A Novel Hybrid Ultrasound Abrasive-Driven Electrochemical Surface Finishing Technique for Additively Manufactured Ti6Al4V Parts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Measurement of Polarization Curve
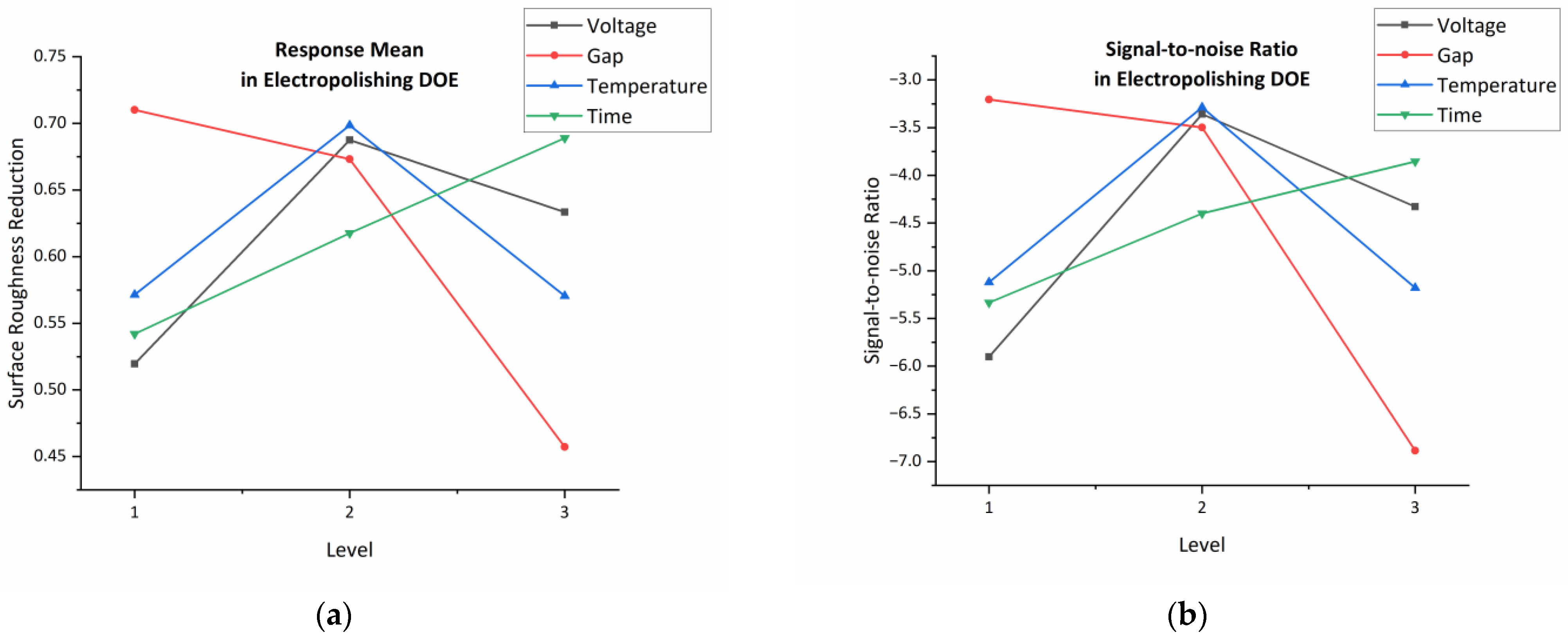
2.3. Optimization of Electropolishing Parameters
2.4. Experimental Study on Ultrasound and Abrasion
2.5. Optimization of Hybrid Surface Finishing
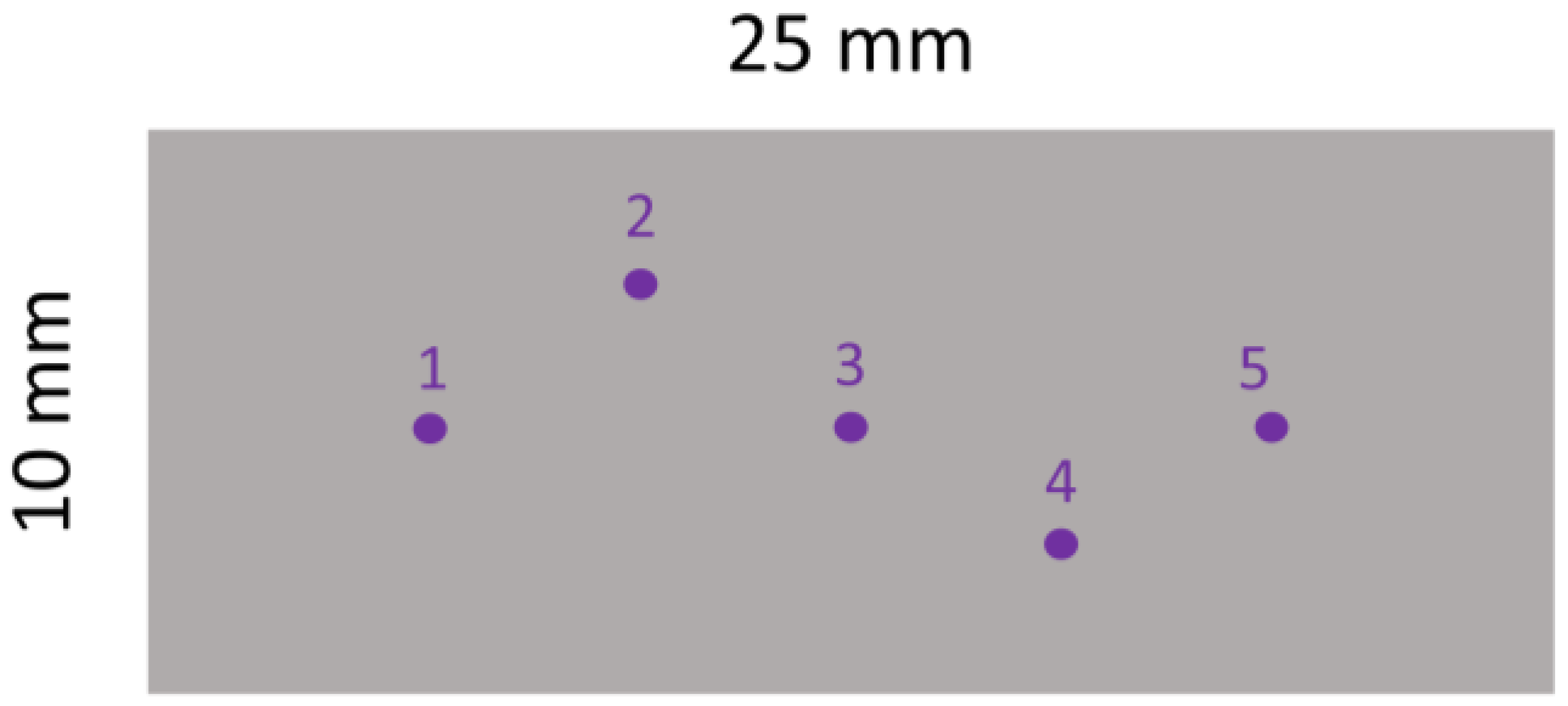
2.6. Surface Roughness Characterization
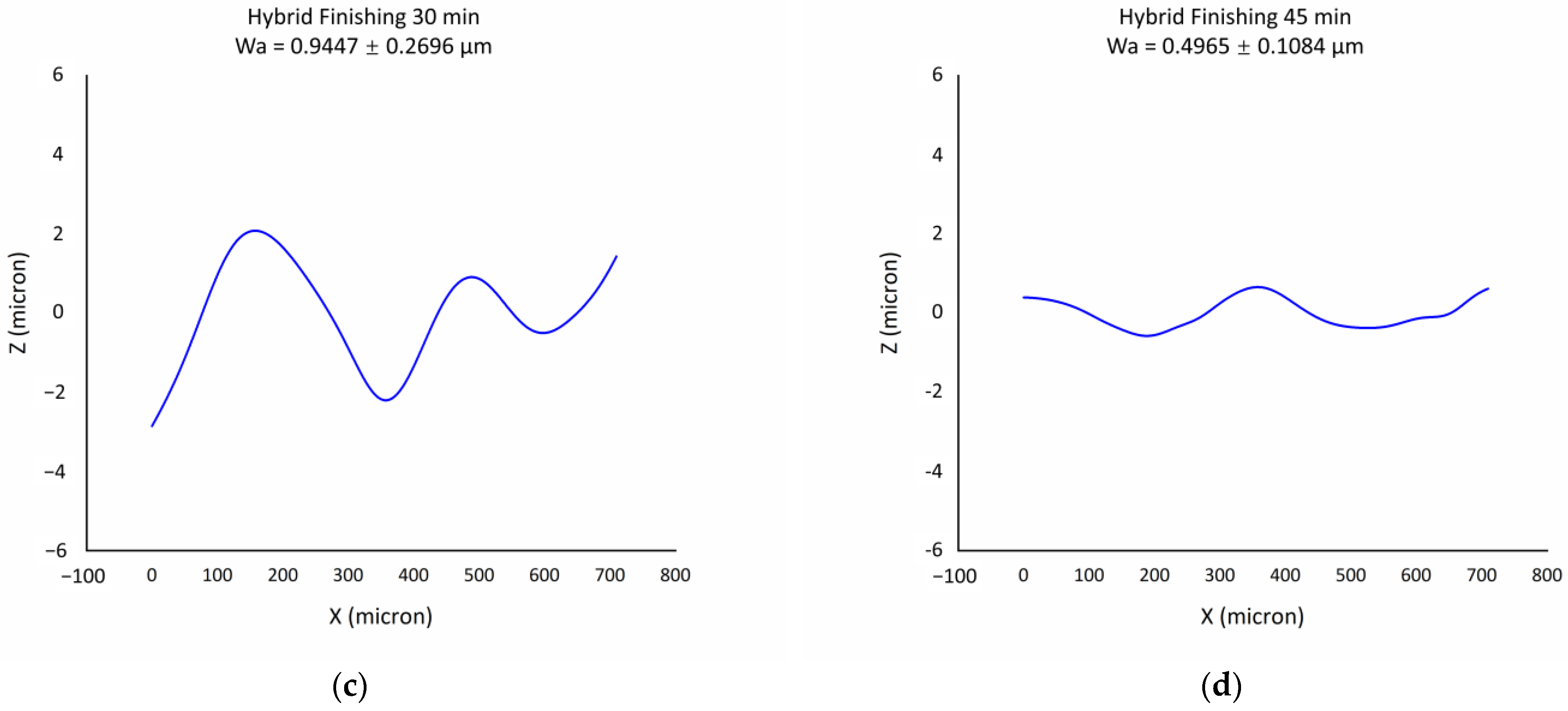
2.7. Investigation of Surface Waviness
3. Results
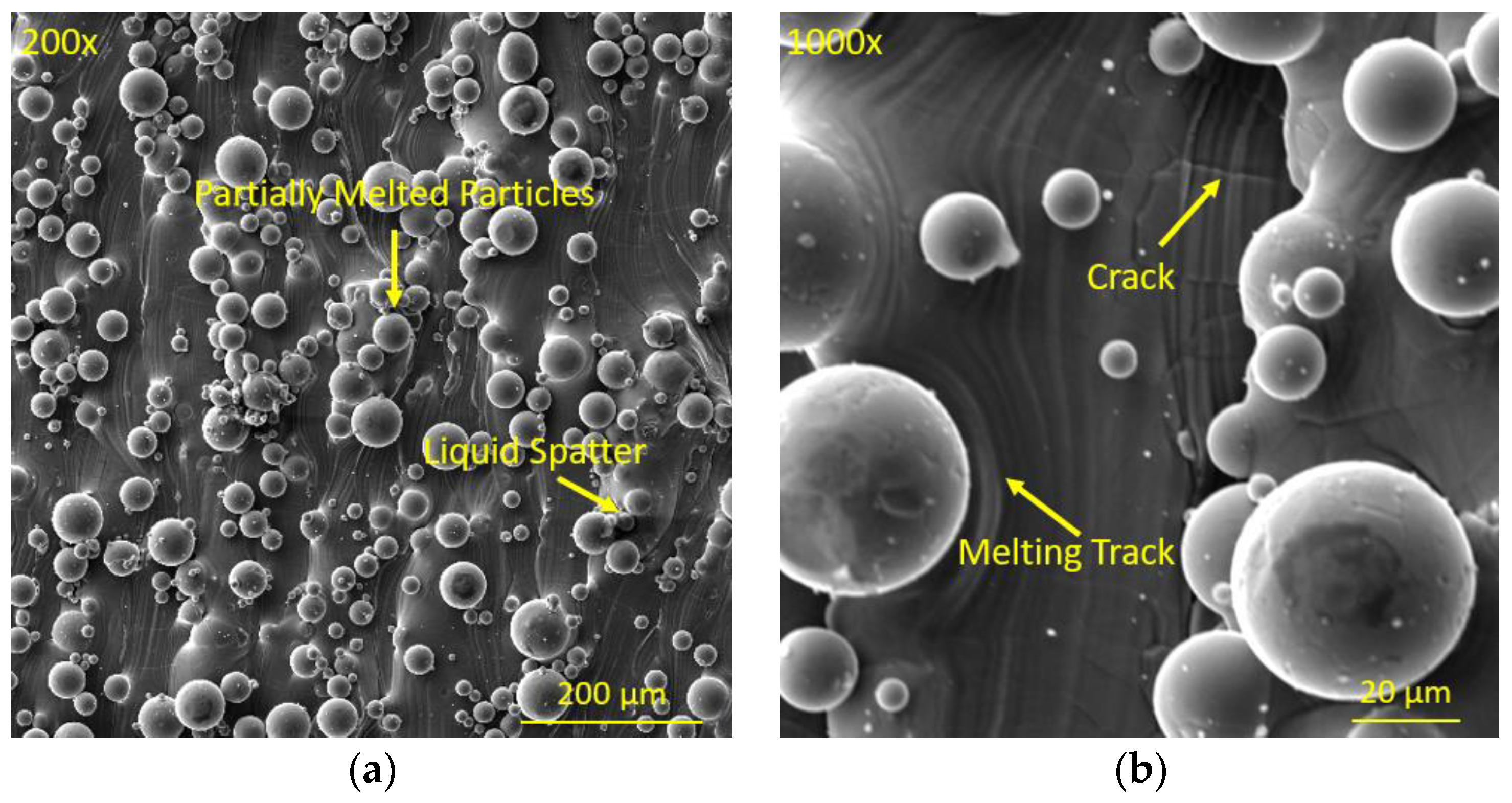
3.1. Surface Analysis of As-Built Samples
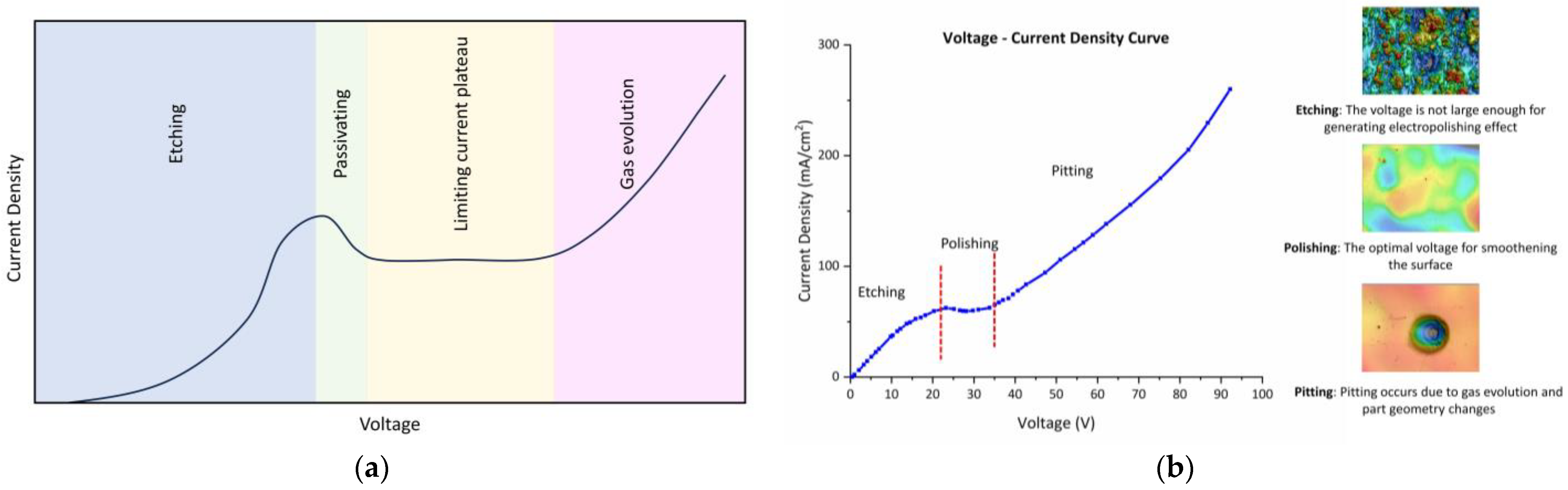
3.2. Polarization Curve
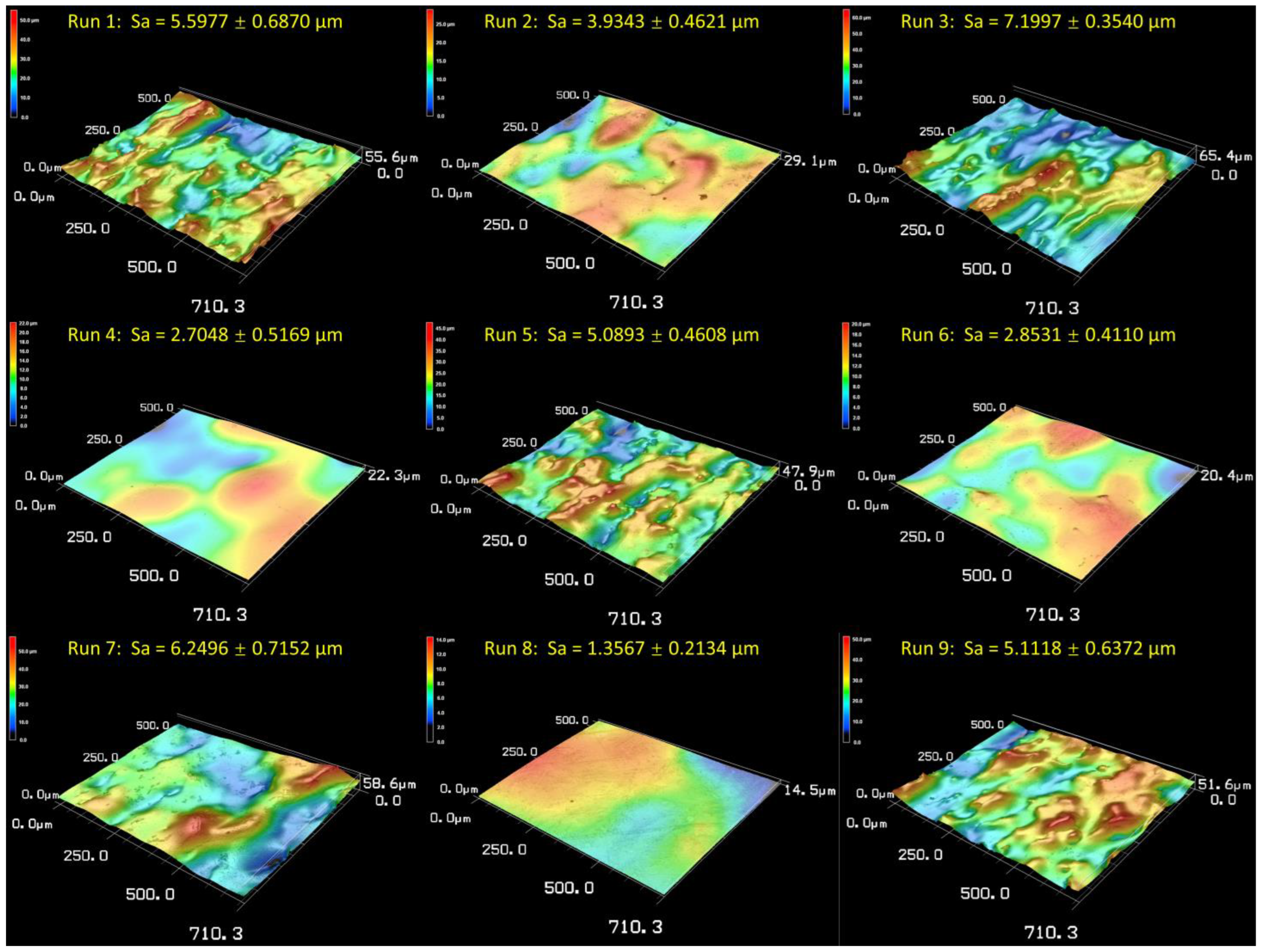
3.3. Analysis of Electropolishing
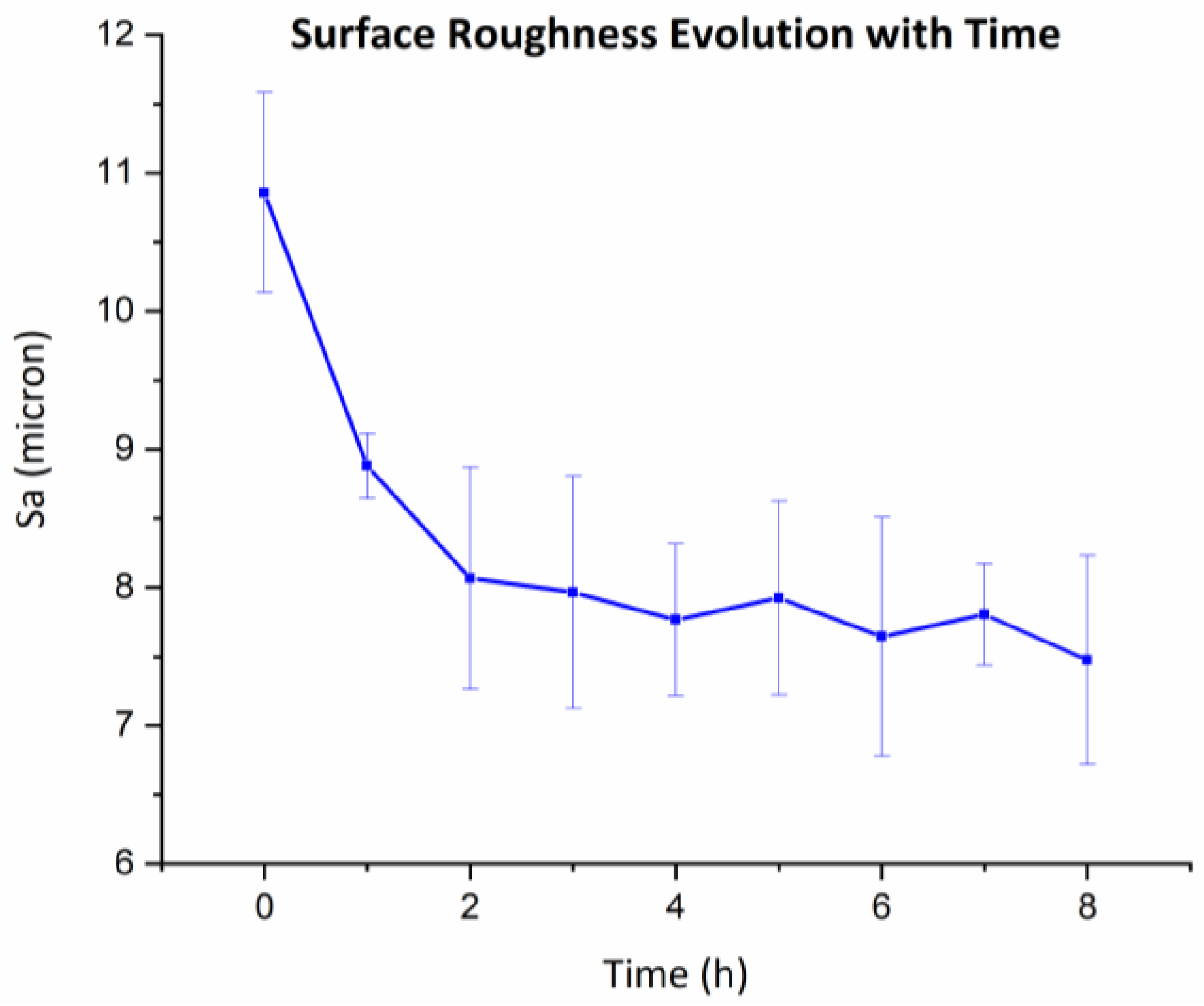
3.4. Effect of Ultrasound and Abrasion on Surface Finishing
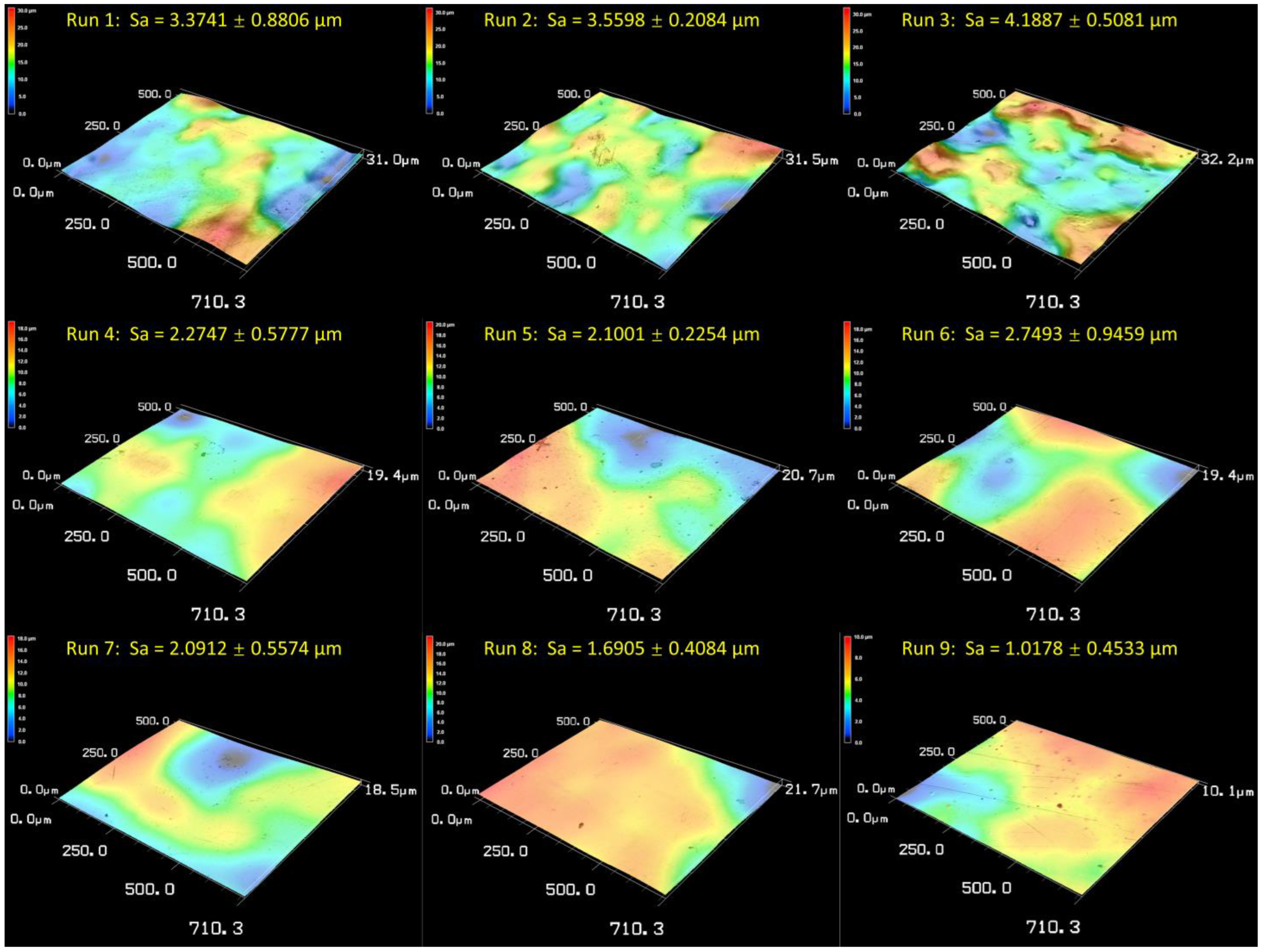
3.5. Analysis of Hybrid Finishing Technique
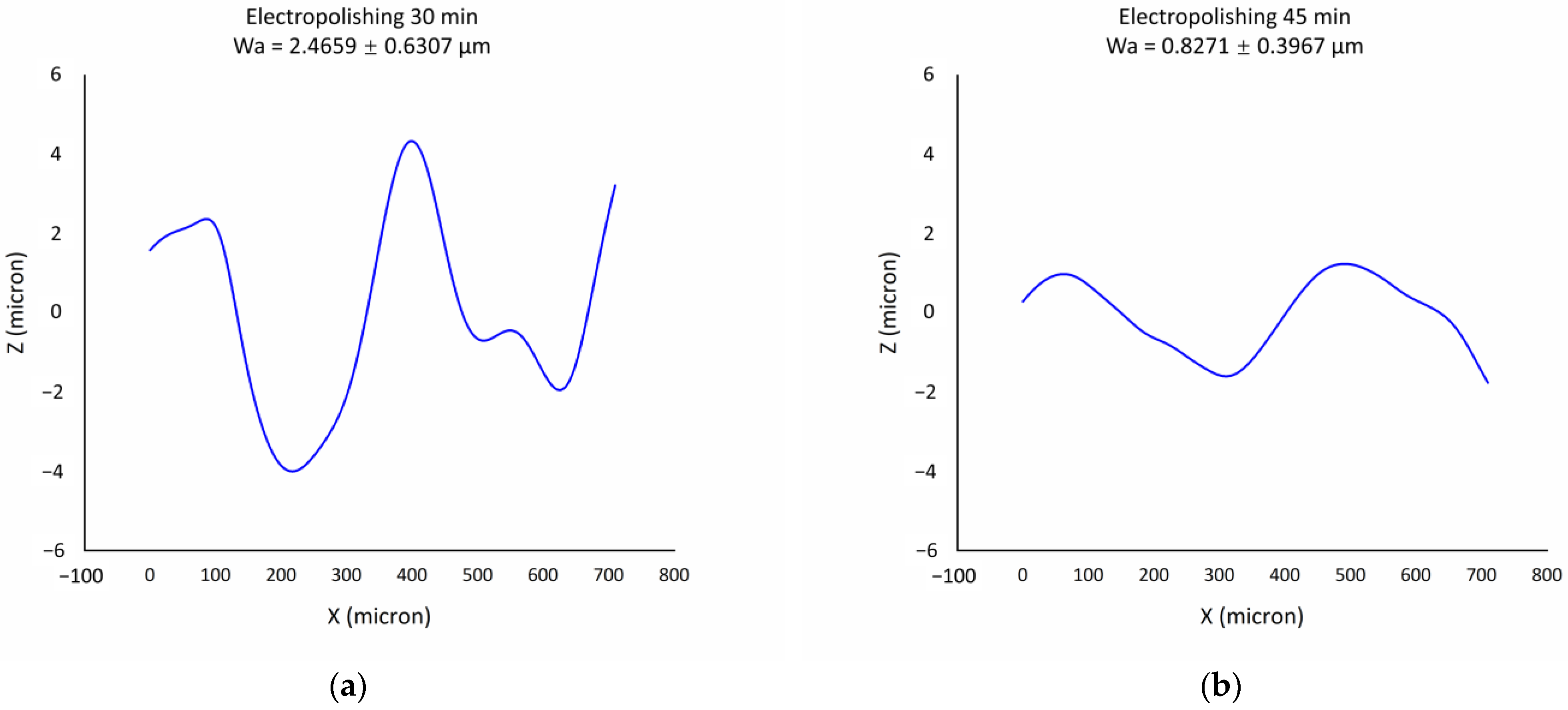
3.6. Comparison between Electropolished Surface and Hybrid Finished Surface
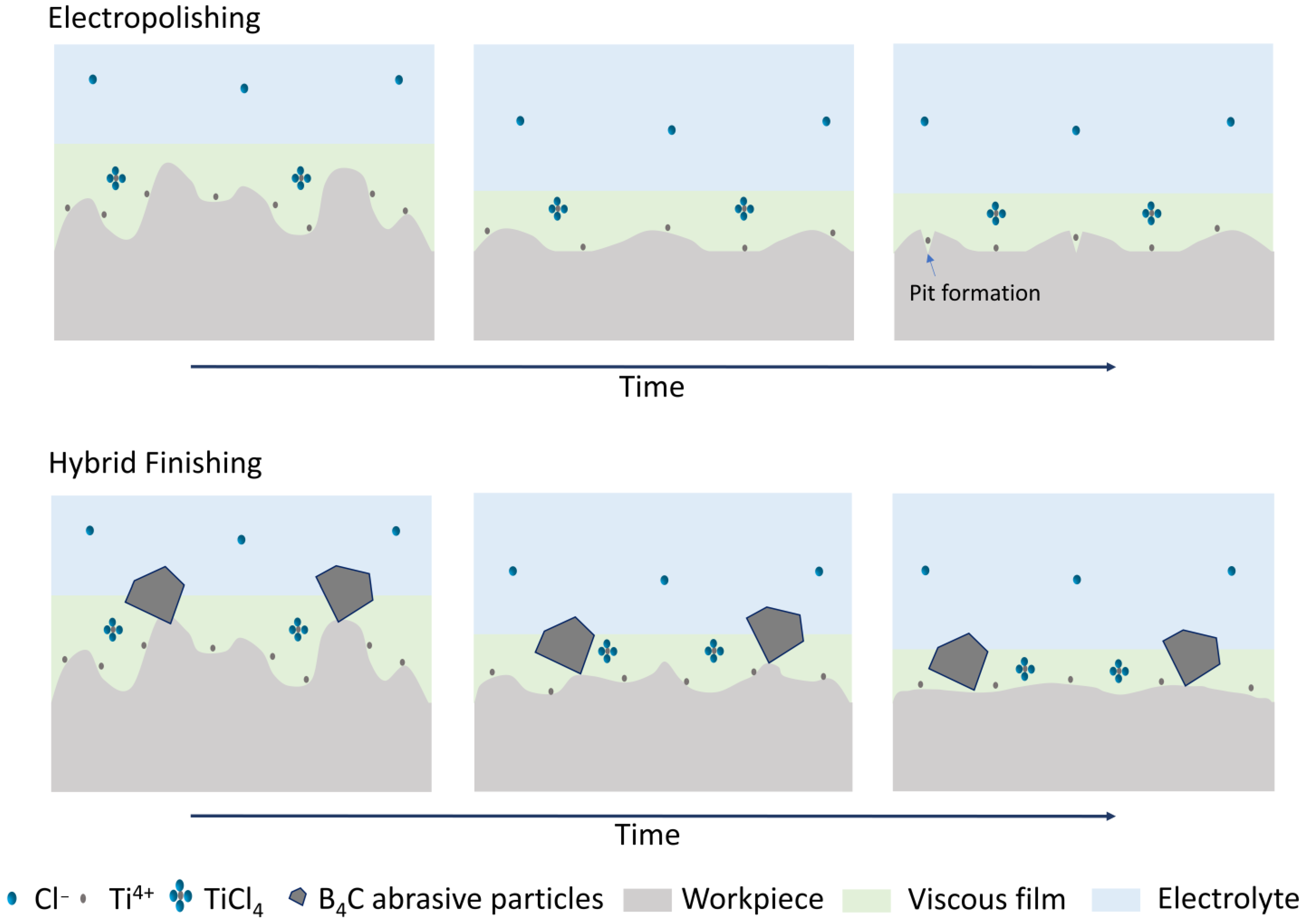
4. Discussion
5. Conclusions
- (1)
- A smooth and reflective surface could be achieved after both the electropolishing and hybrid treatment, with the optimal Sa values of 1.36 ± 0.21 µm and 1.02 ± 0.45 µm, respectively.
- (2)
- No evidence showed that the combination of ultrasound and abrasion is capable of effectively reducing surface roughness.
- (3)
- The introduction of ultrasound and abrasion into electropolishing has the capability to reduce the surface waviness further compared to electropolishing, with the Wa value being 0.83 ± 0.40 µm for electropolishing and 0.50 ± 0.11 µm for hybrid finishing after 45 min.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toyserkani, E.; Sarker, D.; Ibhadode, O.O.; Liravi, F.; Russo, P.; Taherkhani, K. Metal Additive Manufacturing; Wiley: Hoboken, NJ, USA, 2022; pp. 330–337. [Google Scholar] [CrossRef]
- Casalino, G.; Karamimoghadam, M.; Contuzzi, N. Metal Wire Additive Manufacturing: A Comparison between Arc Laser and Laser/Arc Heat Sources. Inventions 2023, 8, 52. [Google Scholar] [CrossRef]
- Snyder, J.C.; Thole, K.A. Understanding Laser Powder Bed Fusion Surface Roughness. J. Manuf. Sci. Eng. 2020, 142, 071003. [Google Scholar] [CrossRef]
- Hassanin, H.; Elshaer, A.; Benhadj-Djilali, R.; Modica, F.; Fassi, I. Surface Finish Improvement of Additive Manufactured Metal Parts. In Micro and Precision Manufacturing; Engineering Materials; Gupta, K., Ed.; Springer: Cham, Switzerland, 2018; pp. 145–164. [Google Scholar] [CrossRef]
- Sanaei, N.; Fatemi, A. Analysis of the Effect of Surface Roughness on Fatigue Performance of Powder Bed Fusion Additive Manufactured Metals. Theor. Appl. Fract. Mech. 2020, 108, 102638. [Google Scholar] [CrossRef]
- Toloei, A.; Stoilov, V.; Northwood, D. The Relationship between Surface Roughness and Corrosion. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition (IMECE), San Diego, CA, USA, 15–21 November 2013. [Google Scholar] [CrossRef]
- Beaucamp, A.T.; Namba, Y.; Charlton, P.; Jain, S.; Graziano, A.A. Finishing of Additively Manufactured Titanium Alloy by Shape Adaptive Grinding (SAG). Surf. Topogr. Metrol. Prop. 2015, 3, 024001. [Google Scholar] [CrossRef]
- Bagehorn, S.; Wehr, J.; Maier, H.J. Application of Mechanical Surface Finishing Processes for Roughness Reduction and Fatigue Improvement of Additively Manufactured Ti-6Al-4V Parts. Int. J. Fatigue 2017, 102, 135–142. [Google Scholar] [CrossRef]
- AlMangour, B.; Yang, J.M. Integration of Heat Treatment with Shot Peening of 17-4 Stainless Steel Fabricated by Direct Metal Laser Sintering. JOM 2017, 69, 2309–2313. [Google Scholar] [CrossRef]
- Lee, J.Y.; Nagalingam, A.P.; Yeo, S.H. A Review on the State-of-the-Art of Surface Finishing Processes and Related ISO/ASTM Standards for Metal Additive Manufactured Components. Virtual Phys. Prototyp. 2021, 16, 68–96. [Google Scholar] [CrossRef]
- Sunay, N.; Kaya, M.; Kaynak, Y. Chemical Post-Processing Methods for Enhancing Surface Properties of Parts Fabricated by Additive Manufacturing: A Review. Sigma J. Eng. Nat. Sci. 2020, 38, 2027–2042. [Google Scholar]
- Bezuidenhout, M.; Haar, G.T.; Becker, T.; Rudolph, S.; Damm, O.; Sacks, N. The Effect of HF-HNO3 Chemical Polishing on the Surface Roughness and Fatigue Life of Laser Powder Bed Fusion Produced Ti6Al4V. Mater. Today Commun. 2020, 25, 101396. [Google Scholar] [CrossRef]
- Urlea, V.; Brailovski, V. Electropolishing and Electropolishing-related Allowances for IN625 Alloy Components Fabricated by Laser Powder-Bed Fusion. Int. J. Adv. Manuf. Technol. 2017, 92, 4487–4499. [Google Scholar] [CrossRef]
- Maleki, E.; Bagherifard, S.; Bandini, M.; Guagliano, M. Surface Post-Treatments for Metal Additive Manufacturing: Progress, Challenges, and Opportunities. Addit. Manuf. 2021, 37, 101619. [Google Scholar] [CrossRef]
- Dixit, N.; Sharma, V.; Kumar, P. Research Trends in Abrasive Flow Machining: A Systematic Review. J. Manuf. Process. 2021, 64, 1434–1461. [Google Scholar] [CrossRef]
- Peng, C.; Fu, Y.; Wei, H.; Li, S.; Wang, X.; Gao, H. Study on Improvement of Surface Roughness and Induced Residual Stress for Additively Manufactured Metal Parts by Abrasive Flow Machining. Procedia CIRP 2018, 71, 386–389. [Google Scholar] [CrossRef]
- François, M.; Han, S.; Segonds, F.; Dupuy, C.; Rivette, M.; Turpault, S.; Mimouna, M.; Salvatore, F.; Rech, J.; Peyre, P. Electromagnetic Performance of Ti6Al4V and AlSi7Mg0.6 Waveguides with Laser Beam Melting (LBM) Produced and Abrasive Flow Machining (AFM) Finished Internal Surfaces. J. Electromagn. Waves Appl. 2021, 35, 2510–2526. [Google Scholar] [CrossRef]
- Qian, C.; Fan, Z.; Tian, Y.; Liu, Y.; Han, J.; Wang, J. A Review on Magnetic Abrasive Finishing. Int. J. Adv. Manuf. Technol. 2021, 112, 619–634. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, G.; Teng, X.; Du, J.; Jiang, L.; Chen, H.; Liu, N. Investigation and Process Optimization for Magnetic Abrasive Finishing Additive Manufacturing Samples with Different Forming Angles. Int. J. Adv. Manuf. Technol. 2022, 118, 2355–2371. [Google Scholar] [CrossRef]
- Lee, S.; Ahmadi, Z.; Pegues, J.W.; Mahjouri-Samani, M.; Shamsaei, N. Laser Polishing for Improving Fatigue Performance of Additive Manufactured Ti-6Al-4V Parts. Opt. Laser Technol. 2021, 134, 106639. [Google Scholar] [CrossRef]
- Han, W.; Fang, F. Fundamental Aspects and Recent Developments in Electropolishing. Int. J. Mach. Tools Manuf. 2019, 139, 1–23. [Google Scholar] [CrossRef]
- Tyagi, P.; Goulet, T.; Riso, C.; Stephenson, R.; Chuenprateep, N.; Schlitzer, J.; Benton, C.; Garcia-Moreno, F. Reducing the Roughness of Internal Surface of an Additive Manufacturing Produced 316 Steel Component by Chempolishing and Electropolishing. Addit. Manuf. 2019, 25, 32–38. [Google Scholar] [CrossRef]
- Acquesta, A.; Monetta, T. The Electropolishing of Additively Manufactured Parts in Titanium: State of the Art. Adv. Eng. Mater. 2021, 23, 2100545. [Google Scholar] [CrossRef]
- Mingear, J.; Zhang, B.; Hartl, D.; Elwany, A. Effect of Process Parameters and Electropolishing on the Surface Roughness of Interior Channels in Additively Manufactured Nickel-Titanium Shape Memory Alloy Actuators. Addit. Manuf. 2019, 27, 565–575. [Google Scholar] [CrossRef]
- Mohammadian, N.; Turenne, S.; Brailovski, V. Electropolishing of Laser Powder Bed-Fused IN625 Components in an Ionic Electrolyte. J. Manuf. Mater. Process. 2019, 3, 86. [Google Scholar] [CrossRef]
- Jain, S.; Corliss, M.; Tai, B.; Hung, W.N. Electrochemical Polishing of Selective Laser Melted Inconel 718. Procedia Manuf. 2019, 34, 239–246. [Google Scholar] [CrossRef]
- Ali, U.; Fayazfar, H.; Ahmed, F.; Toyserkani, E. Internal Surface Roughness Enhancement of Parts Made by Laser Powder-Bed Fusion Additive Manufacturing. Vacuum 2020, 177, 109314. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Che, S.; Tian, Y. Electrochemical Polishing of Additively Manufactured Ti–6Al–4V Alloy. Met. Mater. Int. 2020, 26, 783–792. [Google Scholar] [CrossRef]
- Alrbaey, K.; Wimpenny, D.I.; Al-Barzinjy, A.A.; Moroz, A. Electropolishing of Re-Melted SLM Stainless Steel 316L Parts Using Deep Eutectic Solvents: 3 × 3 Full Factorial Design. J. Mater. Eng. Perform. 2016, 25, 2836–2846. [Google Scholar] [CrossRef]
- Zhao, C.; Qu, N.; Tang, X. Electrochemical Mechanical Polishing of Internal Holes Created by Selective Laser Melting. J. Manuf. Process. 2021, 64, 1544–1562. [Google Scholar] [CrossRef]
- Rech, J.; Krzak, D.; Roy, F.; Salvatore, F.; Gidon, A.; Guérin, S. A New Hybrid Electrochemical-Mechanical Process (PEMEC) for Polishing Complex and Rough Parts. CIRP Ann. 2022, 71, 173–176. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Additive Manufacturing of Ti6Al4V Alloy: A Review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Fayazfar, H.; Rishmawi, I.; Vlasea, M. Electrochemical-Based Surface Enhancement of Additively Manufactured Ti-6Al-4V Complex Structures. J. Mater. Eng. Perform. 2021, 30, 2245–2255. [Google Scholar] [CrossRef]
- Aver’yanova, I.; Bogomolov, D.; Poroshin, V. ISO 25178 Standard for Three-Dimensional Parametric Assessment of Surface Texture. Russ. Eng. Res. 2017, 37, 513–516. [Google Scholar] [CrossRef]
- Carter, L.N.; Villapún, V.M.; Grover, L.; Cox, S.C. Exploring the Duality of Powder Adhesion and Underlying Surface Roughness in Laser Powder Bed Fusion Processed Ti-6Al-4V. J. Manuf. Process. 2022, 81, 14–26. [Google Scholar] [CrossRef]
- Gu, D.; Shen, Y. Balling Phenomena in Direct Laser Sintering of Stainless Steel Powder: Metallurgical Mechanisms and Control Methods. Mater. Des. 2009, 30, 2903–2910. [Google Scholar] [CrossRef]
- Jacquet, P.A. Electrolytic Polishing of Metallic Surfaces. Met. Finish. 1949, 47, 48–54. [Google Scholar]
- Wang, G.; Liu, Z.; Niu, J.; Huang, W.; Wang, B. Effect of Electrochemical Polishing on Surface Quality of Nickel-Titanium Shape Memory Alloy after Milling. J. Mater. Res. Technol. 2020, 9, 253–262. [Google Scholar] [CrossRef]
- Su, K.; Wu, J.; Xia, D. Dual Role of Microparticles in Synergistic Cavitation–Particle Erosion: Modeling and Experiments. Wear 2021, 470–471, 203633. [Google Scholar] [CrossRef]
- Szwajczakaf, E.; Szymański, A. On the Relation Between Mobility of lons and Viscosity. The Walden’s Rule. Mol. Cryst. Liq. Cryst. 1986, 139, 253–261. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, X.; Wang, J.; Gong, T. Numerical Study of the Synergistic Effect of Cavitation and Micro-Abrasive Particles. Ultrason. Sonochem. 2022, 89, 106119. [Google Scholar] [CrossRef]







| Run | Voltage (V) | Temperature (°C) | Gap (mm) | Time (min) |
|---|---|---|---|---|
| 1 | 25 | 25 | 5 | 15 |
| 2 | 25 | 40 | 10 | 30 |
| 3 | 25 | 55 | 15 | 45 |
| 4 | 30 | 25 | 10 | 45 |
| 5 | 30 | 40 | 15 | 15 |
| 6 | 30 | 55 | 5 | 30 |
| 7 | 35 | 25 | 15 | 30 |
| 8 | 35 | 40 | 5 | 45 |
| 9 | 35 | 55 | 10 | 15 |
| Run | Time (min) | Ultrasonic Amplitude (%) | Abrasive Concentration (%) |
|---|---|---|---|
| 1 | 15 | 70 | 5 |
| 2 | 15 | 80 | 10 |
| 3 | 15 | 90 | 15 |
| 4 | 30 | 70 | 10 |
| 5 | 30 | 80 | 15 |
| 6 | 30 | 90 | 5 |
| 7 | 45 | 70 | 15 |
| 8 | 45 | 80 | 5 |
| 9 | 45 | 90 | 10 |
| Sample Name | Type of Treatment | Time | Other Parameters |
|---|---|---|---|
| E-30 | Electropolishing | 30 min | Voltage 30 V, Temperature 40 ± 3 °C, |
| E-45 | Electropolishing | 45 min | Inter-electrode gap 5 mm, |
| H-30 | Hybrid finishing | 30 min | Ultrasonic Amplitude 80%, |
| H-45 | Hybrid finishing | 45 min | Abrasive Concentration 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Toyserkani, E. A Novel Hybrid Ultrasound Abrasive-Driven Electrochemical Surface Finishing Technique for Additively Manufactured Ti6Al4V Parts. Inventions 2024, 9, 45. https://doi.org/10.3390/inventions9020045
Sun M, Toyserkani E. A Novel Hybrid Ultrasound Abrasive-Driven Electrochemical Surface Finishing Technique for Additively Manufactured Ti6Al4V Parts. Inventions. 2024; 9(2):45. https://doi.org/10.3390/inventions9020045
Chicago/Turabian StyleSun, Manyou, and Ehsan Toyserkani. 2024. "A Novel Hybrid Ultrasound Abrasive-Driven Electrochemical Surface Finishing Technique for Additively Manufactured Ti6Al4V Parts" Inventions 9, no. 2: 45. https://doi.org/10.3390/inventions9020045








