Fabrication of Sn(IV)porphyrin-Imbedded Silica Aerogel Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of SnP@SiA
2.2. Preparation of SiA
2.3. Preparation of TMS@SiA
2.4. Photocatalytic Degradation Experiment
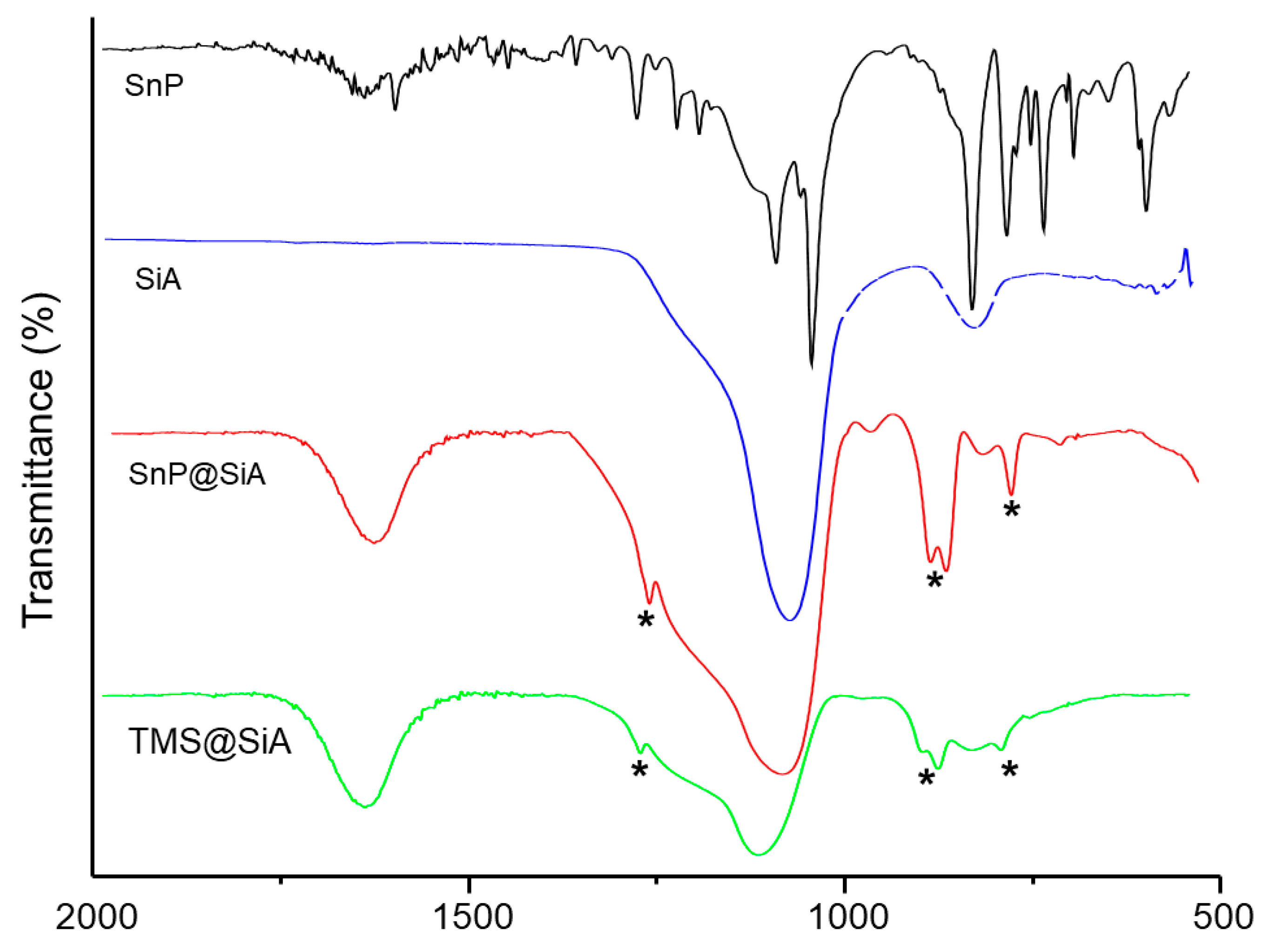
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4265. [Google Scholar] [CrossRef] [PubMed]
- Sonu, S.S.; Rai, N.; Chauhan, I. Multifunctional Aerogels: A comprehensive review on types, synthesis and applications of aerogels. J. Sol-Gel Sci. Technol. 2023, 105, 324–336. [Google Scholar]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef]
- Akhter, F.; Soomro, S.A.; Inglezakis, V.J. Silica aerogels; a review of synthesis, applications and fabrication of hybrid composites. J. Porous Mater. 2021, 28, 1387–1400. [Google Scholar] [CrossRef]
- Meti, P.; Wang, Q.; Mahadik, D.B.; Lee, K.-Y.; Gong, Y.-D.; Park, H.-H. Evolutionary Progress of Silica Aerogels and Their Classification Based on Composition: An Overview. Nanomaterials 2023, 13, 1498. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Ali, A.; Petrik, S.; Coufal, R.; Adach, K.; Fijalkowski, M. Aerogels for Biomedical, Energy and Sensing Applications. Gels 2021, 7, 264. [Google Scholar] [CrossRef]
- Merillas, B.; Vareda, J.P.; Martín-de León, J.; Rodríguez-Pérez, M.Á.; Durães, L. Thermal Conductivity of Nanoporous Materials: Where Is the Limit? Polymers 2022, 14, 2556. [Google Scholar] [CrossRef]
- Bandi, S.; Bell, M.; Schiraldi, D.A. Temperature-responsive clay aerogel—polymer composites. Macromolecules 2005, 38, 9216–9220. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Matyáš, J.; Qafoku, N.P.; Kruger, A.A. Silver-functionalized silica aerogels and their application in the removal of iodine from aqueous environments. J. Hazard. Mater. 2019, 379, 119364. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M.; Mahmood, A.; Kejzlar, P. Photocatalytic Behaviour of Zinc Oxide Nanostructures on Surface Activation of Polymeric Fibres. Polymers 2021, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.; Ahmad, A.; Khan, S.A.; Ahmad, A.; Abuzinadah, M.F.; Karim, S.; Jamal, Q.M.S. Biomedical applications of aerogel. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–48. [Google Scholar]
- Siddique, J.A.; Ansari, S.P.; Yadav, M. Carbon aerogel composites for gas sensing. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 49–73. [Google Scholar]
- Ansari, S.P.; Husain, A.; Shariq, M.U.; Ansari, M.O. Conducting polymer-based aerogels for energy and environmental remediation. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–86. [Google Scholar]
- Li, Z.; Zhao, S.; Koebel, M.K.; Malfait, W.J. Silica aerogels with tailored chemical functionality. Mater. Des. 2020, 193, 108833. [Google Scholar] [CrossRef]
- Hori, T.; Aratani, N.; Takagi, A.; Matsumoto, T.; Kawai, T.; Yoon, M.-C.; Yoon, Z.C.; Cho, S.; Kim, D.; Osuka, A. Giant Porphyrin Wheels with Large Electronic Coupling as Models of Light-Harvesting Photosynthetic Antenna. Chem. Eur. J. 2006, 12, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Drain, C.M.; Hupp, J.T.; Suslick, K.S.; Wasielewski, M.R.; Chen, X. A perspective on four new porphyrin-based functional materials and devices. J. Porphyr. Phthalocyanines 2002, 6, 243–258. [Google Scholar] [CrossRef]
- Milic, T.N.; Chi, N.; Yablon, D.G.; Flynn, G.W.; Batteas, J.D.; Drain, C.M. Controlled hierarchical self-assembly and deposition of nanoscale photonic materials. Angew. Chem. Int. Ed. 2002, 41, 2117–2119. [Google Scholar] [CrossRef]
- Suslick, K.S.; Rakow, N.A.; Kosal, M.E.; Chou, J.-H. The materials chemistry of porphyrins and metalloporphyrins. J. Porphyr. Phthalocyanines 2000, 4, 407–413. [Google Scholar] [CrossRef]
- Chou, J.-H.; Nalwa, H.S.; Kosal, M.E.; Rakow, N.A.; Suslick, K.S. Applications of Porphyrins and Metalloporphyrins to Materials Chemistry. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 6, pp. 43–131. [Google Scholar]
- Hasobe, T. Photo-and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef]
- Nikolaou, V.; Charalambidis, G.; Coutsolelos, A.G. Photocatalytic hydrogen production of porphyrin nanostructures: Spheres vs. fibrils, a case study. Chem. Commun. 2021, 57, 4055–4058. [Google Scholar] [CrossRef]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for Energy Conversion and Storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef]
- Farinone, M.; Urbańska, K.; Pawlicki, M. BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives. Molecules 2020, 25, 4523. [Google Scholar] [CrossRef]
- Grigore, M.E.; Ion, R.-M.; Iancu, L. Tailored porphyrin–gold nanoparticles for biomedical applications. J. Porphyr. Phthalocyanines 2019, 23, 766–780. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.-H.; Wang, L.-N. Porphyrin-Based Organophotocatalysts. In Phthalocyanines and Some Current Applications; Yilmaz, Y., Ed.; IntechOpen: London, UK, 2017; Chapter 6; pp. 119–151. [Google Scholar]
- Ribeiro, S.M.; Serra, A.C.; Gonsalves, A.M.A.R. Covalently immobilized porphyrins on silica modified structures as photooxidation catalysts. J. Mol. Catal. A 2010, 326, 121–127. [Google Scholar] [CrossRef]
- Bedioui, F. Zeolite-encapsulated and clay-intercalated metal porphyrin, phthalocyanine and Schiff-base complexes as models for biomimetic oxidation catalysts: An overview. Coord. Chem. Rev. 1995, 144, 39–68. [Google Scholar] [CrossRef]
- Skrobot, F.; Valente, A.; Neves, G.; Rosa, I.; Rocha, J.; Cavaleiro, J. Monoterpenes oxidation in the presence of Y zeolite-entrapped manganese (III) tetra (4-N-benzylpyridyl) porphyrin. J. Mol. Catal. A 2003, 201, 211–222. [Google Scholar] [CrossRef]
- Maldotti, A.; Molinari, A.; Andreotti, L.; Fogagnolo, M.; Amadelli, R. Novel reactivity of photoexcited iron porphyrins caged into a polyfluoro sulfonated membrane in catalytic hydrocarbon oxygenation. Chem. Commun. 1998, 507–508. [Google Scholar] [CrossRef]
- Baskaran, D.; Mays, J.W.; Zhang, X.P.; Bratcher, M.S. Carbon Nanotubes with Covalently Linked Porphyrin Antennae: Photoinduced Electron Transfer. J. Am. Chem. Soc. 2005, 127, 6916–6917. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Hong, W.; Zong, R.; Yao, W.; Zhu, Y. Visible light photoactivity enhancement via CuTCPP hybridized g-C3N4 nanocomposite. Appl. Catal. B 2015, 166, 366–373. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Douvas, A.M.; Georgiadou, D.G.; Constantoudis, V.; Davazoglou, D.; Kennou, S.; Palilia, L.C.; Daphnomili, D.; Coutsolelos, A.G.; Argitis, P. Large work function shift of organic semiconductors inducing enhanced interfacial electron transfer in organic optoelectronics enabled by porphyrin aggregated nanostructures. Nano Res. 2014, 7, 679–693. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Li, Y.; Li, Y. Aggregate Nanostructures of Organic Molecular Materials. Acc. Chem Res. 2010, 43, 1496–1508. [Google Scholar] [CrossRef]
- Lee, S.J.; Hupp, J.T.; Nguyen, S.T. Growth of Narrowly Dispersed Porphyrin Nanowires and Their Hierarchical Assembly into Macroscopic Columns. J. Am. Chem. Soc. 2008, 130, 9632–9633. [Google Scholar] [CrossRef]
- Yoon, S.M.; Hwang, I.C.; Kim, K.S.; Choi, H.C. Synthesis of single-crystal tetra (4-pyridyl) porphyrin rectangular nanotubes in the vapor phase. Angew. Chem. Int. Ed. 2009, 48, 2506–2509. [Google Scholar] [CrossRef]
- Lehn, J.-M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int. Ed. Engl. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef] [PubMed]
- Durot, S.; Taesch, J.; Heitz, V. Multiporphyrinic Cages: Architectures and Functions. Chem. Rev. 2014, 114, 8542–8578. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin Supramolecular 1D Structures via Surfactant-Assisted Self-Assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin Nanotubes by Ionic Self-Assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Feng, W.; Khan, A.F.; Nawaz, M.H. Sonication-induced self-assembly of polymeric porphyrin-fullerene: Formation of nanorings. J. Appl. Polym. Sci. 2016, 133, 43537. [Google Scholar] [CrossRef]
- Maeda, H.; Hasegawa, M.; Hashimoto, T.; Kakimoto, T.; Nishio, S.; Nakanishi, T. Nanoscale Spherical Architectures Fabricated by Metal Coordination of Multiple Dipyrrin Moieties. J. Am. Chem. Soc. 2006, 128, 10024–10025. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Milic, T.; Xu, C.; Batteas, J.D.; Drain, C.M. Preparation and Characterization of Porphyrin Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14290–14291. [Google Scholar] [CrossRef]
- Hasobe, T.; Oki, H.; Sandanayakaa, A.S.D.; Murata, H. Sonication-assisted supramolecular nanorods of meso-diaryl-substituted porphyrins. Chem. Commun. 2008, 724–726. [Google Scholar] [CrossRef]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 7261–7277. [Google Scholar] [CrossRef]
- Guo, P.; Chen, P.; Liu, M. Porphyrin Assemblies via a Surfactant-Assisted Method: From Nanospheres to Nanofibers with Tunable Length. Langmuir 2012, 28, 15482–15490. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, P.; Ma, W.; Liu, M. Morphology-dependent supramolecular photocatalytic performance of porphyrin nanoassemblies: From molecule to artificial supramolecular nanoantenna. J. Mater. Chem. 2012, 22, 20243–20249. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Y.; Wang, L.; Zhang, N.; Cao, R.; Bian, K.; Alarid, L.; Haddad, R.E.; Bai, F.; Fan, H. Morphology-Controlled Synthesis and Metalation of Porphyrin Nanoparticles with Enhanced Photocatalytic Performance. Nano Lett. 2016, 16, 6523–6528. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.K.M.; Bampos, N.; Clyde-Watson, Z.; Darling, S.L.; Hawley, J.C.; Kim, H.-J.; Mak, C.C.; Webb, S.J. Axial Coordination Chemistry of Metalloporphyrins. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 3, pp. 1–48. [Google Scholar]
- Arnold, D.P.; Blok, J. The coordination chemistry of tin porphyrin complexes. Coord. Chem. Rev. 2004, 248, 299–319. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Coordination framework materials fabricated by the self-assembly of Sn(IV) porphyrins with Ag(I) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular squares of Sn(IV) porphyrins with Re(I)-corners for the fabrication of self-assembled nanostructures performing photocatalytic degradation of Eriochrome Black T dye. Int. J. Mol. Sci. 2022, 23, 13702. [Google Scholar] [CrossRef]
- Lee, C.-J.; Shee, N.K.; Kim, H.-J. Fabrication and Properties of Sn(IV)Porphyrin-Linked Porous Organic Polymer for Environmental Applications. RSC Adv. 2023, 13, 24077–24085. [Google Scholar] [CrossRef]
- Shee, N.K.; Park, B.-H.; Kim, H.-J. Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes. Molecules 2023, 28, 1886. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.-J. Photocatalytic Hydrogen Production by the Sensitization of Sn(IV)-Porphyrin Embedded in a Nafion Matrix Coated on TiO2. Molecules 2022, 27, 3770. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Morphology-controlled self-assembled nanostructures of complementary metalloporphyrin triads through intermolecular coordination tuning and their photocatalytic degradation for Orange II. Inorg. Chem. Front. 2022, 9, 5538–5548. [Google Scholar] [CrossRef]
- Crossley, M.J.; Thordarson, P.R.; Wu, A.-S. Efficient formation of lipophilic dihydroxotin(IV) porphyrins and bis-porphyrins. J. Chem. Soc. Perkin Trans. 2001, 2294–2302. [Google Scholar] [CrossRef]
- Launer, P.J. Infrared Analysis of Organosilicon Compounds: Spectra-Structure Correlations. In Silicon Compounds: Silanes & Silicones; Gelest, Inc.: Morrisville, PA, USA, 2013. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, M.-G.; Kim, N.-G.; Kim, H.-J. Fabrication of Sn(IV)porphyrin-Imbedded Silica Aerogel Composite. J. Compos. Sci. 2023, 7, 401. https://doi.org/10.3390/jcs7090401
Jo M-G, Kim N-G, Kim H-J. Fabrication of Sn(IV)porphyrin-Imbedded Silica Aerogel Composite. Journal of Composites Science. 2023; 7(9):401. https://doi.org/10.3390/jcs7090401
Chicago/Turabian StyleJo, Min-Gyeong, Nam-Gil Kim, and Hee-Joon Kim. 2023. "Fabrication of Sn(IV)porphyrin-Imbedded Silica Aerogel Composite" Journal of Composites Science 7, no. 9: 401. https://doi.org/10.3390/jcs7090401





