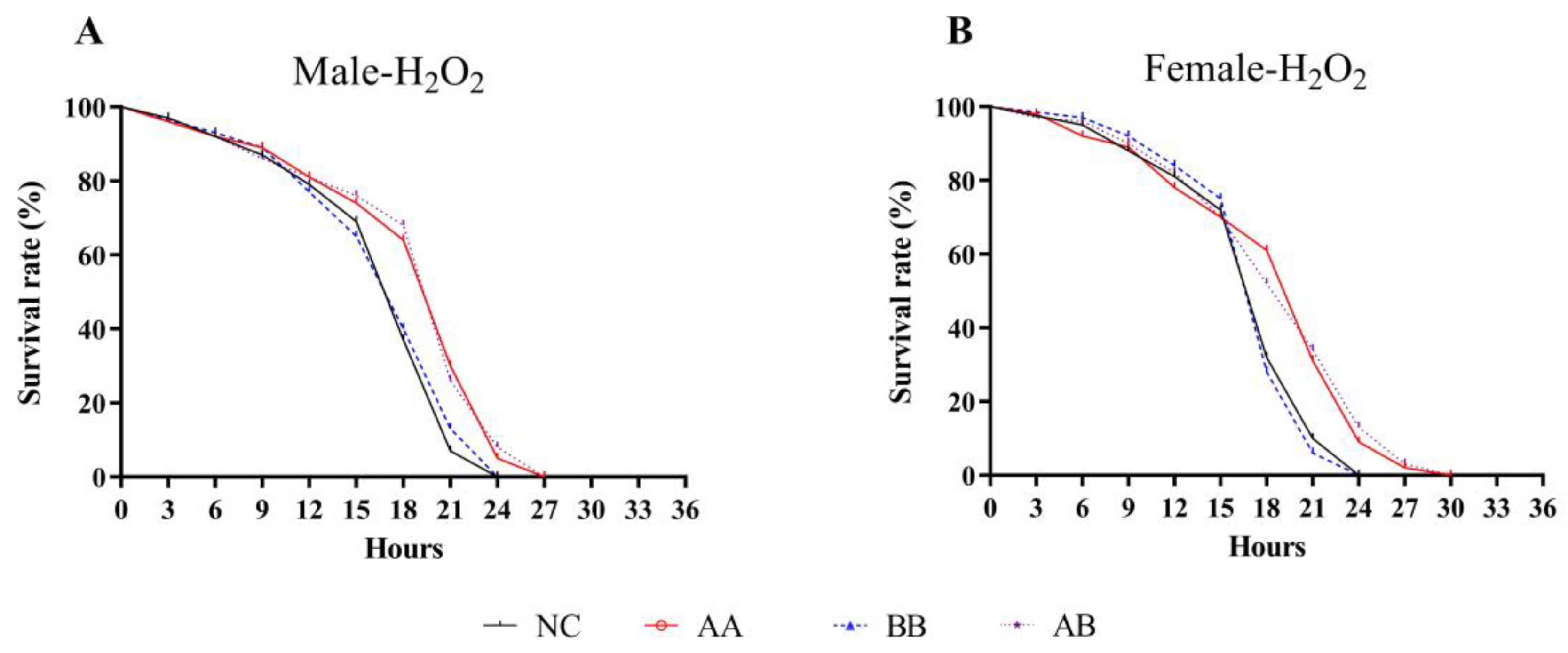
2.4. Hydrogen Peroxide (H2O2) Challenge Assay
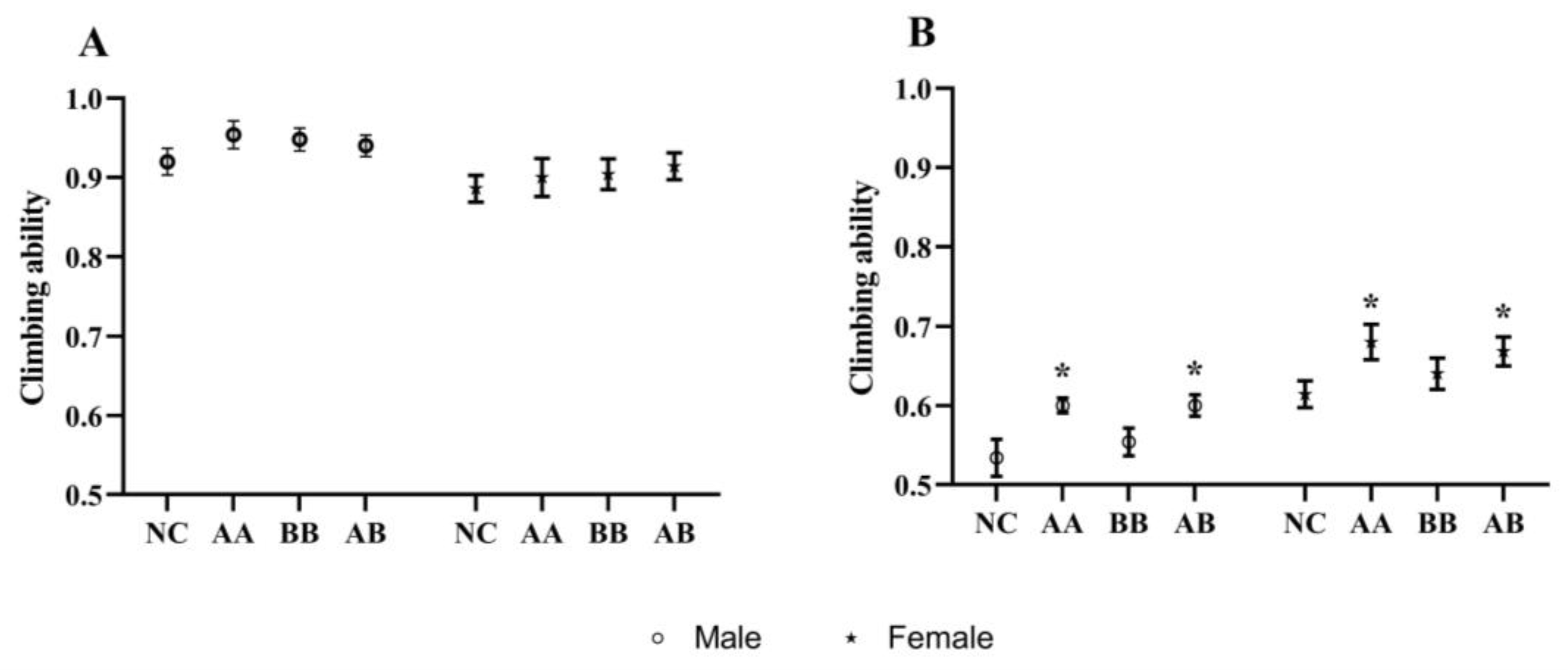
2.5. Climbing Ability Assay
2.6. Animals and Treatment
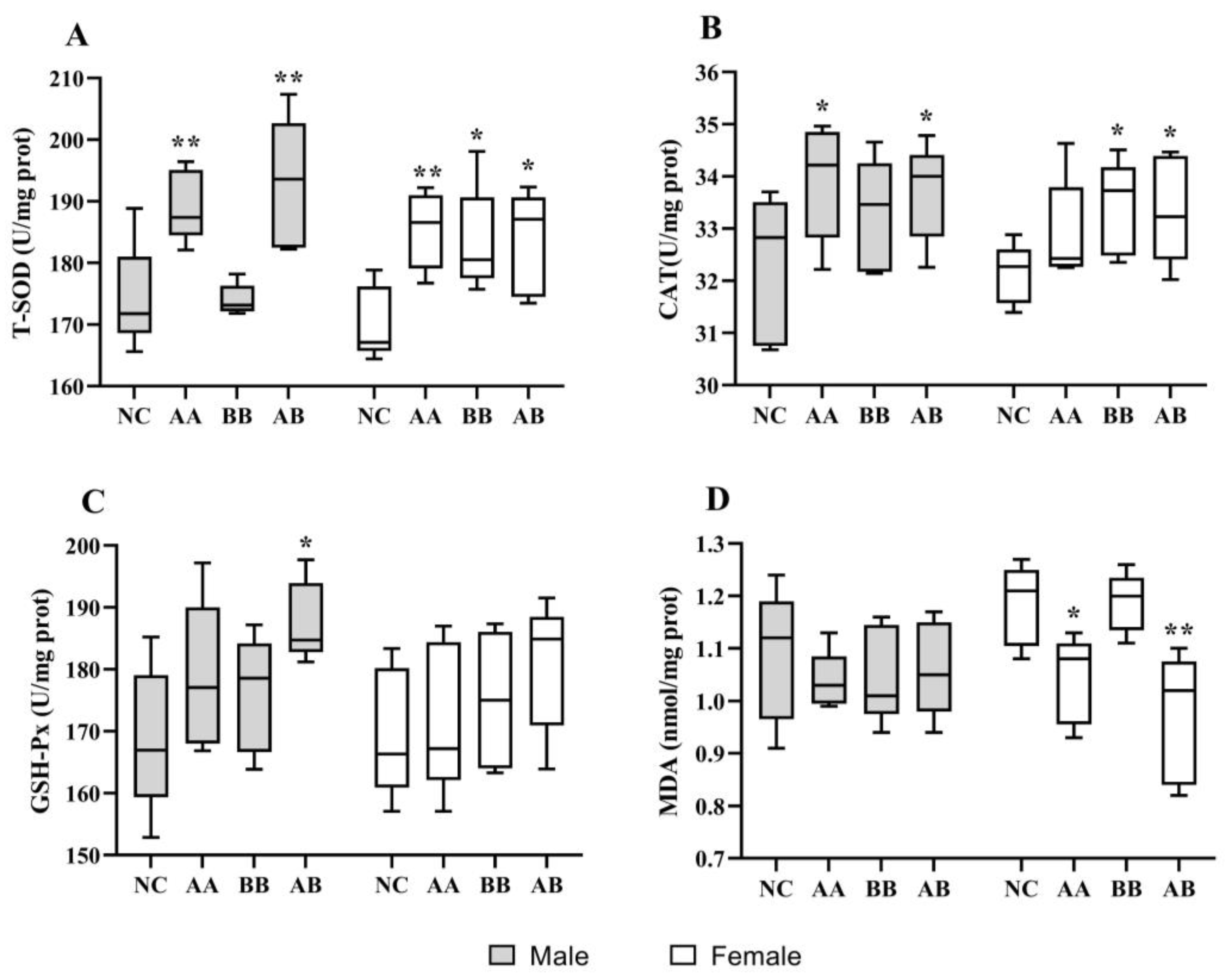
2.7. Biochemical Analysis
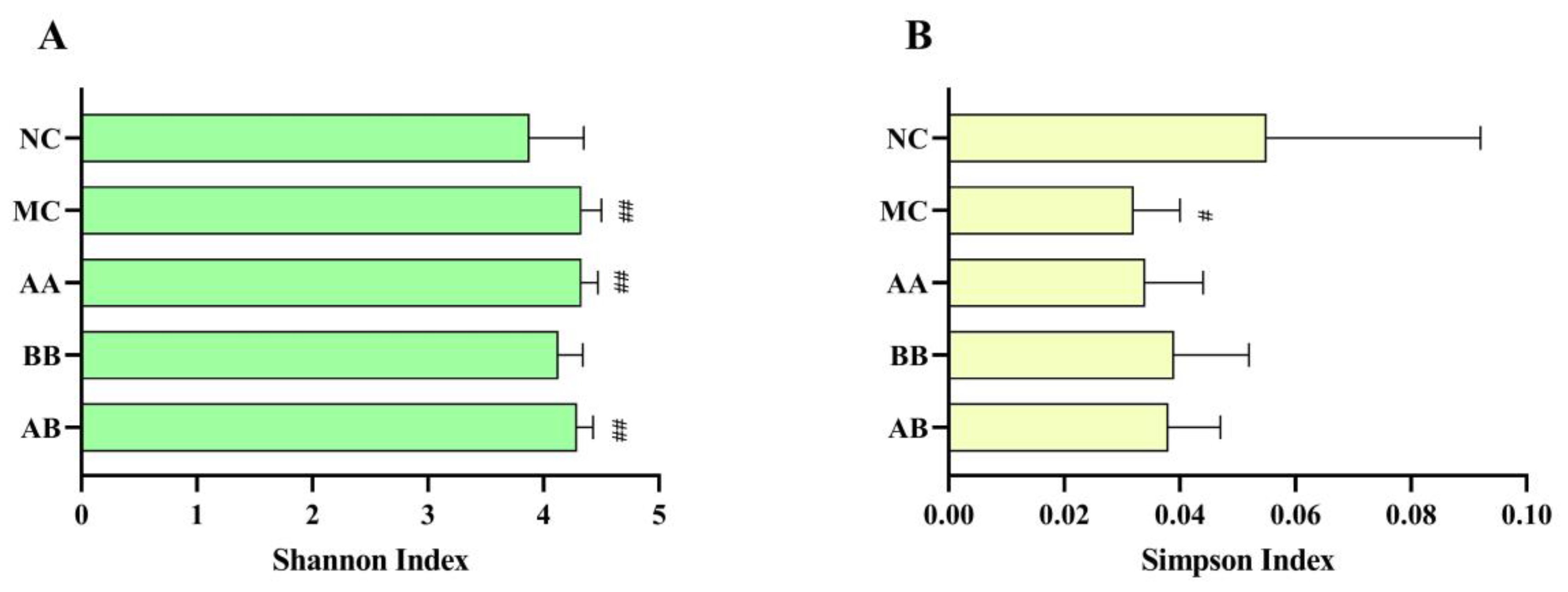
2.8. Intestinal Microbiota Analysis
2.9. Data Analysis
3. Results
3.1. Effect on the Longevity of D. melanogaster
3.2. Effect on H2O2-Induced Intensive Oxidative Stress in D. melanogaster
3.3. Effect on the Climbing Ability of D. melanogaster
3.4. Effect on the Level of Antioxidant Activities in D. melanogaster
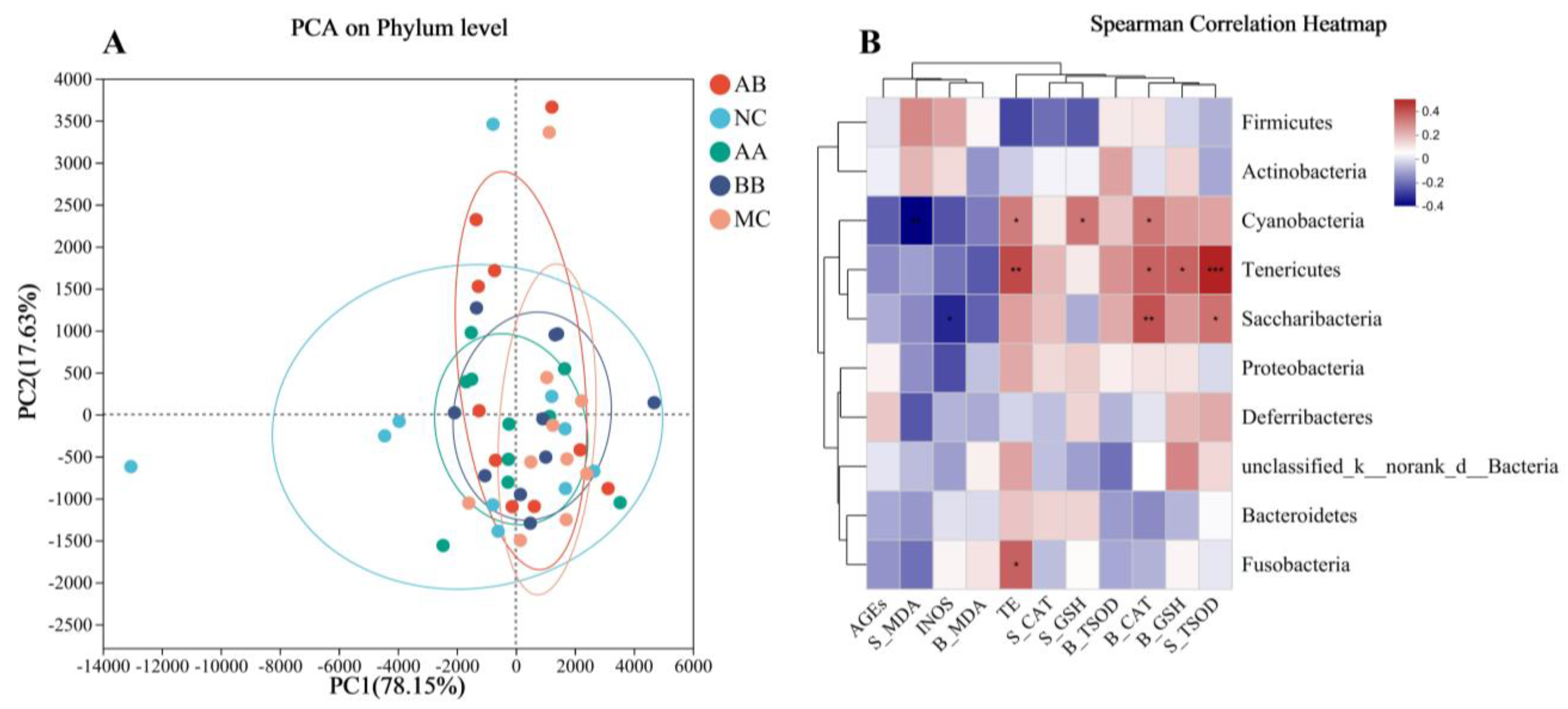
3.5. Effect on the Level of Antioxidant Activities and Aging Biomarkers in Mice
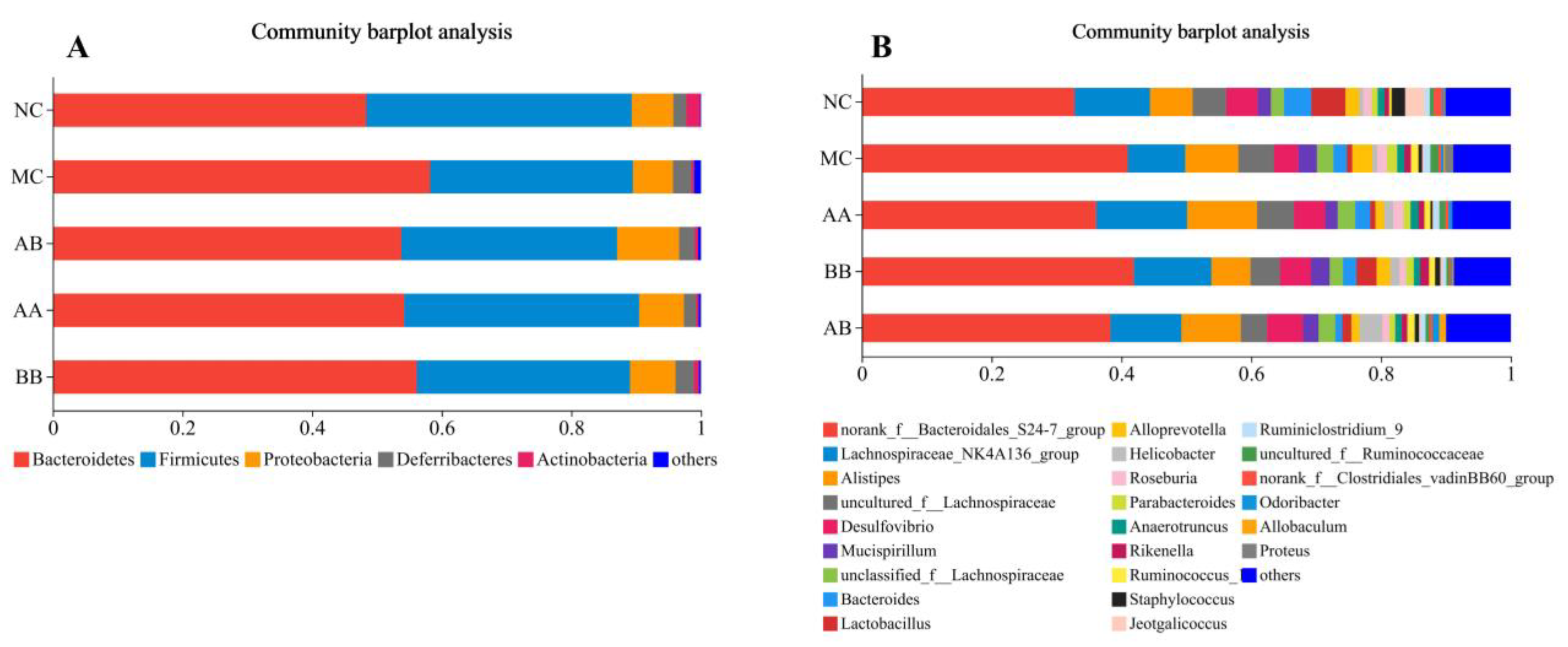
3.6. Effect on the Gut Microbiota Composition of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Simon, M.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Denham, H. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell Senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrosky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Yun, I.J.; Kim, K.H.; Lim, S.H.; Ham, H.J.; Eum, W.S.; Joo, J.H. Amino acid and fatty acid compositions of Agrocybe chaxingu, an edible mushroom. J. Food Compos. Anal. 2011, 24, 175–178. [Google Scholar] [CrossRef]
- Jing, H.J.; Zhang, Q.; Liu, M.; Zhang, J.J.; Zhang, C.; Li, S.S.; Ren, Z.Z.; Gao, Z.; Liu, X.T.; Jia, L. Polysaccharides with antioxidative and antiaging activities from enzymatic-extractable mycelium by Agrocybe aegerita (Brig.) Sing. J. Evid.-Based Complement. Altern. Med. 2018, 2018, 1584647. [Google Scholar] [CrossRef]
- Jing, H.J.; Li, J.; Zhang, J.J.; Wang, W.S.; Li, S.S.; Ren, Z.Z.; Gao, Z.; Song, X.L.; Wang, X.X.; Jia, L. The antioxidative and anti-aging effects of acidic- and alkalic-extractable mycelium polysaccharides by Agrocybe aegerita (Brig.) Sing. Int. J. Biol. Macromol. 2018, 106, 1270–1278. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Zhang, J.H.; Yang, H.L.; Yang, Z.F.; Xue, H.B.; Wu, F.; Wang, Z.Y.J.; Xie, L.; Chen, Y.Y. Acetylation modification and antioxidant activity of polysaccharides from Agrocybe cylindracea. J. Food Meas. Charact. 2022, 16, 1911–1919. [Google Scholar] [CrossRef]
- Du, B.; Peng, F.; Xie, Y.; Wang, H.Y.; Wu, J.H.; Liu, C.; Yang, Y.D. Optimization extraction and antioxidant activity of crude polysaccharide from chestnut mushroom (Agrocybe aegerita) by accelerated solvent extraction combined with response surface methodology (ASE-RSM). Molecules 2022, 27, 2380. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Liu, D.; Chen, Y.H.; Zhong, R.T.; Gao, L.Y.; Yang, C.F.; Ai, C.; El-Seedi, H.R.; Zhao, C. Physicochemical characterization of a polysaccharide from Agrocybe aegirita and its anti-ageing activity. Carbohydr. Polym. 2020, 236, 116056. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wu, L.X.; Tong, A.J.; Zhen, H.M.; Han, D.; Yuan, H.Y.; Li, F.N.; Wang, C.T.; Fan, G.S. Anti-aging effect of Agrocybe aegerita polysaccharide through regulation of oxidative stress and gut microbiota. Foods 2022, 11, 3783. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.X.; Liu, X.Y.; Hu, R.K.; Chen, Y.X.; Xiao, M.F.; Liu, B.; Zeng, F. Prebiotic Agrocybe cylindracea crude polysaccharides combined with Lactobacillus rhamnosus GG postpone aging-related oxidative stress in mice. Food Funct. 2022, 13, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zheng, M.F.; Ye, S.G.; Wu, X.B.; Chen, J.Y. Agrocybe aegerita polysaccharide combined with chemotherapy improves tumor necrosis factor-α and interferon-γ levels in rat esophageal carcinoma. Dis. Esophagus 2013, 26, 859–863. [Google Scholar] [CrossRef]
- Lin, S.L.; Ching, L.T.; Lam, K.; Cheung, P.C.K. Anti-angiogenic effect of water extract from the fruiting body of Agrocybe aegerita. LWT-Food Sci. Technol. 2017, 75, 155–163. [Google Scholar] [CrossRef]
- Li, G.; Liu, X.; Cong, S.; Deng, Y.; Zheng, X. A novel serine protease with anticoagulant and fibrinolytic activities from the fruiting bodies of mushroom Agrocybe aegerita. Int. J. Biol. Macromol. 2021, 168, 631–639. [Google Scholar] [CrossRef]
- Taormina, G.; Ferrante, F.; Vieni, S.; Grassi, N.; Russo, A.; Mirisola, M.G. Longevity: Lesson from model organisms. Genes 2019, 10, 518. [Google Scholar] [CrossRef]
- Weinrich, T.W.; Coyne, A.; Salt, T.E.; Hogg, C.; Jeffery, G. Improving mitochondrial function significantly reduces metabolic, visual, motor and cognitive decline in aged Drosophila melanogaster. Neurobiol. Aging 2017, 60, 34–43. [Google Scholar] [CrossRef]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.F.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. β-glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. Rev. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Liang, J.J.; Zhang, M.N.; Wang, X.N.; Ren, Y.C.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Edible fungal polysaccharides, the gut microbiota, and host health. Carbohydr. Polym. 2021, 273, 118558. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.L.; Guo, D.D.; Fang, L.; Sang, T.T.; Wu, J.J.; Guo, C.J.; Wang, Y.J.; Wang, Y.; Chen, C.J.; Chen, J.J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.X.; Liu, X.; Cheng, Y.W.; Yan, X.M.; Wu, S.C. Gut microbiota and aging. Crit. Rev. Food Sci. 2022, 62, 3509–3534. [Google Scholar] [CrossRef] [PubMed]
- Jayanama, K.; Theou, O. Effects of probiotics and prebiotics on frailty and ageing: A narrative review. Curr. Clin. Pharmacol. 2020, 15, 183–192. [Google Scholar] [CrossRef]
- Chenhuichen, C.; Cabello-Olmo, M.; Barajas, M.; Izquierdo, M.; Ramirez-Velez, R.; Zambom-Ferraresi, F.; Martinez-Velilla, N. Impact of probiotics and prebiotics in the modulation of the major events of the aging process: A systematic review of randomized controlled trials. Exp. Gerontol. 2022, 164, 111809. [Google Scholar] [CrossRef]
- Toward, R.E.; Montandon, S.L.; Walton, G.E.; Gibson, G.R. Effect of prebiotics on the human gut microbiota of elderly persons. Gut Microbes 2012, 3, 57–60. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol.-MYSORE 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Mohanty, D.; Misra, S.; Mohapatra, S.; Sahu, P.S. Prebiotics and synbiotics: Recent concepts in nutrition. Food Biosci. 2018, 26, 152–160. [Google Scholar] [CrossRef]
- Martinez, V.G.; Javadi, C.S.; Ngo, E.; Ngo, L.; Lagow, R.D.; Zhang, B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev. Neurobiol. 2007, 67, 778–791. [Google Scholar] [CrossRef]
- Kidera, H.; Hatabu, T.; Takahashi, K.H. Apoptosis inhibition mitigates aging effects in Drosophila melanogaster. Genetica 2020, 148, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Iliadi, K.G.; Boulianne, G.L. Age-related behavioral changes in Drosophila. Ann. N. Y. Acad. Sci. 2010, 1197, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wickens, A.P. Ageing and the free radical theory. Respir. Physiol. 2001, 128, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Mishra, N.; Dhas, Y. Ageing: Consequences of excessive free radicals and inflammation. Curr. Sci. 2016, 111, 1787–1793. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Morava, E. Galactose supplementation in phosphoglucomutase-1 deficiency; review and outlook for a novel treatable CDG. Mol. Genet. Metab. 2014, 112, 275–279. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. D-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Haider, S.; Liaquat, L.; Shahzad, S.; Sadir, S.; Madiha, S.; Batool, Z.; Tabassum, S.; Saleem, S.; Naqvi, F.; Perveen, T. A high dose of short term exogenous D-galactose administration in young male rats produces symptoms simulating the natural aging process. Life Sci. 2015, 124, 110–119. [Google Scholar] [CrossRef]
- Shwe, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp. Gerontol. 2018, 101, 13–36. [Google Scholar] [CrossRef]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the Balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Zhang, R.F.; Yan, X.Y.; Fan, K.L. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Frimat, M.; Daroux, M.; Litke, R.; Nevière, R.; Tessier, F.J.; Boulanger, E. Kidney, heart and brain: Three organs targeted by ageing and glycation. Clin. Sci. 2017, 131, 1069–1092. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, M.I.; Shcherbakova, D.M.; Dontsova, O.A. Telomerase: Structure, functions, and activity regulation. Biochemistry 2010, 75, 1563–1583. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut microbiota and extreme longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- Tuikhar, N.; Keisam, S.; Labala, R.K.; Imrat; Ramakrishnan, P.; Arunkumar, M.C.; Ahmed, G.; Biagi, E.; Jeyaram, K. Comparative analysis of the gut microbiota in centenarians and young adults shows a common signature across genotypically non-related populations. Mech. Ageing Dev. 2019, 179, 23–35. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, K.A.; Ahn, Y.T.; Jeong, J.J.; Huh, C.S.; Kim, D.H. Comparative analysis of gut microbiota in elderly people of urbanized towns and longevity villages. BMC Microbiol. 2015, 15, 49. [Google Scholar] [CrossRef]
- Kumar, M.; Babaei, P.; Ji, B.; Nielsen, J. Human gut microbiota and healthy aging: Recent developments and future prospective. Nutr. Healthy Aging 2015, 4, 3–16. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Arnoux, J.; O’Toole, P.W. Metagenomic analysis reveals distinct patterns of gut lactobacillus prevalence, abundance, and geographical variation in health and disease. Gut Microbes 2020, 12, 1822729. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.G.; Qi, H.B.; Zhang, Z.Q.; Wang, E.L.; Yun, H.; Yan, H.; Su, X.M.; Liu, Y.Q.; Tang, Z.Z.; Gao, Y.H.; et al. Gut REG3γ-associated Lactobacillus induces anti-inflammatory macrophages to maintain adipose tissue homeostasis. Front. Immunol. 2017, 8, 1063. [Google Scholar] [CrossRef] [PubMed]
- Won, S.M.; Lee, N.Y.; Oh, K.K.; Gupta, H.; Sharma, S.P.; Kim, K.H.; Kim, B.K.; Joung, H.C.; Jeong, J.J.; Ganesan, R.; et al. Gut Lactobacillus and Probiotics Lactobacillus lactis/rhamnosis ameliorate liver fibrosis in prevention and treatment. J. Microbiol. 2023, 61, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, V.; Azcarate-Peril, M.A.; Updegraff, J.; Manderino, L.; Gunstad, J. Randomized clinical trial examining the impact of Lactobacillus rhamnosus GG probiotic supplementation on cognitive functioning in middle-aged and older adults. Neuropsychiatr. Dis. Treat. 2020, 16, 2765–2777. [Google Scholar] [CrossRef]
- Kong, Y.Z.; Olejar, K.J.; On, S.L.W.; Chelikani, V. The potential of Lactobacillus spp. for modulating oxidative stress in the gastrointestinal tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Ambra, R.; Nobili, F.; Garaguso, I.; Raguzzini, A.; Serafini, M. Redox role of Lactobacillus casei Shirota against the cellular damage induced by 2,2 -azobis (2-amidinopropane) dihydrochloride-induced oxidative and inflammatory stress in enterocytes-like epithelial cells. Front. Immunol. 2018, 9, 1131. [Google Scholar] [CrossRef]
- Li, B.L.; Evivie, S.E.; Lu, J.J.; Jiao, Y.H.; Wang, C.F.; Li, Z.Y.; Liu, F.; Huo, G.C. Lactobacillus helveticus KLDS1.8701 alleviates d-galactose-induced aging by regulating Nrf-2 and gut microbiota in mice. Food Funct. 2018, 9, 6587–6599. [Google Scholar] [CrossRef]
- Liu, R.L.; Qiao, Y.; Huang, G.Q.; Qian, W.; Xiong, J.L.; Wang, X.Y.; Zheng, W.J.; Yao, W. Effect of synbiotic containing Bacillus coagulans and Lactulose on gut health in mice with DSS-induced ulcerative colitis. Acta Microbiol. Sin. 2022, 62, 869–881. [Google Scholar] [CrossRef]
- del Campo, F.M.; Magaña, N.V.; Salazar-Félix, N.A.; Rodríguez, M.P.; Romo-Flores, M.D.; Sanabria, L.C.; Campos, E.R.; Manzano, A.M.C. Odoribacter and Anaerotruncus: Gut microbiome signature might be related cognitive impairment in patients on peritoneal dialysis. Nephrol. Dial. Transplant. 2020, 35, 1467. [Google Scholar]
- Hamilton, A.L.; Kamm, M.A.; Ng, S.C.; Morrison, M. Proteus spp. as putative gastrointestinal pathogens. Clin. Microbiol. Rev. 2018, 31, e00085-17. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.J.; Yan, C.C.; Zhang, C.; Pan, W.J.; Zhang, W.N.; Lu, Y.M.; Chen, L.; Chen, Y. Sarcodon aspratus polysaccharides ameliorated obesity-induced metabolic disorders and modulated gut microbiota dysbiosis in mice fed a high-fat diet. Food Funct. 2020, 11, 2588–2602. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Xiao, D.; Liu, W.; Song, Y.F.; Zou, B.R.; Li, L.; Li, P.; Cai, Y.; Liu, D.L.; Liao, Q.F.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.M.; Yu, M.; Li, K.K.; Hu, Y.; Wang, Y.; Xu, X.H.; Qu, J.J. Seleno-lentinan prevents chronic pancreatitis development and modulates gut microbiota in mice. J. Funct. Foods 2016, 22, 177–188. [Google Scholar] [CrossRef]
- Synytsya, A.; Mícková, K.; Synytsya, A.; Jablonsky, I.; Spevácek, J.; Erban, V.; Kováríková, E.; Copíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Yang, W.J.; Pei, F.; Zhao, L.Y.; Hu, Q.H. In vitro fermentation of six kinds of edible mushrooms and its effects on fecal microbiota composition. LWT—Food Sci. Technol. 2018, 96, 627–635. [Google Scholar] [CrossRef]

| Group | Mean Lifespan (d) | Maximum Lifespan (d) | Median Survival (d) | Prolongation of Mean Lifespan (%) | |
|---|---|---|---|---|---|
| Male | NC | 50.72 ± 1.45 | 66.20 ± 0.19 | 55.50 | - |
| AA | 53.83 ± 1.60 | 71.40 ± 0.32 ** | 59.50 | 6.13 | |
| BB | 52.14 ± 1.43 | 67.80 ± 0.42 ** | 57.50 | 2.80 | |
| AB | 54.99 ± 1.52 * | 71.60 ± 0.47 ** | 61.00 | 8.42 | |
| Female | NC | 51.59 ± 1.39 | 67.20 ± 0.24 | 55.50 | - |
| AA | 55.61 ± 1.45 * | 71.00 ± 0.32 ** | 62.00 | 7.79 | |
| BB | 53.39 ± 1.50 | 71.10 ± 0.33 ** | 59.00 | 3.45 | |
| AB | 56.64 ± 1.49 ** | 74.80 ± 0.27 ** | 61.00 | 9.79 | |
| Group | Mean Lifespan (h) | Maximum Lifespan (h) | Median Survival (h) | Prolongation of Mean Lifespan (%) | |
|---|---|---|---|---|---|
| Male | NC | 17.04 ± 0.51 | 23.10 ± 0.43 | 18.00 | - |
| AA | 18.93 ± 0.59 ** | 25.50 ± 0.47 ** | 21.00 | 11.09 | |
| BB | 17.31 ± 0.50 | 24.00 ± 0.00 * | 18.00 | 1.58 | |
| AB | 19.11 ± 0.58 ** | 26.40 ± 0.38 ** | 21.00 | 12.15 | |
| Female | NC | 17.34 ± 0.46 | 24.00 ± 0.00 | 18.00 | - |
| AA | 18.90 ± 0.62 * | 27.30 ± 0.51 ** | 21.00 | 9.00 | |
| BB | 17.46 ± 0.40 | 22.80 ± 0.46 * | 18.00 | 0.69 | |
| AB | 19.11 ± 0.61 * | 27.90 ± 0.43 ** | 21.00 | 10.21 | |
| NC | MC | AA | BB | AB | |
|---|---|---|---|---|---|
| Aging biomarkers | |||||
| AGEs (ng/gprot) | 623.44 ± 30.89 | 878.00 ± 125.55 ## | 763.64 ± 84.36 ## | 750.00 ± 127.71 # | 735.58 ± 86.46 *## |
| iNOS (U/mgprot) | 0.56 ± 0.04 | 0.77 ± 0.10 ## | 0.57 ± 0.07 ** | 0.60 ± 0.07 ** | 0.61 ± 0.06 **# |
| Telomerase (nU/mgprot) | 69.61 ± 3.97 | 57.10 ± 6.92 ## | 61.47 ± 4.42 ## | 60.25 ± 1.88 ## | 62.28 ± 5.53 # |
| Antioxidant activity in brain | |||||
| T-SOD (U/mgprot) | 351.12 ± 15.40 | 287.61 ± 16.00 ## | 335.15 ± 26.44 ** | 333.88 ± 22.48 ** | 336.64 ± 20.41 ** |
| CAT (U/mgprot) | 40.73 ± 0.72 | 37.16 ± 1.74 ## | 39.35 ± 0.63 *# | 38.65 ± 1.22 # | 39.28 ± 1.48 * |
| GSH-Px (U/mgprot) | 707.22 ± 31.20 | 619.59 ± 56.82 ## | 688.71 ± 39.94 * | 671.21 ± 33.01 # | 681.65 ± 46.45 * |
| MDA (nmol/mgprot) | 13.25 ± 1.08 | 19.99 ± 1.93 ## | 16.99 ± 2.25 *## | 16.29 ± 2.64 *# | 14.73 ± 0.83 **# |
| Antioxidant activity in serum | |||||
| T-SOD (U/mL) | 82.23 ± 4.96 | 58.66 ± 4.28 ## | 71.83 ± 4.80 **## | 69.07 ± 3.02 **## | 72.32 ± 3.55 **## |
| CAT (U/mL) | 29.29 ± 1.65 | 22.42 ± 2.59 ## | 25.16 ± 2.43 **# | 25.00 ± 1.88 *## | 27.43 ± 2.80 ** |
| GSH-Px (U/mL) | 358.41 ± 19.33 | 295.88 ± 33.99 ## | 345.29 ± 21.81 ** | 332.13 ± 26.28 * | 339.55 ± 20.00 * |
| MDA (nmol/mL) | 4.80 ± 0.26 | 8.38 ± 1.61 ## | 6.03 ± 1.29 *# | 6.46 ± 0.54 *## | 6.19 ± 0.67 **## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Feng, Y.; Zhen, H.; Zhao, L.; Wu, H.; Liu, B.; Fan, G.; Tong, A. Agrocybe aegerita Polysaccharide Combined with Bifidobacterium lactis Bb-12 Attenuates Aging-Related Oxidative Stress and Restores Gut Microbiota. Foods 2023, 12, 4381. https://doi.org/10.3390/foods12244381
Liu X, Feng Y, Zhen H, Zhao L, Wu H, Liu B, Fan G, Tong A. Agrocybe aegerita Polysaccharide Combined with Bifidobacterium lactis Bb-12 Attenuates Aging-Related Oxidative Stress and Restores Gut Microbiota. Foods. 2023; 12(24):4381. https://doi.org/10.3390/foods12244381
Chicago/Turabian StyleLiu, Xiaoyan, Yanyu Feng, Hongmin Zhen, Lina Zhao, Hongqiang Wu, Bin Liu, Guangsen Fan, and Aijun Tong. 2023. "Agrocybe aegerita Polysaccharide Combined with Bifidobacterium lactis Bb-12 Attenuates Aging-Related Oxidative Stress and Restores Gut Microbiota" Foods 12, no. 24: 4381. https://doi.org/10.3390/foods12244381





