Controlling Surface Wettability and Plasmonic Resonance of Au/ZnO Heterostructured Films
Abstract
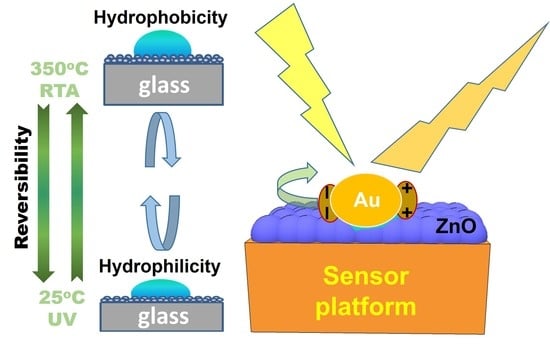
:1. Introduction
2. Experiments and Film Structures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Siddiqi, H.M.; Habib, A.; Shahid, M.; Afzal, A. ZnO/Zn(OH)2 nanoparticles and self-cleaning coatings for the photocatalytic degradation of organic pollutants. Front. Environ. Sci. 2022, 10, 965925. [Google Scholar] [CrossRef]
- Zhao, F.; Lin, J.; Lei, Z.; Yi, Z.; Qin, F.; Zhang, J.; Liu, L.; Wu, X.; Yang, W.; Wu, P. Realization of 18.97% theoretical efficiency of 0.9 μm thick c-Si/ZnO heterojunction ultrathin-film solar cells via surface plasmon resonance enhancement. Phys. Chem. Chem. Phys. 2022, 24, 4871–4880. [Google Scholar] [CrossRef] [PubMed]
- Mardosaitė, R.; Jurkevičiutė, A.; Račkauskas, S. Superhydrophobic ZnO Nanowires: Wettability Mechanisms and Functional Applications. Cryst. Growth Des. 2021, 21, 4765–4779. [Google Scholar] [CrossRef]
- Mei, G.S.; Menon, P.S.; Hegde, G. ZnO for performance enhancement of surface plasmon resonance biosensor: A review. Mater. Res. Express 2020, 7, 012003. [Google Scholar] [CrossRef]
- Cestellos-Blanco, S.; Zhang, H.; Kim, J.M.; Shen, Y.-X.; Yang, P. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis. Nat. Catal. 2020, 3, 245–255. [Google Scholar] [CrossRef]
- Gupta, A.K.; Hsu, C.-H.; Chen, C.-H.; Purwidyantri, A.; Prabowo, B.A.; Wang, J.-L.; Tian, Y.-C.; Lai, C.-S. Au-spotted zinc oxide nano-hexagonrods structure for plasmon-photoluminescence sensor. Sens. Actuators B Chem. 2019, 290, 100–109. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Purwidyantri, A.; Liu, K.-C. Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dixit, T.; Palani, I.; Nakamura, D.; Higashihata, M.; Singh, V. Utilization of surface plasmon resonance of Au/Pt nanoparticles for highly photosensitive ZnO nanorods network based plasmon field effect transistor. Phys. E 2017, 93, 97–104. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.; Tian, L.; Wang, S.; Gao, N.; Zhang, W.; Wang, P.; Yin, X.; Li, G. Facile fabrication of highly controllable gating systems based on the combination of inverse opal structure and dynamic covalent chemistry. Nanoscale 2017, 9, 7268–7275. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.-Q.; Chen, Z.; Chi, L. Recent Advances in TiO2-Based Nanostructured Surfaces with Controllable Wettability and Adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Cheng, H.F.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability for selective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.W.; Wei, D.H.; Wu, S.H.; Chen, Y.Y.; Yao, Y.D. Photoluminescence and wettability control of NiFe/ZnO heterostructure bilayer films. RSC Adv. 2015, 5, 96705–96713. [Google Scholar] [CrossRef]
- Peng, K.-Y.; Ho, Y.-H.; Wei, D.-H.; Yu, Y.-C.; Yao, Y.-D.; Tian, W.-C.; Wei, P.-K. Efficiency enhancement of organic light-emitting devices by using honeycomb metallic electrodes and two-dimensional photonic crystal arrays. Org. Electron. 2014, 15, 3043–3051. [Google Scholar] [CrossRef]
- Lin, C.-A.; Tsai, D.-S.; Chen, C.-Y.; He, J.-H. Significant enhancement of yellow–green light emission of ZnO nanorod arrays using Ag island films. Nanoscale 2011, 3, 1195–1199. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.C.; Losurdo, M.; Kim, T.-H.; Giangregorio, M.; Bruno, G.; Everitt, H.O.; Brown, A.S. Plasmonic Gallium Nanoparticles on Polar Semiconductors: Interplay between Nanoparticle Wetting, Localized Surface Plasmon Dynamics, and Interface Charge. Langmuir 2009, 25, 924–930. [Google Scholar] [CrossRef]
- Wei, D.H.; Tong, S.K.; Chen, S.C.; Hao, Y.H.; Wu, M.R.; Yang, C.J.; Huang, R.T.; Chung, R.J. Tuning surface plasmonic resonance and surface wettability of Au/CrN films by nitrogen-containing gas. Nanomaterials 2022, 12, 2575. [Google Scholar] [CrossRef]
- Li, Q.; Meng, J.; Huang, J.; Li, Z. Plasmon-Induced Pyro-Phototronic Effect Enhancement in Self-Powered UV–Vis Detection with a ZnO/CuO p–n Junction Device. Adv. Funct. Mater. 2021, 32, 2108903. [Google Scholar] [CrossRef]
- Wei, D.H.; Lin, T.K.; Liang, Y.C.; Chang, H.W. Formation and Application of Core-Shell of FePt-Au Magnetic-Plasmonic Nanoparticles. Front. Chem. 2021, 9, 653718. [Google Scholar] [CrossRef]
- Pan, K.-Y.; Wei, D.-H. Optoelectronic and Electrochemical Properties of Vanadium Pentoxide Nanowires Synthesized by Vapor-Solid Process. Nanomaterials 2016, 6, 140. [Google Scholar] [CrossRef]
- Chen, X.Q.; Wu, Z.S.; Liu, D.D.; Gao, Z.Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.F.; Jia, R.; Gao, N.K.; Yan, W.S.; Zhang, L.; Li, X.; Liu, D. Near-infrared promoted wettability recovery of superhydrophilic ZnO. J. Phys. Chem. C 2017, 121, 12745–12749. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, S.; Xu, W.; Cheng, Y. Controllable wettability and morphology of electrodeposited surfaces on zinc substrates. Appl. Surf. Sci. 2016, 360, 904–914. [Google Scholar] [CrossRef]
- Li, J.; Jing, Z.; Yang, Y.; Zha, F.; Yan, L.; Lei, Z. Reversible low adhesive to high adhesive superhydrophobicity transition on ZnO nanoparticle surfaces. Appl. Surf. Sci. 2014, 289, 1–5. [Google Scholar] [CrossRef]
- Li, H.; Zheng, M.J.; Liu, S.D.; Ma, L.; Zhu, C.Q.; Xiong, Z.Z. Reversible surface wettability transition between superhydrophobicity and superhydrophilicity on hierarchical micro/nanostructure ZnO mesh films. Surf. Coat. Technol. 2013, 224, 88–92. [Google Scholar] [CrossRef]
- Xu, C.; Fang, L.; Wu, F.; Huang, Q.; Yin, B. Wetting behavior of triethoxyoctylsilane modified ZnO nanowire films. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 48–53. [Google Scholar] [CrossRef]
- Myint, M.T.Z.; Kumar, N.S.; Hornyak, G.L.; Dutta, J. Hydrophobic/hydrophilic switching on zinc oxide micro-textured surface. Appl. Surf. Sci. 2013, 264, 344–348. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, P.; Chen, W.; Pan, S.; Chen, D. Hemocompatibility of ZnO thin films prepared by filtered cathodic vacuum arc deposition. Vacuum 2013, 89, 220–224. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.Y.; Lin, W.; Moon, K.S.; Wong, C.P. Reversible superhydrophobic–superhydrophilic transition of ZnO nanorod/epoxy composite films. ACS Appl. Mater. Interfaces 2012, 4, 3959–3964. [Google Scholar] [CrossRef]
- Fatemi, H.; Khodadadi, A.A.; Firooz, A.A.; Mortazavi, Y. Apple—Biomorphic synthesis of porous ZnO nanostructures for glucose direct electrochemical biosensor. Curr. Appl. Phys. 2012, 12, 1033–1038. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Zhao, D.; Li, B.; Zhang, Z.; Jiang, M.; Shen, D. High responsivity ZnO nanowires based UV detector fabricated by the dielectrophoresis method. Sens. Actuators B Chem. 2012, 166–167, 12–16. [Google Scholar] [CrossRef]
- Jindal, K.; Tomar, M.; Gupta, V. Inducing electrocatalytic functionality in ZnO thin film by N doping to realize a third generation uric acid biosensor. Biosens. Bioelectron. 2014, 55, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Chi, P.-W.; Wei, D.-H. Investigations on the Crystallographic Orientation Induced Surface Morphology Evolution of ZnO Thin Films and Their Wettability and Conductivity. J. Phys. Chem. C 2016, 120, 8210–8219. [Google Scholar] [CrossRef]
- Chao, C.H.; Weng, W.J.; Wei, D.H. Enhanced UV photodetector response and recovery times using a non-polar ZnO sensing layer. J. Vac. Sci. Technol. A 2016, 34, 02D106. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Chi, P.-W.; Su, C.-W.; Wei, D.-H. Control of hydrophobic surface and wetting states in ultra-flat ZnO films by GLAD method. Appl. Surf. Sci. 2017, 404, 380–387. [Google Scholar] [CrossRef]
- Chi, P.-W.; Su, C.-W.; Wei, D.-H. Internal stress induced natural self-chemisorption of ZnO nanostructured films. Sci. Rep. 2017, 7, 43281. [Google Scholar] [CrossRef] [Green Version]
- Azimi, G.; Dhiman, R.; Kwon, H.-M.; Paxson, A.T.; Varanasi, K.K. Hydrophobicity of rare-earth oxide ceramics. Nat. Mater. 2013, 12, 315–320. [Google Scholar] [CrossRef]
- Navale, Y.; Navale, S.; Ramgir, N.; Stadler, F.; Gupta, S.; Aswal, D.; Patil, V. Zinc oxide hierarchical nanostructures as potential NO2 sensors. Sens. Actuators B Chem. 2017, 251, 551–563. [Google Scholar] [CrossRef]
- Rezaie, M.N.; Manavizadeh, N.; Abadi, E.M.N.; Nadimi, E.; Boroumand, F.A. Comparison study of transparent RF-sputtered ITO/AZO and ITO/ZnO bilayers for near UV-OLED applications. Appl. Surf. Sci. 2017, 392, 549–556. [Google Scholar] [CrossRef]
- Quan, Z.; Liu, X.; Qi, Y.; Song, Z.; Qi, S.; Zhou, G.; Xu, X. Robust room temperature ferromagnetism and band gap tuning in nonmagnetic Mg doped ZnO films. Appl. Surf. Sci. 2017, 399, 751–757. [Google Scholar] [CrossRef]
- Chi, P.-W.; Wei, D.-H. Dielectric enhancement with low dielectric loss in textured ZnO films inserted with NiFe. J. Mater. Chem. C 2017, 5, 1394–1401. [Google Scholar] [CrossRef]
- Chi, P.-W.; Wei, D.-H.; Yu, C.-C.; Yao, Y.-D. Magnetic-control-electric and reversal behavior of ZnO/NiFe/ZnO multilayer films. AIP Adv. 2017, 7, 056309. [Google Scholar] [CrossRef] [Green Version]
- Alema, F.; Ledyaev, O.; Miller, R.; Beletsky, V.; Osinsky, A.; Schoenfeld, W.V. Growth of high Mg content wurtzite MgZnO epitaxial films via pulsed metal organic chemical vapor deposition. J. Cryst. Growth 2016, 435, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Opel, M.; Geprägs, S.; Althammer, M.; Brenninger, T.; Gross, R. Laser molecular beam epitaxy of ZnO thin films and heterostructures. J. Phys. D Appl. Phys. 2014, 47, 034002. [Google Scholar] [CrossRef] [Green Version]
- Flickyngerova, S.; Netrvalova, M.; Novotny, I.; Bruncko, J.; Gaspierik, P.; Sutta, P.; Tvarozek, V. Ion sputter etching of ZnO:Ga thin film surfaces. Vacuum 2012, 86, 703–706. [Google Scholar] [CrossRef]
- Montero, M.M.; Borras, A.; Saghi, Z.; Espinos, J.P.; Barranco, A.; Cotrino, J.; Elipe, A.R.G. Vertical and tilted Ag-NPs@ZnO nanorods by plasma-enhanced chemical vapor deposition. Nanotechnology 2012, 23, 255303. [Google Scholar] [CrossRef]
- Chao, C.-H.; Chen, M.-Y.; Lin, C.-R.; Yu, Y.-C.; Yao, Y.-D.; Wei, D.-H. Postannealing Effect at Various Gas Ambients on Ohmic Contacts of Pt/ZnO Nanobilayers toward Ultraviolet Photodetectors. Int. J. Photoenergy 2013, 2013, 372869. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.-H.; Wei, D.-H. Growth of non-polar ZnO thin films with different working pressures by plasma enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2014, 53, 11RA05. [Google Scholar] [CrossRef]
- Chao, C.-H.; Wei, D.-H. Synthesis and Characterization of High c-axis ZnO Thin Film by Plasma Enhanced Chemical Vapor Deposition System and its UV Photodetector Application. J. Vis. Exp. 2015, 104, e53097. [Google Scholar] [CrossRef]
- Chi, P.W.; Su, C.W.; Jhuo, B.H.; Wei, D.H. Photoirradiation Caused Controllable Wettability Switching of Sputtered Highly Aligned c-Axis-Oriented Zinc Oxide Columnar Films. Int. J. Photoenergy 2014, 2014, 765209. [Google Scholar] [CrossRef] [Green Version]
- Vayssieres, L. Growth of Arrayed Nanorods and Nanowires of ZnO from Aqueous Solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Tong, S.-K.; Chi, P.-W.; Kung, S.-H.; Wei, D.-H. Tuning bandgap and surface wettability of NiFe2O4 driven by phase transition. Sci. Rep. 2018, 8, 1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, S.-K.; Wei, D.-H. Ultraviolet induced switchable surface wetting behavior of NiFe2O4. Jpn. J. Appl. Phys. 2019, 58, SAAD08. [Google Scholar] [CrossRef]
- Tong, S.-K.; Chang, J.-H.; Hao, Y.-H.; Wu, M.-R.; Wei, D.-H.; Chueh, Y.-L. Optimum resistive switching characteristics of NiFe2O4 by controlling film thickness. Appl. Surf. Sci. 2021, 564, 150091. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Oparicheva, U.G.; Grishina, A.E.; Maevskaya, M.V.; Emeline, A.V.; Bahnemann, D.W. Dependences of ZnO Photoinduced Hydrophilic Conversion on Light Intensity and Wavelengths. J. Phys. Chem. C 2015, 119, 9824–9828. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Lv, Y.; Sun, Y.; Zhu, Y. Performance Enhancement of ZnO Photocatalyst via Synergic Effect of Surface Oxygen Defect and Graphene Hybridization. Langmuir 2013, 29, 3097–3105. [Google Scholar] [CrossRef]
- Choi, A.; Kim, K.; Jung, H.-I.; Lee, S.Y. ZnO nanowire biosensors for detection of biomolecular interactions in enhancement mode. Sens. Actuators B Chem. 2010, 148, 577–582. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Ding, H.; Xiong, H.-M. Folic acid functionalized ZnO quantum dots for targeted cancer cell imaging. Nanotechnology 2015, 26, 305702. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.M.; Ibraheem, I.J.; Obaid, A.S.; Bououdina, M. Nanostructured ZnO-based biosensor: DNA immobilization and hybridization. Sens. Bio-Sens. Res. 2017, 15, 46–52. [Google Scholar] [CrossRef]
- Tripathy, N.; Kim, D.-H. Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 2018, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, N.; Sharma, N.; Nerthigan, Y.; Wu, H.-F. Self-assembled diphenylalanine-zinc oxide hybrid nanostructures as a highly selective luminescent biosensor for trypsin detection. Appl. Surf. Sci. 2021, 554, 149600. [Google Scholar] [CrossRef]
- Tan, C.L.; Jang, S.J.; Lee, Y.T. Localized Surface Plasmon Resonance with Broadband Ultralow Reflectivity from Metal Nanoparticles on Glass and Silicon Subwavelength Structures. Opt. Express 2012, 20, 17448. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yang, H.I.; Choi, W. Moreover, the interface trap density between MoS2 and ALD Al2O3 is expected to increase after Al2O3 encapsulation. Appl. Phys. Lett. 2018, 113, 133104. [Google Scholar] [CrossRef]
- Giannini, V.; Fernández-Domínguez, A.I.; Heck, S.C.; Maier, S.A. Plasmonic Nanoantennas: Fundamentals and Their Use in Controlling the Radiative Properties of Nanoemitters. Chem. Rev. 2011, 111, 3888–3912. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-Enhanced Raman Scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Scott, J.F. UV Resonant Raman Scattering in ZnO. Phys. Rev. B 1970, 2, 1209–1211. [Google Scholar] [CrossRef]
- Alim, K.A.; Fonoberov, V.A.; Balandin, A.A. Origin of the optical phonon frequency shifts in ZnO quantum dots. Appl. Phys. Lett. 2005, 86, 053103. [Google Scholar] [CrossRef]
- Hong, D.-Y.; Kim, S.K.; Kwon, Y.-U. Synergistic Effects between Gold Nanoparticles and Nanostructured Platinum Film in Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2015, 119, 22611–22617. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-C.; Wei, D.-H. Controlling Surface Wettability and Plasmonic Resonance of Au/ZnO Heterostructured Films. J. Compos. Sci. 2022, 6, 328. https://doi.org/10.3390/jcs6110328
Chen S-C, Wei D-H. Controlling Surface Wettability and Plasmonic Resonance of Au/ZnO Heterostructured Films. Journal of Composites Science. 2022; 6(11):328. https://doi.org/10.3390/jcs6110328
Chicago/Turabian StyleChen, Sheng-Chiang, and Da-Hua Wei. 2022. "Controlling Surface Wettability and Plasmonic Resonance of Au/ZnO Heterostructured Films" Journal of Composites Science 6, no. 11: 328. https://doi.org/10.3390/jcs6110328