Polyhedral Oligomeric Sesquioxane Cross-Linked Chitosan-Based Multi-Effective Aerogel Preparation and Its Water-Driven Recovery Mechanism
Abstract
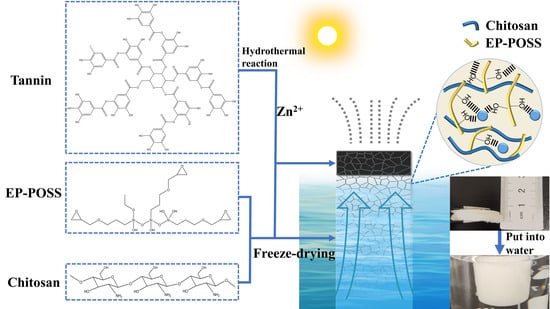
:1. Introduction
2. Results and Discussion
2.1. PCS Morphology and Structure
2.2. Water-Driven Recovery Properties and Mechanism of PCS

2.3. Mechanical Properties of PCS
2.4. Water Transport Properties of PCS
2.5. Photothermal Evaporation Properties of PCH
2.6. Photothermal Purification Performance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PCH
4.2.1. Preparation of the EP-POSS
4.2.2. Preparation of the PCS
4.2.3. Construction of Photothermal Layers of HA-Zn2+ Complexes
4.3. Structure Characterizations
4.4. Water Absorption Test
4.5. Evaporation Performance Test
4.6. Artificial Seawater Purification Test
4.7. Antimicrobial Performance Test
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, W.; Xu, Y.; Ma, X.; Tian, Z.; Zhang, C.; Han, J.; Han, X.; He, S.; Duan, G.; Li, Y. Cellulose-based interfacial solar evaporators: Structural regulation and performance manipulation. Adv. Funct. Mater. 2023, 33, 202302351. [Google Scholar] [CrossRef]
- Kim, H.; Rao, S.R.; Kapustin, E.A.; Zhao, L.; Yang, S.; Yaghi, O.M.; Wang, E.N. Adsorption-based atmospheric water harvesting device for arid climates. Nat. Commun. 2018, 9, 1191. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Z.; Xiao, C.; Liu, F.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. High-performance salt-rejecting and cost-effective superhydrophilic porous monolithic polymer foam for solar steam generation. ACS Appl. Mater. Interfaces 2020, 12, 16308–16318. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, P.; Yang, C.; Liao, Q.; Hao, X.; Huang, Y.; Zhang, M.; Wang, X.; Lin, T.; Cheng, H.; et al. Janus-interface engineering boosting solar steam towards high-efficiency water collection. Energy Environ. Sci. 2021, 14, 5330–5338. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Z.C.; Cai, Z.R.; Ganapathy, T.; Fang, N.X.; Li, C.X.; Yu, C.L.; Zhang, Y.; Song, Y.L. Highly efficient three-dimensional solar evaporator for high salinity desalination by localized crystallization. Nat. Commun. 2020, 11, 521. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Wu, P.; Zhao, J.; Yang, X.; Owens, G.; Xu, H. Enhancing solar steam generation using a highly thermally conductive evaporator support. Sci. Bull. 2021, 66, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ran, X.; Zhang, Z.; Zhong, M.; Wang, D.; Li, P.; Fan, Z. Designing a solar interfacial evaporator based on tree structures for great coordination of water transport and salt rejection. Mater. Horiz. 2023, 10, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Chen, C.; He, S.; Hitz, E.M.; Wang, Y.; Gan, W.; Mi, R.; Hu, L. A high-performance self-regenerating solar evaporator for continuous water desalination. Adv. Mater. 2019, 31, e1900498. [Google Scholar] [CrossRef]
- Li, C.; Jiang, D.; Huo, B.; Ding, M.; Huang, C.; Jia, D.; Li, H.; Liu, C.-Y.; Liu, J. Scalable and robust bilayer polymer foams for highly efficient and stable solar desalination. Nano Energy 2019, 60, 841–849. [Google Scholar] [CrossRef]
- He, F.; Han, M.; Zhang, J.; Wang, Z.; Wu, X.; Zhou, Y.; Jiang, L.; Peng, S.; Li, Y. A simple, mild and versatile method for preparation of photothermal woods toward highly efficient solar steam generation. Nano Energy 2020, 71, 104650. [Google Scholar] [CrossRef]
- Liu, T.; Gou, S.; He, Y.; Fang, S.; Zhou, L.; Gou, G.; Liu, L. N-methylene phosphonic chitosan aerogels for efficient capture of Cu2+ and Pb2+ from aqueous environment. Carbohydr. Polym. 2021, 269, 118355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Shi, R.; Chen, L.; Fan, M. A robust salt-tolerant superoleophobic chitosan/nanofibrillated cellulose aerogel for highly efficient oil/water separation. Carbohydr. Polym. 2018, 200, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Guo, Z.; Li, F.; Zhang, H.; Bai, F.; Wang, L. Chitosan-based bifunctional composite aerogel combining absorption and phototherapy for bacteria elimination. Carbohydr. Polym. 2020, 247, 116739. [Google Scholar] [CrossRef] [PubMed]
- Moghtader, F.; Solakoglu, S.; Piskin, E. Alginate- and chitosan-modified gelatin hydrogel microbeads for delivery of E. coli phages. Gels 2024, 10, 244. [Google Scholar] [CrossRef]
- Venkatesan, R.; Vetcher, A.A.; Al-Asbahi, B.A.; Kim, S.-C. Chitosan-based films blended with tannic acid and moringa oleifera for application in food packaging: The preservation of strawberries (Fragaria ananassa). Polymers 2024, 16, 937. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, J.; You, F.; Yao, C.; Yang, H.; Chen, R.; Yu, P. Chitosan/clay aerogel: Microstructural evolution, flame resistance and sound absorption. Appl. Clay Sci. 2022, 228, 106624. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S. Supramolecular systems based on chitosan and chemically functionalized nanocelluloses as protective and reinforcing fillers of paper structure. Carbohydr. Polym. Technol. Appl. 2023, 6, 100380. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Ruiz-García, C.; Fernandes, F.M.; Dico, G.L.; Lisuzzo, L.; Prevot, V.; Darder, M.; Aranda, P. Sepiolite-hydrogels: Synthesis by ultrasound irradiation and their use for the preparation of functional clay-based nanoarchitectured materials. Front. Chem. 2021, 9, 733105. [Google Scholar] [CrossRef]
- Menshutina, N.; Majouga, A.; Uvarova, A.; Lovskaya, D.; Tsygankov, P.; Mochalova, M.; Abramova, O.; Ushakova, V.; Morozova, A.; Silantyev, A. Chitosan aerogel particles as nasal drug delivery systems. Gels 2022, 8, 796. [Google Scholar] [CrossRef]
- Yudaev, P.; Semenova, A.; Chistyakov, E. Gel based on modified chitosan for oil spill cleanup. J. Appl. Polym. Sci. 2024, 141, e54838. [Google Scholar] [CrossRef]
- Bidgoli, H.; Khodadadi, A.A.; Mortazavi, Y. A hydrophobic/oleophilic chitosan-based sorbent: Toward an effective oil spill remediation technology. J. Environ. Chem. Eng. 2019, 7, 103340. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Mu, M.; Feng, C.; Chuan, D.; Ren, Y.; Wang, X.; Fan, R.; Yan, J.; Guo, G. MXene-based polysaccharide aerogel with multifunctional enduring antimicrobial effects for infected wound healing. Int. J. Biol. Macromol. 2024, 261, 129238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiang, Y.; Zhang, H.; Zhu, T.; Chen, S.; Li, J.; Du, J.; Yan, X. A multifunctional chitosan composite aerogel based on high density amidation for chronic wound healing. Carbohydr. Polym. 2023, 321, 121248. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, J.; Cao, X.; Xie, F.; Yang, H.; Wang, C.; Bittencourt, C.; Li, W. Regenerated cellulose/chitosan composite aerogel with highly efficient adsorption for anionic dyes. Int. J. Biol. Macromol. 2023, 244, 125067. [Google Scholar] [CrossRef]
- Ko, E.; Kim, H. Preparation of chitosan aerogel crosslinked in chemical and ionical ways by non-acid condition for wound dressing. Int. J. Biol. Macromol. 2020, 164, 2177–2185. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Synthesis of imidazolium-crosslinked chitosan aerogel and its prospect as a dye removing adsorbent. RSC Adv. 2016, 6, 56544–56548. [Google Scholar] [CrossRef]
- Ozimek, J.; Pielichowski, K. Recent advances in polyurethane/poss hybrids for biomedical applications. Molecules 2022, 27, 40. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Wang, B.T.; Zhang, Y.; Zhang, P.; Fang, Z.P. Functionalization of polyhedral oligomeric silsesquioxanes with bis(hydroxyethyl) ester and preparation of the corresponding degradable nanohybrids. Chin. Chem. Lett. 2012, 23, 1083–1086. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, Z. Effect of poly(ethylene glycol)-polyhedral oligomeric silsesquioxanes on the thermal and mechanical properties of biodegradable poly(l-lactide). Compos. Commun. 2017, 3, 11–13. [Google Scholar] [CrossRef]
- Yu, B.; Yuen, A.C.Y.; Xu, X.; Zhang, Z.-C.; Yang, W.; Lu, H.; Fei, B.; Yeoh, G.H.; Song, P.; Wang, H. Engineering mxene surface with poss for reducing fire hazards of polystyrene with enhanced thermal stability. J. Hazard. Mater. 2021, 401, 123342. [Google Scholar] [CrossRef]
- Teng, S.; Jiang, Z.; Qiu, Z. Effect of different POSS structures on the crystallization behavior and dynamic mechanical properties of biodegradable Poly(ethylene succinate). Polymer 2019, 163, 68–73. [Google Scholar] [CrossRef]
- Hawashi, M.; Altway, A.; Widjaja, T.; Gunawan, S. Optimization of process conditions for tannin content reduction in cassava leaves during solid state fermentation using Saccharomyces cerevisiae. Heliyon 2019, 5, e02298. [Google Scholar] [CrossRef]
- Yan, W.; Shi, M.; Dong, C.; Liu, L.; Gao, C. Applications of tannic acid in membrane technologies: A review. Adv. Colloid Interface Sci. 2020, 284, 102267. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, H.; Li, S.; Huang, L.; Zhang, R.; Zhang, L.; Yu, A.; Duan, B. Polyphenol-driving assembly for constructing chitin-polyphenol-metal hydrogel as wound dressing. Carbohydr. Polym. 2022, 290, 119444. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ma, C.; Chen, Z.; Zhang, X.; Niu, N.; Li, J.; Liu, S.; Li, S. Biomass-derived solar-to-thermal materials: Promising energy absorbers to convert light to mechanical motion. J. Mater. Chem. A 2019, 7, 4002–4008. [Google Scholar] [CrossRef]
- Karakochuk, C.D.; Murphy, H.M.; Whitfield, K.C.; Barr, S.I.; Vercauteren, S.M.; Talukder, A.; Porter, K.; Kroeun, H.; Eath, M.; McLean, J.; et al. Elevated levels of iron in groundwater in prey veng province in cambodia: A possible factor contributing to high iron stores in women. J. Water Health 2015, 13, 575–586. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, L.; Liu, J.; He, S.; Shao, W. Sustainable, highly efficient and superhydrophobic fluorinated silica functionalized chitosan aerogel for gravity-driven oil/water separation. Gels 2021, 7, 66. [Google Scholar] [CrossRef]
- Sang, Y.; Miao, P.; Chen, T.; Zhao, Y.; Chen, L.; Tian, Y.; Han, X.; Gao, J. Fabrication and evaluation of graphene oxide/hydroxypropyl cellulose/chitosan hybrid aerogel for 5-fluorouracil release. Gels 2022, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Wang, B.; Hu, Y.; Xu, D. Light-responsive nanocomposites combining graphene oxide with poss based on host-guest chemistry. Chin. Chem. Lett. 2019, 30, 717–720. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Fu, Z.; Wang, D.; Wang, C.; Li, J. Fluorescence response mechanism of green synthetic carboxymethyl chitosan-Eu3+ aerogel to acidic gases. Int. J. Biol. Macromol. 2021, 192, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jiang, X.; Wang, J.; Hu, R. Degradable composite aerogel with excellent water-absorption for trace water removal in oil and oil-in-water emulsion filtration. Front. Mater. 2022, 9, 1093164. [Google Scholar] [CrossRef]
- Ye, S.; Liu, Y.; Feng, J. Low-density, mechanical compressible, water-induced self-recoverable graphene aerogels for water treatment. ACS Appl. Mater. Interfaces 2017, 9, 22456–22464. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, X.; Li, Y.; Wang, Y.; Yu, H.; Li, Z.; Zhou, Y. Regulating and controlling the microstructure of nanocellulose aerogels by varying the intensity of hydrogen bonds. ACS Sustain. Chem. Eng. 2023, 11, 1581–1590. [Google Scholar] [CrossRef]
- Guo, R.; Hou, X.; Zhao, D.; Wang, H.; Shi, C.; Zhou, Y. Mechanical stability and biological activity of Mg–Sr co-doped bioactive glass/chitosan composite scaffolds. J. Non-Cryst. Solids 2022, 583, 121481. [Google Scholar] [CrossRef]
- Chen, L.; He, X.; Liu, H.; Qian, L.; Kim, S.H. Water adsorption on hydrophilic and hydrophobic surfaces of silicon. J. Phys. Chem. C 2018, 122, 11385–11391. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, J.; Zhao, Y.; Zhang, D.; Tong, L.; Gao, F.; Liu, C.; Chen, F. Starch-based shape memory sponge for rapid hemostasis in penetrating wounds. J. Mater. Chem. B 2023, 11, 852–864. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Ma, P.X.; Guo, B. Rapid thermal responsive conductive hybrid cryogels with shape memory properties, photothermal properties and pressure dependent conductivity. J. Colloid Interface Sci. 2018, 526, 281–294. [Google Scholar] [CrossRef]
- Xuan, H.; Du, Q.; Li, R.; Shen, X.; Zhou, J.; Li, B.; Jin, Y.; Yuan, H. Shape-memory-reduced graphene/chitosan cryogels for non-compressible wounds. Int. J. Mol. Sci. 2023, 24, 1389. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cranston, E.D. Chemically cross-linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Peng, Z.; Yu, C.; Zhong, W. Facile preparation of a 3D porous aligned graphene-based wall network architecture by confined self-assembly with shape memory for artificial muscle, pressure sensor, and flexible supercapacitor. ACS Appl. Mater. Interfaces 2022, 14, 17739–17753. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, Y.; Lai, W.; Huang, F.; Ou, A.; Qin, R.; Liu, X.; Wang, X. Ester crosslinking enhanced hydrophilic cellulose nanofibrils aerogel. ACS Sustain. Chem. Eng. 2018, 6, 11979–11988. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zou, F.; Chen, S.; Wang, Y. Development of polyhydroxyalkanoate-based polyurethane with water-thermal response shape-memory behavior as new 3D elastomers scaffolds. Polymers 2019, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, H.; Xu, Z.; Chi, H.; Li, X.; Wang, S.; Zhang, T.; Zhao, Y. Underwater mechanically tough, elastic, superhydrophilic cellulose nanofiber-based aerogels for water-in-oil emulsion separation and solar steam generation. ACS Appl. Nano Mater. 2021, 4, 8979–8989. [Google Scholar] [CrossRef]
- Wang, J.; Du, W.; Zhang, Z.; Gao, W.; Li, Z. Biomass/polyhedral oligomeric silsesquioxane nanocomposites: Advances in preparation strategies and performances. J. Appl. Polym. Sci. 2020, 138, 49641. [Google Scholar] [CrossRef]
- Sun, X.; Tian, Q.; Xue, Z.; Zhang, Y.; Mu, T. The dissolution behaviour of chitosan in acetate-based ionic liquids and their interactions: From experimental evidence to density functional theory analysis. RSC Adv. 2014, 4, 30282–30291. [Google Scholar] [CrossRef]
- Nguyen, H.G.; Nguyen, T.A.H.; Do, D.B.; Pham, X.N.; Nguyen, T.H.; Nghiem, H.L.T.; Nguyen, M.V.; Pham, T.T. Natural cellulose fiber-derived photothermal aerogel for efficient and sustainable solar desalination. Langmuir 2023, 39, 6780–6793. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Z.; Ni, L.; Qi, L.; Yang, Y.; Zhou, Y.; Qi, J.; Li, J. Nanofiber-constructed aerogel solar evaporator for efficient salt resistance and volatile removal. ACS ES&T Eng. 2023, 3, 2027–2037. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, H.; Sun, J.; Wang, Y.; Gao, C.; Liu, Y. A silsesquioxane-based flexible polyimide aerogel with high hydrophobicity and good adsorption for liquid pollutants in wastewater. J. Mater. Sci. 2021, 56, 3576–3588. [Google Scholar] [CrossRef]
- Gao, H.; Yang, M.; Dang, B.; Luo, X.; Liu, S.; Li, S.; Chen, Z.; Li, J. Natural phenolic compound–iron complexes: Sustainable solar absorbers for wood-based solar steam generation devices. RSC Adv. 2020, 10, 1152–1158. [Google Scholar] [CrossRef]
- Zhang, P.; Piao, X.; Guo, H.; Xiong, Y.; Cao, Y.; Yan, Y.; Wang, Z.; Jin, C. A multi-function bamboo-based solar interface evaporator for efficient solar evaporation and sewage treatment. Ind. Crop. Prod. 2023, 200, 116823. [Google Scholar] [CrossRef]
- He, M.; Alam, K.; Liu, H.; Zheng, M.; Zhao, J.; Wang, L.; Liu, L.; Qin, X.; Yu, J. Textile waste derived cellulose based composite aerogel for efficient solar steam generation. Compos. Commun. 2021, 28, 100936. [Google Scholar] [CrossRef]
- Li, S.; He, Y.; Guan, Y.; Liu, X.; Liu, H.; Xie, M.; Zhou, L.; Wei, C.; Yu, C.; Chen, Y. Cellulose nanofibril-stabilized pickering emulsion and in situ polymerization lead to hybrid aerogel for high-efficiency solar steam generation. ACS Appl. Polym. Mater. 2020, 2, 4581–4591. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Ma, D.; Yuan, Y.; Yao, J.; Zhang, W.; Su, H.; Su, Y.; Gu, J.; Zhang, D. Simultaneously achieving thermal insulation and rapid water transport in sugarcane stems for efficient solar steam generation. J. Mater. Chem. A 2019, 7, 9034–9039. [Google Scholar] [CrossRef]
- Liu, M.; He, X.; Gu, J.; Li, Z.; Liu, H.; Liu, W.; Zhang, Y.; Zheng, M.; Yu, J.; Wang, L.; et al. Cotton fiber-based composite aerogel derived from waste biomass for high-performance solar-driven interfacial evaporation. Ind. Crop. Prod. 2024, 211, 118220. [Google Scholar] [CrossRef]
- Meng, R.; Lyu, J.; Zou, L.; Zhong, Q.; Liu, Z.; Zhu, B.; Chen, M.; Zhang, L.; Chen, Z. CNT-based gel-coated cotton fabrics for constructing symmetrical evaporator with up/down inversion property for efficient continuous solar desalination. Desalination 2023, 554, 116494. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, Y.; Wang, W.; Ding, X.; Yu, D. High-efficiency solar evaporator prepared by one-step carbon nanotubes loading on cotton fabric toward water purification. Sci. Total Environ. 2019, 698, 134136. [Google Scholar] [CrossRef]
- Li, W.; Jian, H.; Wang, W.; Yu, D. Highly efficient solar vapour generation via self-floating three-dimensional Ti2O3-based aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 634, 128031. [Google Scholar] [CrossRef]
- Zhang, X.; Pi, M.; Lu, H.; Li, M.; Wang, X.; Wang, Z.; Ran, R. A biomass hybrid hydrogel with hierarchical porous structure for efficient solar steam generation. Sol. Energy Mater. Sol. Cells 2022, 242, 111742. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.; Liu, D.; Liu, S.; Lei, D.; Zheng, L.; Wei, Q.; Gao, M. Zinc-based metal organic framework with antibacterial and anti-inflammatory properties for promoting wound healing. Regen. Biomater. 2022, 9, rbac019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, W.; Wu, M.; Liu, Z.; Yu, G.; Cui, Q.; Liu, C.; Fatehi, P.; Li, B. Robust, scalable, and cost-effective surface carbonized pulp foam for highly efficient solar steam generation. ACS Appl. Mater. Interfaces 2023, 15, 7414–7426. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ma, M.; Shen, Y.; Zhao, Z.; Wang, X.; Wang, J.; Pan, J.; Wang, D.; Wang, C.; Li, J. Polyhedral Oligomeric Sesquioxane Cross-Linked Chitosan-Based Multi-Effective Aerogel Preparation and Its Water-Driven Recovery Mechanism. Gels 2024, 10, 279. https://doi.org/10.3390/gels10040279
Liu Y, Ma M, Shen Y, Zhao Z, Wang X, Wang J, Pan J, Wang D, Wang C, Li J. Polyhedral Oligomeric Sesquioxane Cross-Linked Chitosan-Based Multi-Effective Aerogel Preparation and Its Water-Driven Recovery Mechanism. Gels. 2024; 10(4):279. https://doi.org/10.3390/gels10040279
Chicago/Turabian StyleLiu, Yang, Mingjian Ma, Yuan Shen, Zhengdong Zhao, Xuefei Wang, Jiaqi Wang, Jiangbo Pan, Di Wang, Chengyu Wang, and Jian Li. 2024. "Polyhedral Oligomeric Sesquioxane Cross-Linked Chitosan-Based Multi-Effective Aerogel Preparation and Its Water-Driven Recovery Mechanism" Gels 10, no. 4: 279. https://doi.org/10.3390/gels10040279