Model-Optimizing Radiofrequency Parameters of 3D Finite Element Analysis for Ablation of Benign Thyroid Nodules
Abstract
:1. Introduction
2. Materials and Methods
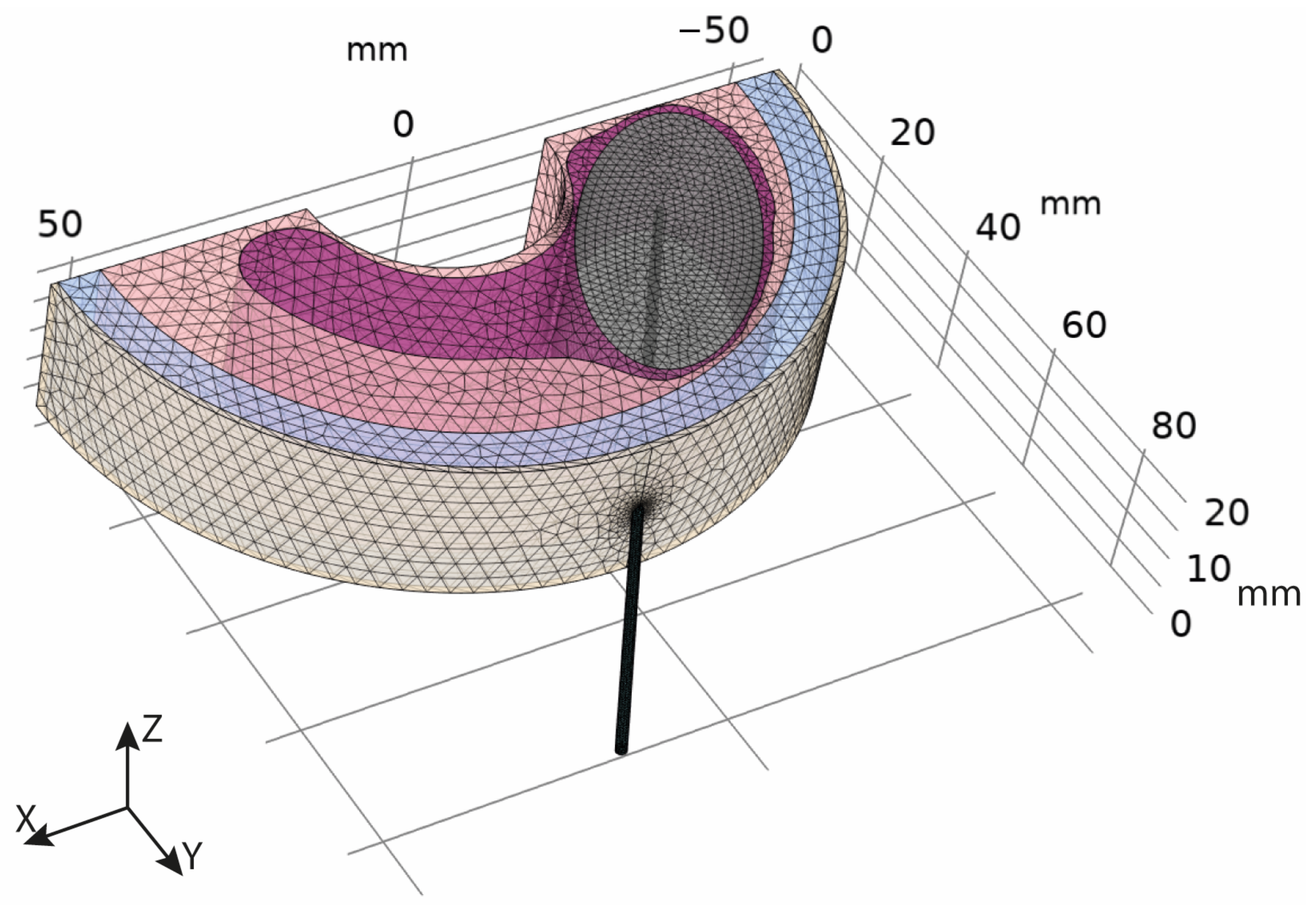
2.1. Geometric Model
2.2. Mathematical Model
2.3. Model Parameters
| Skin | Fat | Muscle | Thyroid | Nodule | ||
|---|---|---|---|---|---|---|
| Thermal conductivity | k (W/m∙K) | 0.37 [31,37] | 0.21 [31,37] | 0.49 [31,37] | 0.52 [31,37] | 0.89 [31] |
| Density | ρ (kg/m3) | 1109 [31,37] | 911 [31,37] | 1090 [31,37] | 1050 [31,37] | 1050 [31] |
| Specific heat at constant pressure | cp (J/kg∙K) | 3391 [31,37] | 2348 [31,37] | 3421 [31,37] | 3609 [31,37] | 3770 [31] |
| Electrical conductivity | σ (S/m) | 4.09∙10−3 [37] | 4.37∙10−2 [37] | 4.43∙10−1 [37] | 5.64∙10−1 [37] | 4.81∙10−1 [38] |
| Relative permittivity | εr (-) | 1.06∙103 [37] | 57.3 [37] | 3.77∙103 [37] | 2.18∙103 [37] | 2.18∙103 [37] |
| Blood perfusion | ωb (s−1) | 0.00196 [39] | 5.01∙10−4 [39] | 7.08∙10−4 [39] | 0.098 [39] | 0.0096 [31] 0.021 [40] |
| Metabolic heat | Qm (W/m3) | 1829.85 [31] | 464.61 [31] | 1046 [31] | 4200 [31] | 42,000 [31] |
| Frequency factor | A (s−1) | 4.575∙1072 [41] | 4.43∙1016 [24] | 2.94∙1039 [42] | 7.39∙1039 [28] | 7.39∙1039 [28] |
| Activation Energy | ΔE (J/mol) | 4.71∙105 [41] | 1.3∙105 [24] | 2.596∙105 [42] | 2.577∙105 [28] | 2.577∙105 [28] |
| Electrode Active Tip | Electrode Shaft | ||
|---|---|---|---|
| Thermal conductivity | k (W/m∙K) | 400 | 0.026 |
| Density | ρ (kg/m3) | 8960 | 1150 |
| Specific heat at constant pressure | cp (J/kg∙K) | 385 | 1700 |
| Electrical conductivity | σ (S/m) | 5.99∙107 | 1∙10−5 |
| Relative permittivity | εr (-) | 1 | 1 |
2.4. Optimization Model
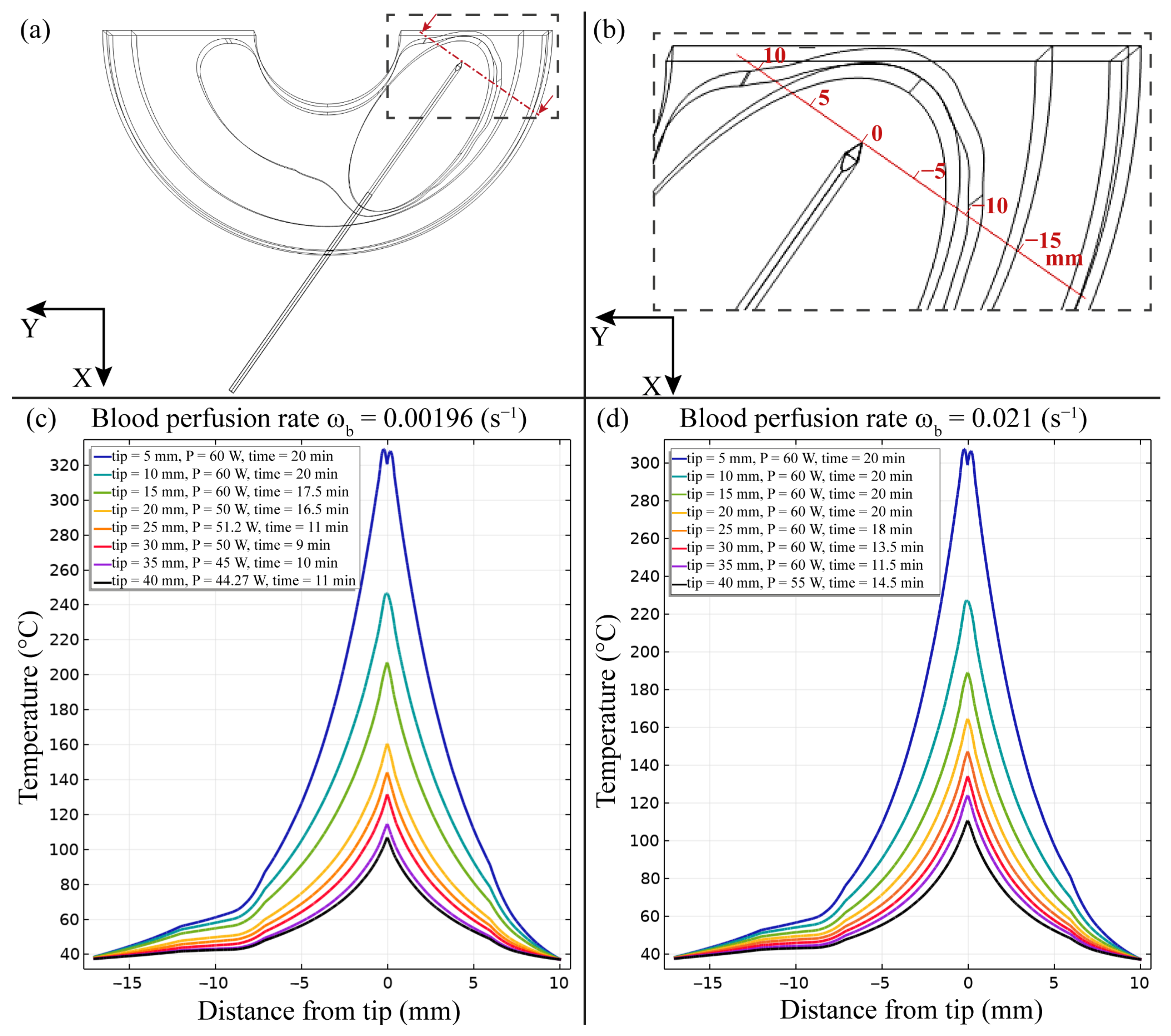
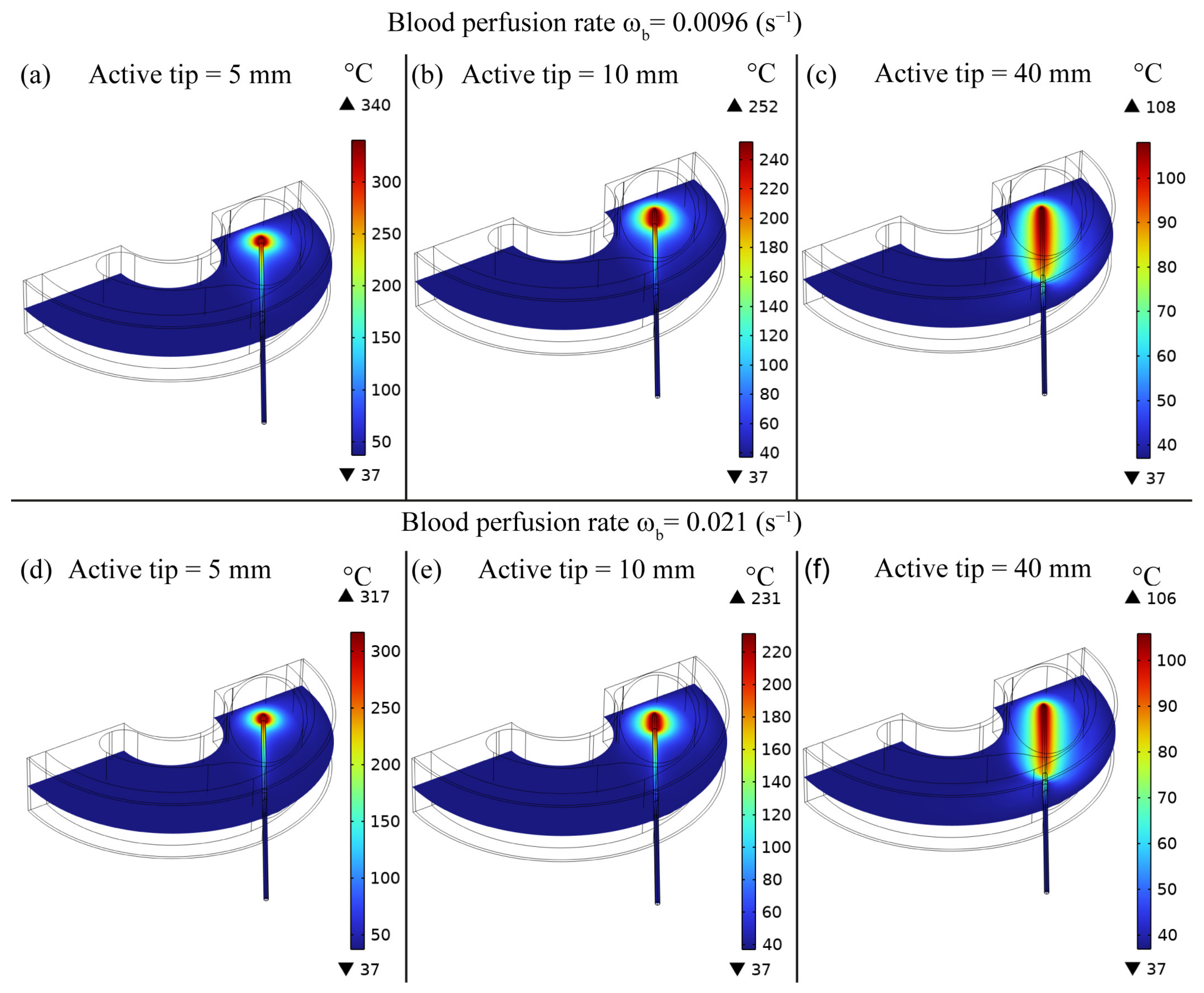
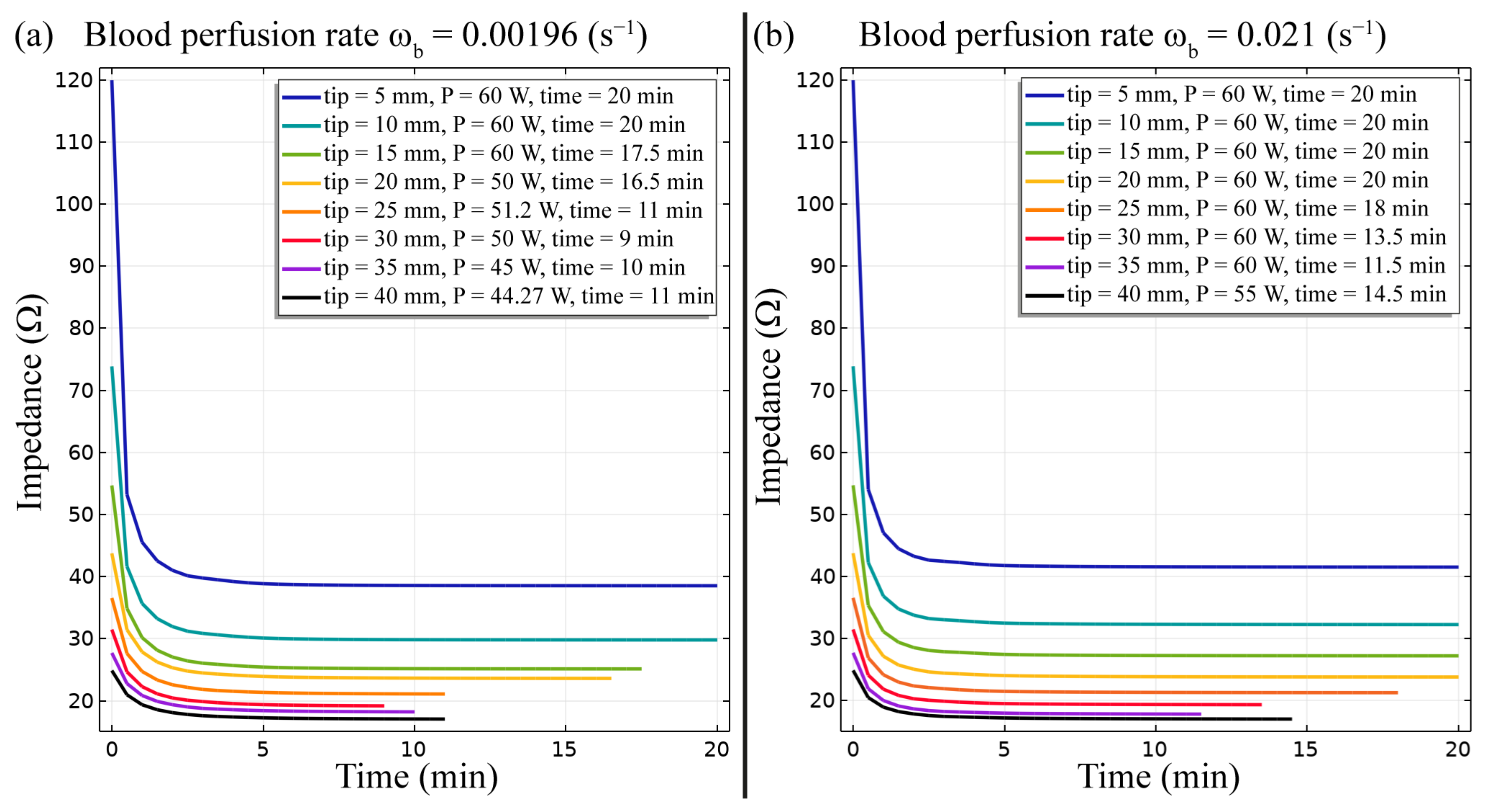
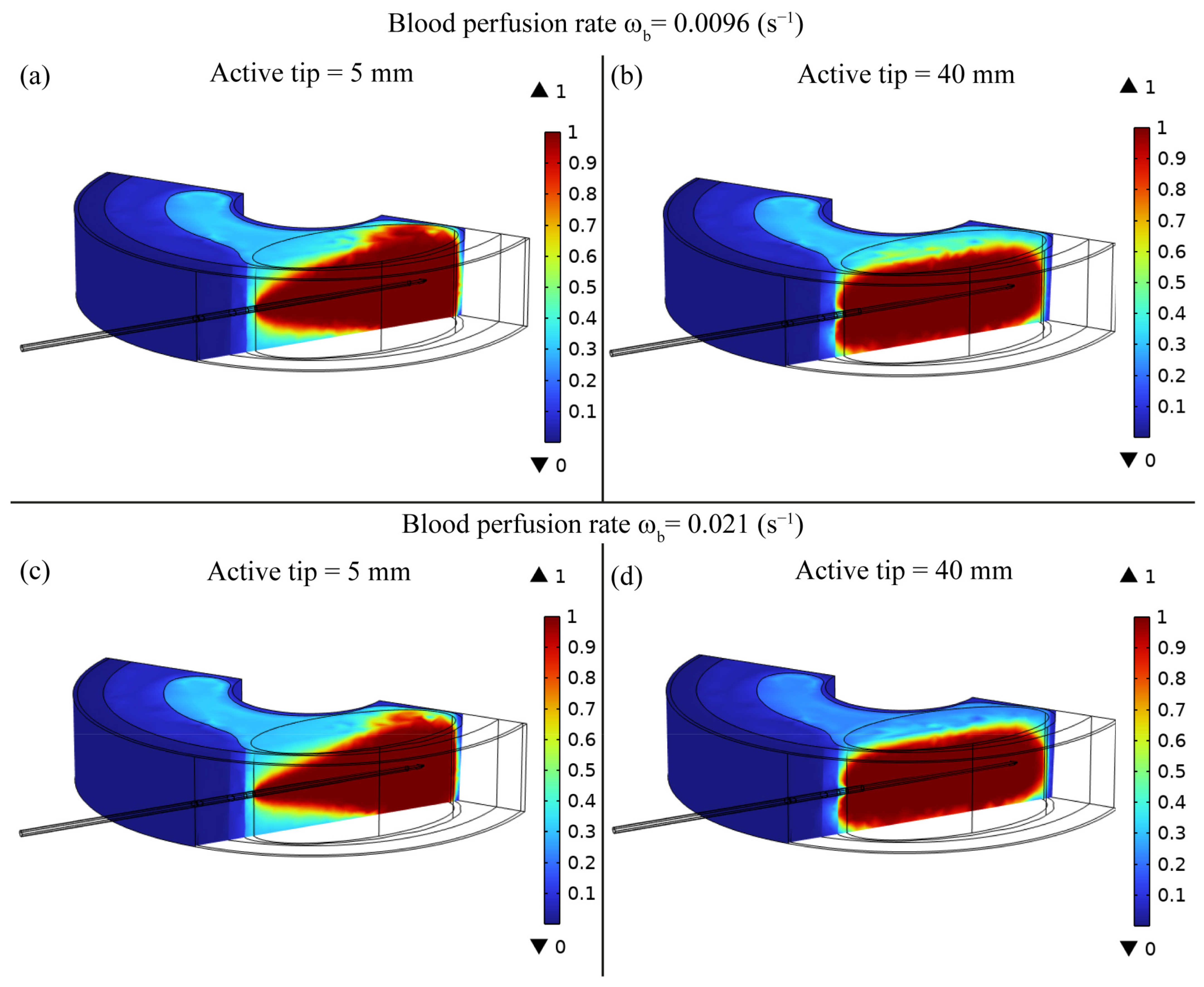
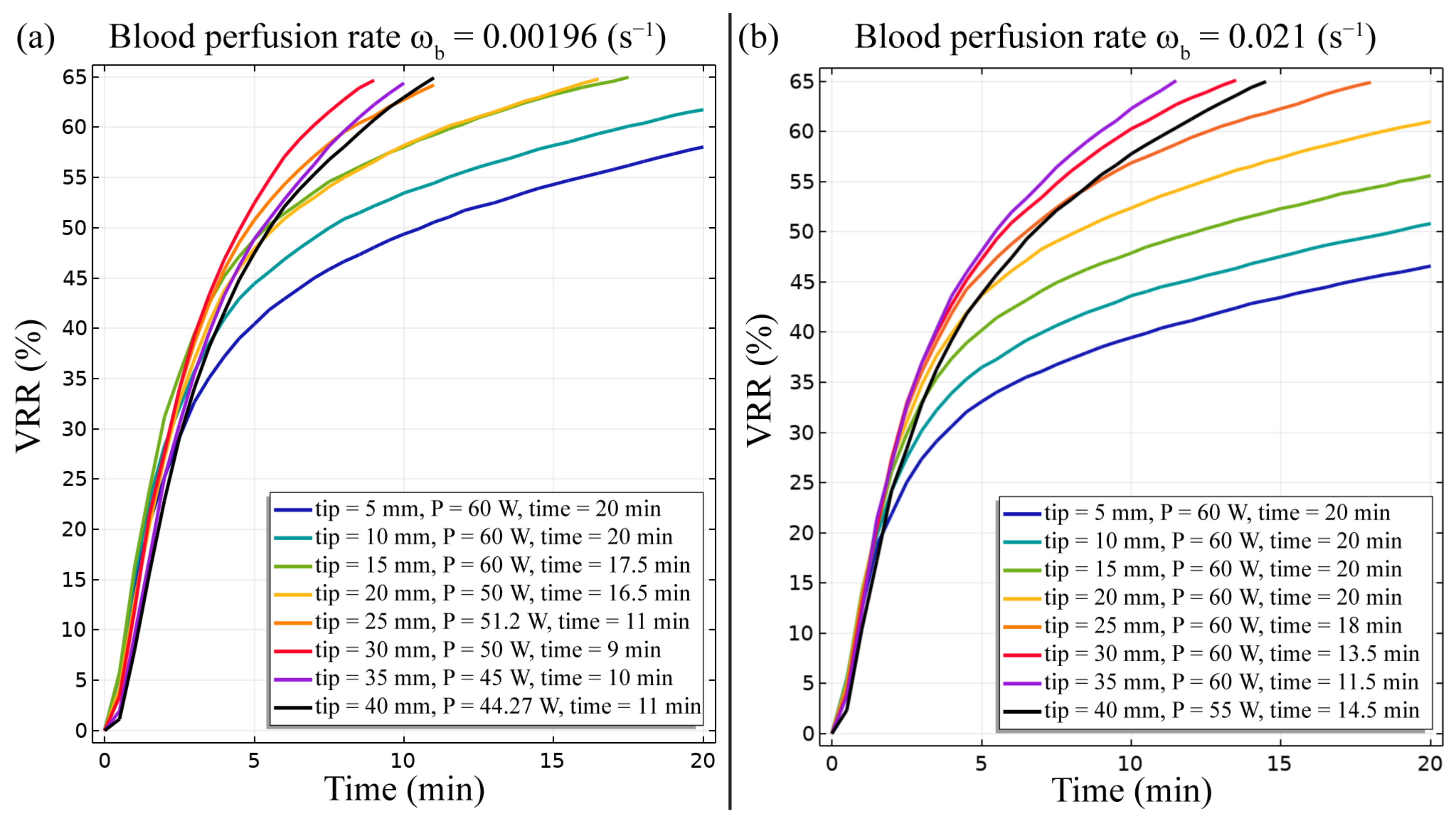
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, S.L.; Baek, J.H.; Lee, J.H.; Shong, Y.K.; Sung, J.Y.; Kim, K.S.; Lee, D.; Kim, J.-H.; Baek, S.M.; Sim, J.S.; et al. Efficacy and Safety of Radiofrequency Ablation for Benign Thyroid Nodules: A Prospective Multicenter Study. Korean J. Radiol. 2018, 19, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; Monpeyssen, H.; Frasoldati, A.; Hegedüs, L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur. Thyroid J. 2020, 9, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Bini, F.; Andrioli, M.; Giovanella, L.; Thorel, M.F.; Ceriani, L.; Valabrega, S.; Lenzi, A.; Drudi, F.M.; Marinozzi, F.; et al. Analysis of Tissue Surrounding Thyroid Nodules by Ultrasound Digital Images. Endocrine 2015, 48, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Damião, C.P.; Montero, J.R.G.; Moran, M.B.H.; da Cruz Filho, R.A.; Fontes, C.A.P.; Lima, G.A.B.; Conci, A. On the Possibility of Using Temperature to Aid in Thyroid Nodule Investigation. Sci. Rep. 2020, 10, 21010. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Wang, C.K.; Wang, W.H.; Kuo, C.Y.; Chiang, P.L.; Lin, A.N.; Baek, J.H.; Wu, M.H.; Cheng, K.L. Multicenter Study of Benign Thyroid Nodules with Radiofrequency Ablation: Results of 762 Cases over 4 Years in Taiwan. J. Pers. Med. 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Cova, L.; Monaco, C.G.; Sconfienza, L.M.; Corbetta, S.; Benedini, S.; Ambrogi, F.; Milani, V.; Baroli, A.; Ierace, T.; et al. Benign Thyroid Nodules Treatment Using Percutaneous Laser Ablation (PLA) and Radiofrequency Ablation (RFA). Int. J. Hyperth. 2017, 33, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Deandrea, M.; Trimboli, P.; Mormile, A.; Cont, A.T.; Milan, L.; Buffet, C.; Giovanella, L.; Limone, P.P.; Poirée, S.; Leenhardt, L.; et al. Determining an Energy Threshold for Optimal Volume Reduction of Benign Thyroid Nodules Treated by Radiofrequency Ablation. Eur. Radiol. 2021, 31, 5189–5197. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Deandrea, M. Treating Thyroid Nodules by Radiofrequency: Is the Delivered Energy Correlated with the Volume Reduction Rate? A Pilot Study. Endocrine 2020, 69, 682–687. [Google Scholar] [CrossRef]
- Deandrea, M.; Garino, F.; Alberto, M.; Garberoglio, R.; Rossetto, R.; Bonelli, N.; Spiezia, S.; De Santis, M.; Monti, S.; Deiana, M.G.; et al. Radiofrequency Ablation for Benign Thyroid Nodules According to Different Ultrasound Features: An Italian Multicentre Prospective Study. Eur. J. Endocrinol. 2019, 180, 79–87. [Google Scholar] [CrossRef]
- Lee, M.T.; Wang, C.Y. Radiofrequency Ablation in Nodular Thyroid Diseases. J. Med. Ultrasound 2013, 21, 62–70. [Google Scholar] [CrossRef]
- Jin, H.; Fan, J.; Lu, L.; Cui, M. A Propensity Score Matching Study Between Microwave Ablation and Radiofrequency Ablation in Terms of Safety and Efficacy for Benign Thyroid Nodules Treatment. Front. Endocrinol. 2021, 12, 584972. [Google Scholar] [CrossRef]
- Trimboli, P.; Bini, F.; Marinozzi, F.; Baek, J.H.; Giovanella, L. High-Intensity Focused Ultrasound (HIFU) Therapy for Benign Thyroid Nodules without Anesthesia or Sedation. Endocrine 2018, 61, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Pelloni, F.; Bini, F.; Marinozzi, F.; Giovanella, L. High-Intensity Focused Ultrasound (HIFU) for Benign Thyroid Nodules: 2-Year Follow-up Results. Endocrine 2019, 65, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Piccardo, A.; Pezzoli, C.; Bini, F.; Ricci, R.; Ruberto, T.; Trimboli, P. Comparison of High Intensity Focused Ultrasound and Radioiodine for Treating Toxic Thyroid Nodules. Clin. Endocrinol. 2018, 89, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Bini, F.; Trimboli, P.; Marinozzi, F.; Giovanella, L. Treatment of Benign Thyroid Nodules by High Intensity Focused Ultrasound (HIFU) at Different Acoustic Powers: A Study on in-Silico Phantom. Endocrine 2018, 59, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Tufano, R.P.; Pace-Asciak, P.; Russell, J.O.; Suárez, C.; Randolph, G.W.; López, F.; Shaha, A.R.; Mäkitie, A.; Rodrigo, J.P.; Kowalski, L.P.; et al. Update of Radiofrequency Ablation for Treating Benign and Malignant Thyroid Nodules. The Future Is Now. Front. Endocrinol. 2021, 12, 698689. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Baek, J.H.; Che, Y.; Chou, Y.H.; Fukunari, N.; Kim, J.H.; Lin, W.C.; My, L.T.; Na, D.G.; Quek, L.H.H.; et al. Radiofrequency Ablation of Benign Thyroid Nodules: Recommendations from the Asian Conference on Tumor Ablation Task Force—Secondary Publication. J. Med. Ultrasound 2021, 29, 77–83. [Google Scholar] [CrossRef]
- Garberoglio, R.; Aliberti, C.; Appetecchia, M.; Attard, M.; Boccuzzi, G.; Boraso, F.; Borretta, G.; Caruso, G.; Deandrea, M.; Freddi, M.; et al. Radiofrequency Ablation for Thyroid Nodules: Which Indications? The First Italian Opinion Statement. J. Ultrasound 2015, 18, 423–430. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, Y.P.; Tang, X.Y.; Ding, M.; He, Y.; Li, P.; Zhai, B. Significance of Radiofrequency Ablation in Large Solid Benign Thyroid Nodules. Front. Endocrinol. 2022, 13, 902484. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.X.; Nguyen, V.V.H.; Nguyen, H.T.; Nguyen, D.T.; Le, C. Van. Efficacy and Safety of Single-Session Radiofrequency Ablation in Treating Benign Thyroid Nodules: A Short-Term Prospective Cohort Study. Int. J. Endocrinol. 2021, 2021, 7556393. [Google Scholar] [CrossRef]
- Zhang, B.; Moser, M.A.J.; Zhang, E.M.; Luo, Y.; Liu, C.; Zhang, W. A Review of Radiofrequency Ablation: Large Target Tissue Necrosis and Mathematical Modelling. Phys. Medica 2016, 32, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Repaka, R. Temperature-Controlled Radiofrequency Ablation of Different Tissues Using Two-Compartment Models. Int. J. Hyperth. 2017, 33, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Melnik, R. Thermal Ablation of Biological Tissues in Disease Treatment: A Review of Computational Models and Future Directions. Electromagn. Biol. Med. 2020, 39, 49–88. [Google Scholar] [CrossRef] [PubMed]
- Servin, F.; Collins, J.A.; Heiselman, J.S.; Frederick-Dyer, K.C.; Planz, V.B.; Geevarghese, S.K.; Brown, D.B.; Miga, M.I. Fat Quantification Imaging and Biophysical Modeling for Patient-Specific Forecasting of Microwave Ablation Therapy. Front. Physiol. 2022, 12, 820251. [Google Scholar] [CrossRef] [PubMed]
- Chagas Paz, A.A.; de Souza, M.A.; Brock, P.W.; Ferreira Mercuri, E.G. Finite Element Analysis to Predict Temperature Distribution in the Human Neck with Abnormal Thyroid: A Proof of Concept. Comput. Methods Programs Biomed. 2022, 227, 107234. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, H.; Wu, S.; Jiang, T.; Zhou, Z.; Bai, Y. Numerical Evaluation of Ablation Zone under Different Tip Temperatures during Radiofrequency Ablation. Math. Biosci. Eng. 2019, 16, 2514–2531. [Google Scholar] [CrossRef] [PubMed]
- Chang, I. Finite Element Analysis of Hepatic Radiofrequency Ablation Probes Using Temperature-Dependent Electrical Conductivity. Biomed. Eng. Online 2003, 2, 12. [Google Scholar] [CrossRef]
- Jin, C.; He, Z.; Liu, J. MRI-Based Finite Element Simulation on Radiofrequency Ablation of Thyroid Cancer. Comput. Methods Programs Biomed. 2014, 113, 529–538. [Google Scholar] [CrossRef]
- Namakshenas, P.; Mojra, A. Numerical Study of Non-Fourier Thermal Ablation of Benign Thyroid Tumor by Focused Ultrasound (FU). Biocybern. Biomed. Eng. 2019, 39, 571–585. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, A.; Mojra, A.; Hooman, K. A Porous Medium Approach to Thermal Analysis of Focused Ultrasound for Treatment of Thyroid Nodules. Appl. Acoust. 2021, 182, 108236. [Google Scholar] [CrossRef]
- González, J.R.; Damião, C.; Moran, M.; Pantaleão, C.A.; Cruz, R.A.; Balarini, G.A.; Conci, A. A Computational Study on the Role of Parameters for Identification of Thyroid Nodules by Infrared Images (And Comparison with Real Data). Sensors 2021, 21, 4459. [Google Scholar] [CrossRef] [PubMed]
- Bahramian, F.; Mojra, A. Analysis of Thyroid Thermographic Images for Detection of Thyroid Tumor: An Experimental-Numerical Study. Int. J. Numer. Method. Biomed. Eng. 2019, 35, e3192. [Google Scholar] [CrossRef] [PubMed]
- Bini, F.; Pica, A.; Azzimonti, L.; Giusti, A.; Ruinelli, L.; Marinozzi, F.; Trimboli, P. Artificial Intelligence in Thyroid Field. A Comprehensive Review. Cancers 2021, 13, 4740. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.M.; Baek, J.H.; Na, D.G.; Suh, C.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H. Diagnostic Performance of Practice Guidelines for Thyroid Nodules: Thyroid Nodule Size versus Biopsy Rates. Radiology 2019, 291, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Viduetsky, A.; Herrejon, C.L. Sonographic Evaluation of Thyroid Size: A Review of Important Measurement Parameters. J. Diagn. Med. Sonogr. 2019, 35, 206–210. [Google Scholar] [CrossRef]
- Rossmanna, C.; Haemmerich, D. Review of Temperature Dependence of Thermal Properties, Dielectric Properties, and Perfusion of Biological Tissues at Hyperthermic and Ablation Temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies. 1996. Available online: https://www.semanticscholar.org/paper/Compilation-of-the-Dielectric-Properties-of-Body-at-Gabriel/6de149eb3f64b7341e832023c3bf2a6eac3c8ed0 (accessed on 6 August 2023).
- Huang, S.; Cai, W.; Han, S.; Lin, Y.; Wang, Y.; Chen, F.; Shao, G.; Liu, Y.; Yu, X.; Cai, Z.; et al. Differences in the Dielectric Properties of Various Benign and Malignant Thyroid Nodules. Med. Phys. 2021, 48, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Hasgalla, P.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues, Version 4.1. 2022. Available online: https://itis.swiss/virtual-population/tissue-properties/overview/ (accessed on 15 May 2023).
- Tegler, L.; Gillquist, J.; Anderberg, B.; Lundström, B.; Johansson, H. Thyroid Blood Flow Rate in Man. Electromagnetic Flowmetry during Operation in Euthyroid Normal Gland, Nontoxic Goiter, and Hyperthyroidism. J. Endocrinol. Investig. Off. J. Ital. Soc. Endocrinol. 1981, 4, 335–341. [Google Scholar] [CrossRef]
- Xu, F.; Seffen, K.A.; Lu, T.J. Temperature-Dependent Mechanical Behaviors of Skin Tissue. Int. J. Comput. Sci. 2008, 35, 92–101. [Google Scholar]
- Pearce, J.A. Models for Thermal Damage in Tissues: Processes and Applications. Crit. Rev. Biomed. Eng. 2010, 38, 1–20. [Google Scholar] [CrossRef]
- Powell, M.J.D. The BOBYQA Algorithm for Bound Constrained Optimization without Derivatives; Report NA2009/06; University of Cambridge: Cambridge, UK, 2009. [Google Scholar]
- Wong, K.P.; Lang, B.H.H. Use of Radiofrequency Ablation in Benign Thyroid Nodules: A Literature Review and Updates. Int. J. Endocrinol. 2013, 2013, 428363. [Google Scholar] [CrossRef]
- Berjano, E.J. Theoretical Modeling for Radiofrequency Ablation: State-of-the-Art and Challenges for the Future. Biomed. Eng. Online 2006, 5, 24. [Google Scholar] [CrossRef]
- Tang, Y.D.; Jin, T.; Flesch, R.C.C. Effect of Mass Transfer and Diffusion of Nanofluid on the Thermal Ablation of Malignant Cells during Magnetic Hyperthermia. Appl. Math. Model. 2020, 83, 122–135. [Google Scholar] [CrossRef]
- Pérez, J.J.; González-Suárez, A.; Berjano, E. Numerical Analysis of Thermal Impact of Intramyocardial Capillary Blood Flow during Radiofrequency Cardiac Ablation. Int. J. Hyperth. 2018, 34, 243–249. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bini, F.; Pica, A.; Marinozzi, F.; Giusti, A.; Leoncini, A.; Trimboli, P. Model-Optimizing Radiofrequency Parameters of 3D Finite Element Analysis for Ablation of Benign Thyroid Nodules. Bioengineering 2023, 10, 1210. https://doi.org/10.3390/bioengineering10101210
Bini F, Pica A, Marinozzi F, Giusti A, Leoncini A, Trimboli P. Model-Optimizing Radiofrequency Parameters of 3D Finite Element Analysis for Ablation of Benign Thyroid Nodules. Bioengineering. 2023; 10(10):1210. https://doi.org/10.3390/bioengineering10101210
Chicago/Turabian StyleBini, Fabiano, Andrada Pica, Franco Marinozzi, Alessandro Giusti, Andrea Leoncini, and Pierpaolo Trimboli. 2023. "Model-Optimizing Radiofrequency Parameters of 3D Finite Element Analysis for Ablation of Benign Thyroid Nodules" Bioengineering 10, no. 10: 1210. https://doi.org/10.3390/bioengineering10101210






