Properties of Pectin Extracted from Vietnamese Mango Peels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Pectin Extraction from Dried Mango Peel Powder
2.3. Pectin Yield
2.4. Measurement of the Degree of Esterification
2.5. Determination of Water Holding Capacity (WHC) and Solubility of Mango Peel Pectin
2.6. Determination of the Emulsion Activity and Emulsion Stability
2.7. Rheological Analysis
2.8. Determination of Viscosity-average Molecular Mass
2.9. Statistical Analysis
3. Results and Discussion
3.1. Pectin Yield
3.2. Water Holding Capacity (WHC)
3.3. Solubility
3.4. Degree of Esterification
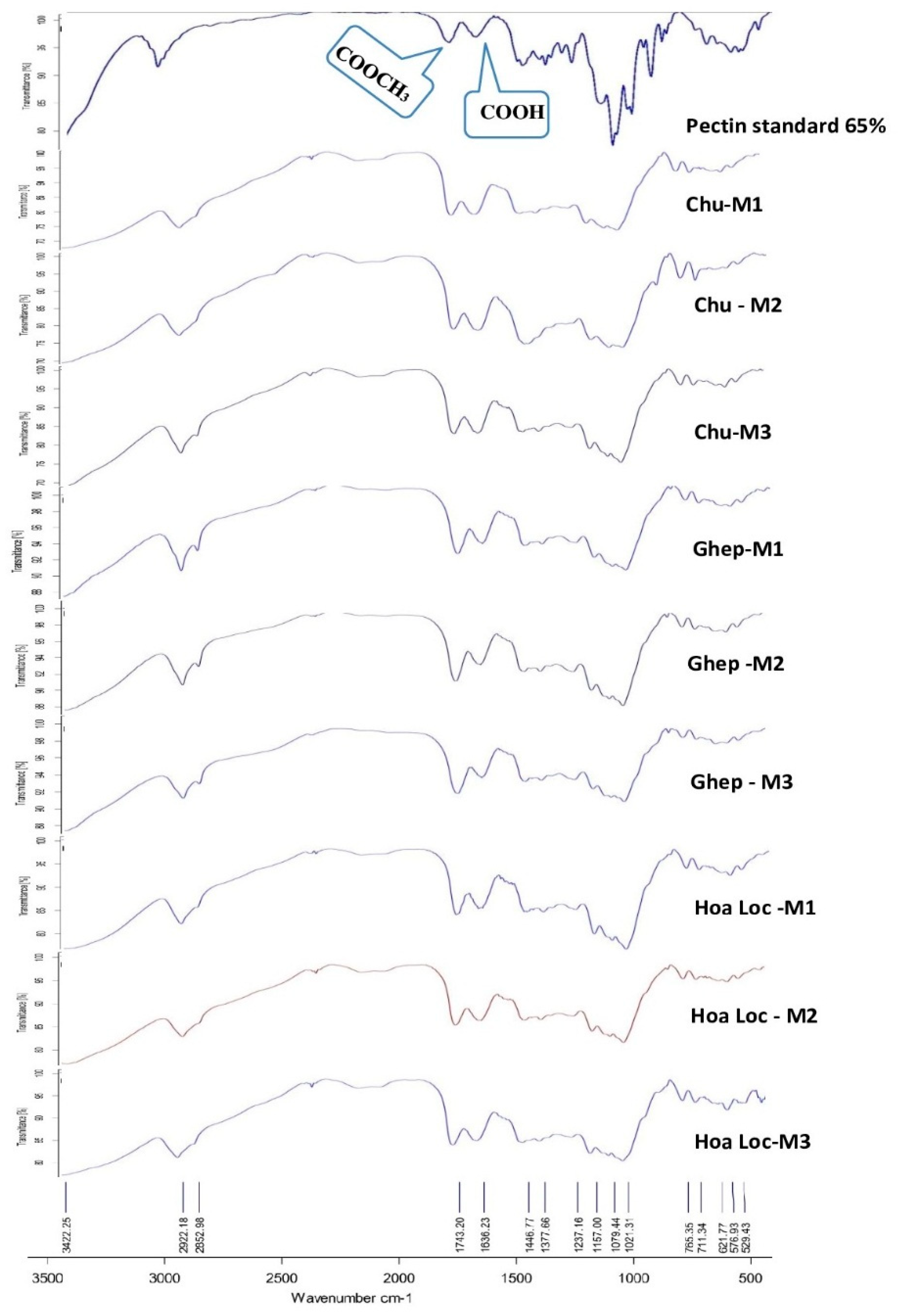
3.5. FTIR Analysis
3.6. Emulsifying Properties
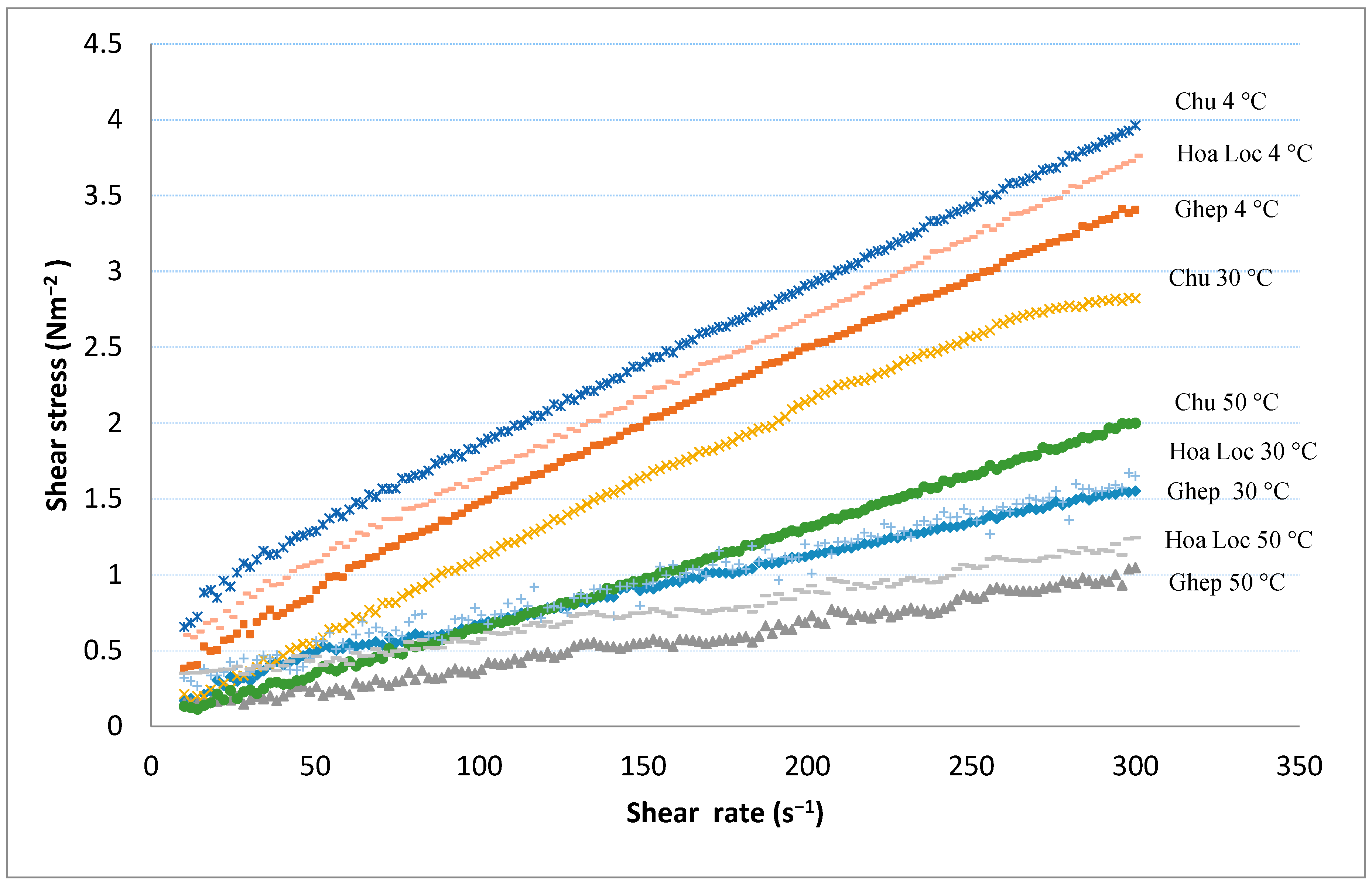
3.7. Rheological Properties
3.8. Viscosity-Average Molecular Weight
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Koubala, B.; Kansci, G.; Mbome, L.I.; Crepeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Effect of extraction conditions on some physicochemical characteristics of pectins from “Amelioree” and “Mango” mango peels. Food Hydrocoll. 2008, 22, 1345–1351. [Google Scholar] [CrossRef]
- Elkner, K.; Kosson, R. Dietary fiber content and its fractional composition in cabbage as affected by cultivar earliness and sauerkraut storage period. Veg. Crops Res. Bull. 2008, 69, 165–175. [Google Scholar]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ. Int. J. 2003, 3, 206–228. [Google Scholar]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Functional properties and characterization of dietary fiber from Mangifera pajang Kort. fruit pulp. J. Agric. Food Chem. 2011, 59, 3980–3985. [Google Scholar] [CrossRef]
- Nguyen, H.V.H.; Savage, G.P. The effects of temperature and pH on the extraction of oxalate and pectin from green kiwifruit (Actinidia deliciosa L.), golden kiwifruit (Actinidia chinensis L.), kiwiberry (Actinidia arguta) and persimmon (Diospyros kaki). Int. J. Food Sci. Tech 2013, 48, 794–800. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of “Tommy Atkins” mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef]
- El Bulk, R.E.; Babiker, E.F.E.; El Tinay, A.H. Changes in chemical composition of guava fruits during development and ripening. Food Chem. 1997, 59, 395–399. [Google Scholar] [CrossRef]
- Zhou, H.C.; Li, G.; Zhao, X.; Li, L.J. Comparative analysis of polygalacturonase in the fruit of strawberry cultivars. Genet. Mol. Res. 2015, 14, 12776–12787. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Fruits and Fruit Products. In Food Chemistry, 4th Revised and Extended edn; Belitz, H.-D., Grosch, W., Schieberle, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 807–861. [Google Scholar]
- Good Agricultural Practices (GAP) Library 2010. Available online: http://www.gap.org.vn (accessed on 28 October 2017).
- Larrauri, J.A.; Ruperez, P.; Borroto, B.; Saura-Calixto, F. Mango peels as a new tropical fibre: Preparation and characterization. LWT Food Sci. Technol. 1996, 29, 729–733. [Google Scholar] [CrossRef]
- Dorta, E.; Gloria, L.M.; Gonzalez, M. Using drying treatments to stabilize mango peel and seed: Effect on antioxidant activity. LWT Food Sci. Technol. 2012, 45, 261–268. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada Rao, U.J.S. Mango peel dietary fiber: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- The Observatory of Economic Complexity (OEC) Pectic Substances, Pectinates, Pectates. Available online: http://atlas.media.mit.edu/en/profile/hs92/130220/ (accessed on 20 December 2018).
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Goff, H.D. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissamplelospareira) pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Eastwood, M.A.; Robertson, J.A.; Brydon, W.G.; MacDonald, D. Measurement of water—Holding properties of fibre and their faecal bulking ability in man. Br. J. Nutr. 1983, 50, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sciarini, L.S.; Maldonado, F.; Ribotta, P.D.; Perez, G.T.; Leon, A.E. Chemical composition and functional properties of Gleditsia triacanthos gum. Food Hydrocoll. 2009, 23, 306–313. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, S.Q.; Zhang, J.S. Rheological behavior and microstructure of Oviductus Ranae hydrogels. Food Sci. Biotechnol. 2012, 21, 467–474. [Google Scholar] [CrossRef]
- Kar, F.; Arslan, N. Effect of temperature and concentration on viscosity of orange peel pectin solutions and intrinsic viscosity–molecular weight relationship. Carbohydr. Polym. 1999, 40, 277–284. [Google Scholar] [CrossRef]
- Kar, F.; Arslan, N. Characterization of orange peel pectin and effect of sugars, l-ascorbic acid, ammonium persulfate, salts on viscosity of orange peel pectin solutions. Carbohydr. Polym. 1999, 40, 285–291. [Google Scholar] [CrossRef]
- Owens, H.S.; Lotzkar, H.; Schultz, T.H.; Maclay, W.D. Shape and size of pectinic acid deduced from viscometric measurements. J. Am. Chem. Soc. 1946, 68, 1628–1632. [Google Scholar] [CrossRef]
- Proctor, A.; Peng, L.C. Pectin transitions during blueberry fruit development and ripening. J. Food Sci. 1989, 54, 385–387. [Google Scholar] [CrossRef]
- Taylor, J.E. Exotics: Guava. In Biochemistry of Fruit Ripening; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 151–187. [Google Scholar]
- Berardini, N.; Knodler, M.; Schieber, A.; Carle, R. Utilization of mango peels as a source of pectin and polyphenolics. Innov. Food Sci. Emerg. Technol. 2005, 6, 442–452. [Google Scholar] [CrossRef]
- Gwynne, J. Chemistry in Its Element: Compounds. Royal Society of Chemistry. Available online: http://www.rsc.org/chemistryworld/podcast/CIIEcompounds/transcripts/pectin.asp (2015) (accessed on 30 December 2018).
- Figuerola, F.; Hurtado, M.L.; Estevez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Yapo, B.M.; Koffi, K.L. Dietary fiber components in yellow passion fruit rind—A potential fiber source. J. Agric. Food Chem. 2008, 56, 5880–5883. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, N.; Wathelet, B.; Jimenez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fiber composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Chang, K.C. Extraction and physicochemical characterization of pectin from sunflower head residues. J. Food Sci. 1992, 57, 1439–1443. [Google Scholar] [CrossRef]
- Keller, M. Developmental Physiology. In The Science of Grapevines: Anatomy and Physiology; Keller, M., Ed.; Academic Press: Washington, DC, USA, 2015; pp. 196–268. [Google Scholar]
- Kumar, A.; Chauhan, G.S. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr. Polym. 2010, 82, 454–459. [Google Scholar] [CrossRef]
- Ismail, N.S.H.; Ramli, N.; Hani, N.M.; Meon, Z. Extraction and characterization of pectin from dragon fruit (Hylocereus polyrhizus) using various extraction conditions. Sains Malays. 2012, 41, 41–45. [Google Scholar]
- Vriesmann, L.C.; Teofilo, R.F.; de Oliverira Petkowicz, C.L. Extraction and characterization of pectin from cacao pod husks (Theobroma cacao L.) with citric acid. LWT Food Sci. Technol. 2012, 49, 108–116. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Pectin methylesterase activity and other factors affecting pH and titratable acidity in processing tomatoes. Food Chem. 2012, 132, 915–920. [Google Scholar] [CrossRef]
- Ng, J.K.T.; Schroder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus x domestica) fruit growth. BMC Plant Biol. 2013, 13, 1–16. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Lopez-Franco, Y.L.; Gooycolea, F.M.; Lizardi-Mendoza, J. Gum of Prosopis/Acacia Species. In Polysaccharides: Bioactivity and Biotechnology; Kishan Gopal, K., Jean-Michel Mérillon, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–20. [Google Scholar]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Loey, A.V.; Hendrickx, M. The emulsifying and emulsion—Stabilizing properties of pectin: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Fasoli, E.; Righetti, P.G. The peel and pulp of mango fruit: A proteomic samba. Biochim Biophys Acta Proteins Proteom. 2013, 1834, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Abu-Goukh, A.A.; Shattir, A.E.T.; Mahdi, E.F.M. Physico-chemical changes during growth and development of papaya fruit. II: Chemical changes. Agric. Biol. J. N. Am. 2010, 1, 871–877. [Google Scholar] [CrossRef]
- Emaga, T.H.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary fiber components and pectin chemical features of peels during ripening in banana and plaintain varieties. Bioresour. Technol. 2008, 99, 4346–43454. [Google Scholar] [CrossRef] [PubMed]
- Yuliarti, O.; Matia-Merino, L.; Goh, K.K.T.; Mawson, J.; Williams, M.A.K.; Brennan, C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015, 166, 479–485. [Google Scholar] [CrossRef]
- Owens, H.S.; McCready, R.M.; Shepherd, A.D.; Schultz, S.H.; Pippen, E.L.; Swenson, H.A.; Miers, J.C.; Erlandsen, R.F.; Maclay, W.D. Methods Used at Western Regional Research Laboratory for Extraction and Analysis of Pectic Materials; Western Regional Research Laboratory: Albany, CA, USA, 1952; AIC-340. [Google Scholar]
- Morris, G.A.; Foster, T.J.; Harding, S.E. The effect of the degree of esterification on the hydrodynamic properties of citrus pectin. Food Hydrocoll. 2000, 14, 227–235. [Google Scholar] [CrossRef]
| Cultivars Samples | Pectin Yield (g/100 g DW) | Degree of Esterification (%) | Water Holding Capacity (g H2O/1 g pectin) | Solubility (%) | Emulsion Activity (%) | Emulsion Stability (%) | Intrinsic Viscosity (mL/g) | Mw (kDa) |
|---|---|---|---|---|---|---|---|---|
| Ghep | ||||||||
| Pre mature | 27.5 ± 1.2 | 55.8 ± 0.7 | 11.4 ± 0.2 | 81.7 ± 1.3 | 34.2 ± 0.3 | 65.8 ± 9.3 | 46.3 ± 0.1 | 397.0 ± 1.4 |
| Mature | 24.2 ± 0.7 | 55.8 ± 0.2 | 11.6 ± 0.8 | 87.4 ± 1.6 | 31.8 ± 1.7 | 40.2 ± 3.8 | 50.3 ± 0.2 | 444.5 ± 0.8 |
| Ripe | 31.7 ± 1.0 | 55.7 ± 0.5 | 13.6 ± 0.4 | 86.0 ± 1.4 | 29.9 ± 0.4 | 39.5 ± 3.4 | 50.1 ± 0.2 | 434.6 ±1.2 |
| Cat Chu | ||||||||
| Pre mature | 21.0 ± 0.7 | 50.9 ± 0.5 | 10.6 ± 0.3 | 77.4 ± 0.5 | 19.4 ± 4.0 | 35.5 ± 6.0 | 67.2 ± 0.3 | 539.8 ± 1.0 |
| Mature | 19.2 ± 0.4 | 49.6 ± 0.2 | 14.9 ± 1.1 | 79.1 ± 1.3 | 11.8 ± 0.9 | 60.6 ± 2.7 | 73.7 ± 0.4 | 578.0 ± 0.7 |
| Ripe | 26.5 ± 0.3 | 50.3 ± 0.5 | 9.5 ± 0.0 | 82.5 ± 1.2 | 24.2 ± 2.0 | 35.6 ± 0.6 | 71.3 ± 0.5 | 564.0 ± 1.5 |
| Hoa Loc | ||||||||
| Pre mature | 20.5 ± 0.9 | 52.1 ± 0.2 | 11.3 ± 0.3 | 83.4 ± 1.8 | 30.9 ± 1.1 | 85.5 ± 3.1 | 44.5 ± 0.2 | 408.2 ± 0.8 |
| Mature | 18.4 ± 0.8 | 52.4 ± 0.5 | 13.4 ± 0.6 | 85.1 ± 1.2 | 33.8 ± 0.5 | 94.5 ± 3.7 | 52.0 ± 0.3 | 444.6 ± 1.5 |
| Ripe | 24.1 ± 1.2 | 51.0 ± 0.3 | 11.8 ± 0.7 | 84.6 ± 1.3 | 27.6 ± 3.4 | 28.5 ± 5.0 | 50.3 ± 0.2 | 432.8 ± 1.1 |
| Analysis of variance | Significance | |||||||
| Cultivars | ** | ** | ns | ** | ** | * | * | * |
| Maturity stages | ** | ns | * | ** | ns | ** | ns | ns |
| Interaction | ** | ns | ** | ns | ** | ** | * | * |
| LSD cultivars | 3.1 | 0.8 | --- | 2.8 | 4.6 | 21.2 | 1.7 | 9.9 |
| LSD maturity | 4.5 | --- | 1.6 | 3.3 | --- | 19.6 | --- | --- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.D.H.; Nguyen, H.V.H.; Savage, G.P. Properties of Pectin Extracted from Vietnamese Mango Peels. Foods 2019, 8, 629. https://doi.org/10.3390/foods8120629
Nguyen HDH, Nguyen HVH, Savage GP. Properties of Pectin Extracted from Vietnamese Mango Peels. Foods. 2019; 8(12):629. https://doi.org/10.3390/foods8120629
Chicago/Turabian StyleNguyen, Hoa D. H., Ha V. H. Nguyen, and Geoffrey P. Savage. 2019. "Properties of Pectin Extracted from Vietnamese Mango Peels" Foods 8, no. 12: 629. https://doi.org/10.3390/foods8120629




