Kaolin–Polyvinyl Alcohol–Potato Starch Composite Films for Environmentally Friendly Packaging: Optimization and Characterization
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Experimental Design
2.3. Preparation of Films
3. Characterizations
3.1. Thermal Properties
3.2. FTIR Analysis
3.3. Morphological Analysis
3.4. Mechanical Analysis
3.5. Water Absorption
3.6. Moisture Content
3.7. Soil Burial Degradability
4. Results and Discussion
4.1. Statistical Analysis
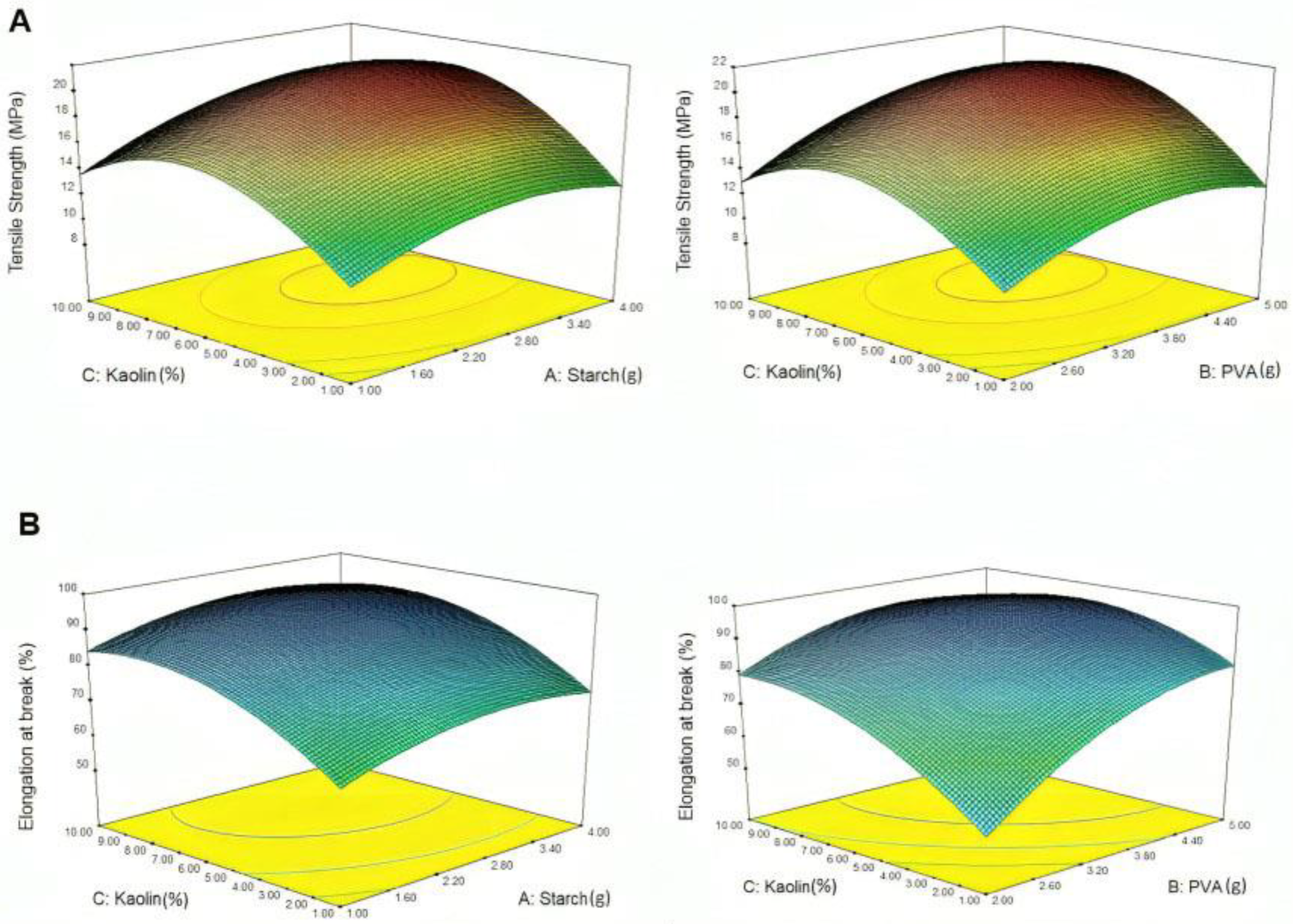
4.2. Effect on Mechanical Properties
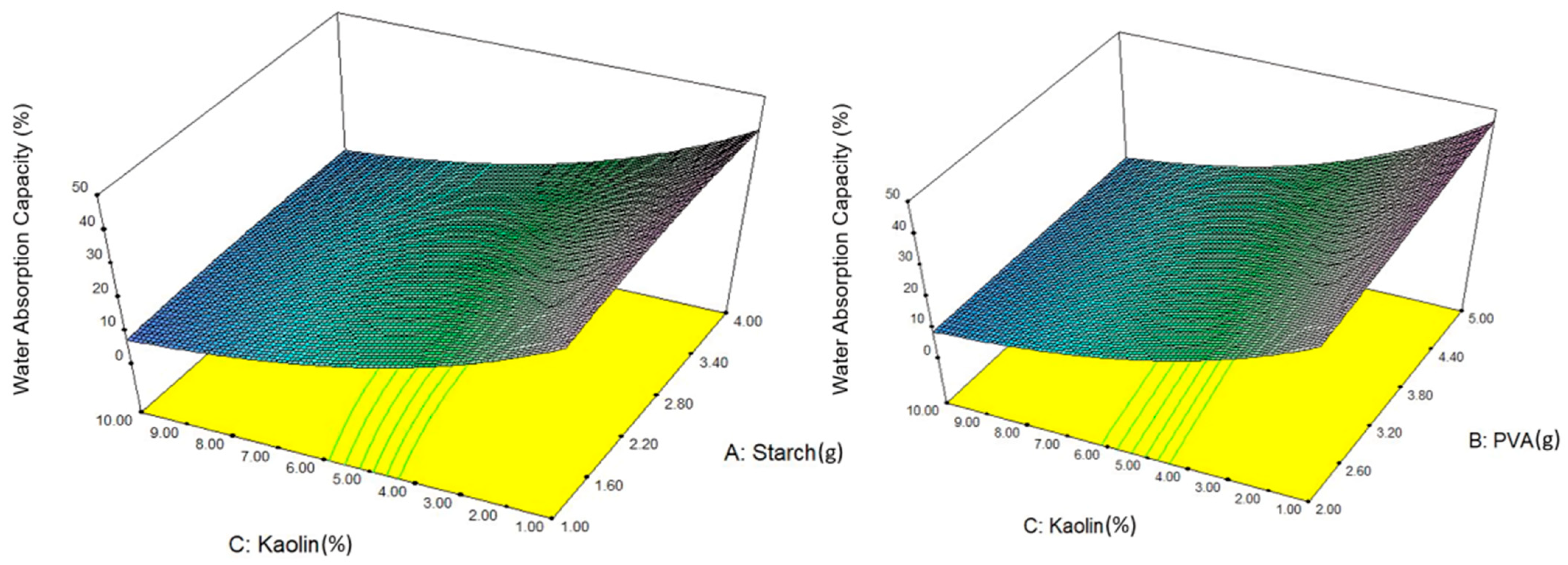
4.3. Effect on Water Absorption Capacity
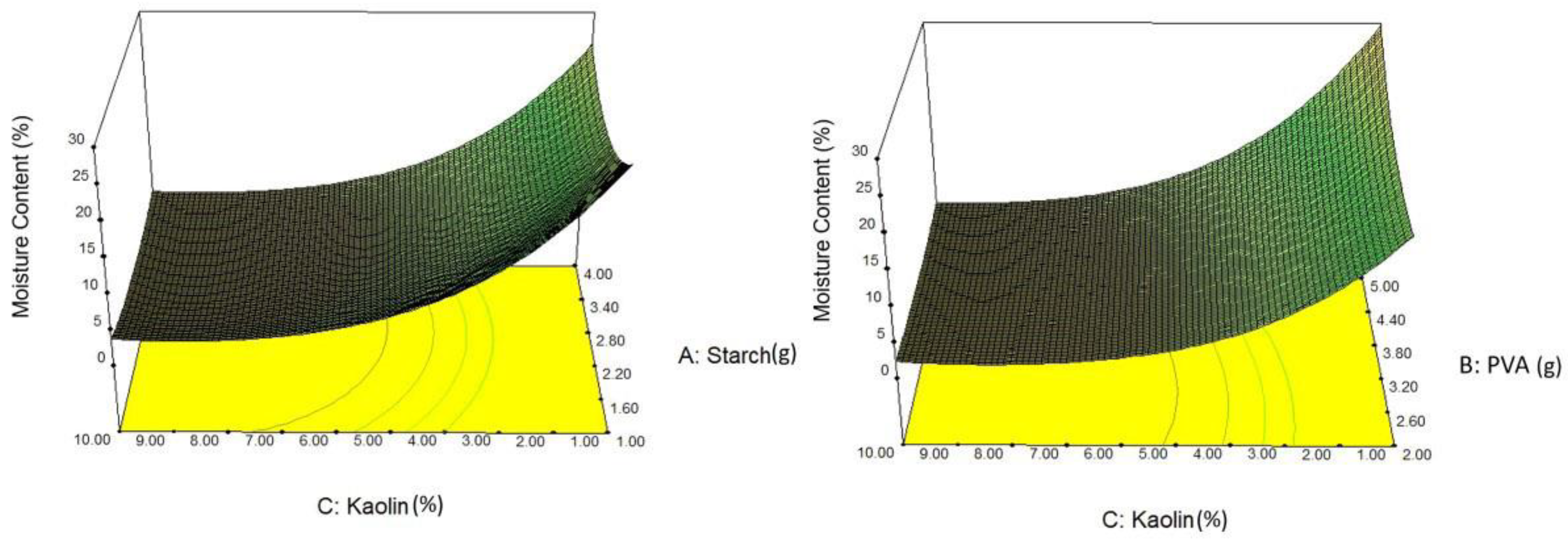
4.4. Effect on Moisture Content
4.5. Effect on Degradability
4.6. Optimization and Validation
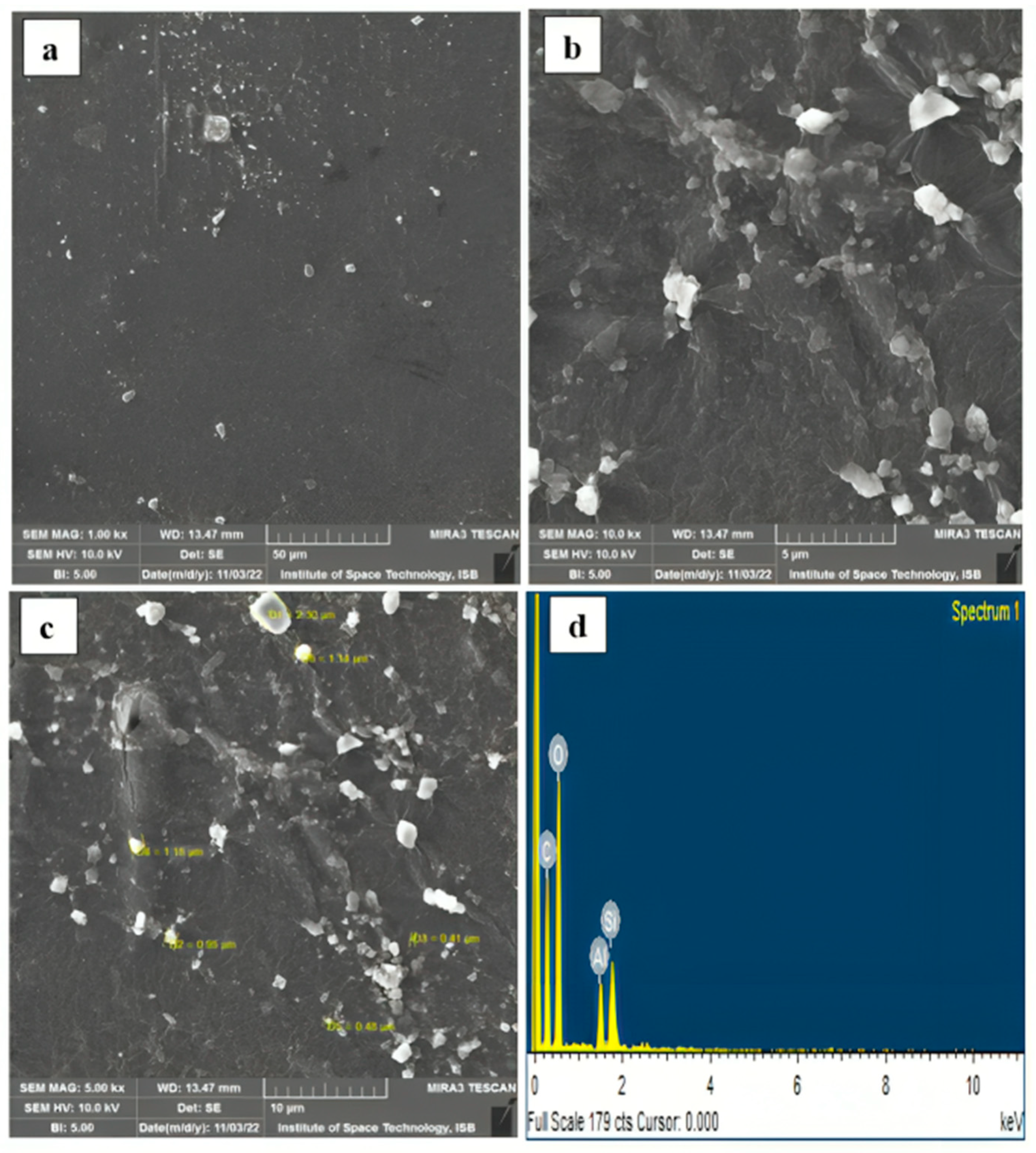
4.7. Structural and Morphological Analysis
5. Conclusions
- The mechanical analysis revealed that the incorporation of kaolin into the PS and PVA matrix has a significant impact. Obtaining the optimum concentration for all components is crucial for achieving a balance between TS and EAB. The optimal level was found to be 5.5 wt% of kaolin, 3.5 g of PVA, and 2.50 g of potato, resulting in good mechanical properties. If the concentrations of PVA and PS fluctuate in conjunction with kaolin, a significant difference in tensile strength (TS) and elongation at break (EAB) is observed.
- The fluctuating concentration of kaolin exhibited a trend of both increase and decrease in the water absorption capacity of the composite films. In this way, the maximum water absorption capacity was observed in Expt 14 of 51% with 0.0 wt% of kaolin, and Expt 2 exhibited a lower water absorption capacity of 6% with 13.07 wt% of kaolin. The absence of kaolin in the PVA/PS matrix demonstrated an improved water absorption capacity because both PVA and PS are hydrophilic in nature and their OH groups align with polymer chains. The higher concentration of kaolin in the second case increased its swelling properties, creating a barrier against water and making the film brittle in nature. This indicates the crucial role of the optimum value of kaolin in achieving a balanced and improved water absorption capacity.
- A similar trend in water absorption properties was observed in the properties of moisture content and degradability. A higher moisture content and degradability were observed in Expt 13 and Expt 16, while Expt 16 and Expt 6 showed lower moisture content and degradability, respectively.
- The FTIR results revealed Si-O bonds and Si-O-Al at 962 cm−1 and 858 cm−1, respectively, confirming the unique 1:1 composition of kaolin. Furthermore, the C=C stretching vibrations in the PVA and PS polymer matrix were observed at 1660 cm−1, representing polymerization reactions and reactivity within the matrix. A significant bond shift was observed when incorporating kaolin into the PVA–PS matrix, where the Si-O-Al bond peak replaced the Si-O-Si peak. This indicates the successful integration of kaolin into the composite, highlighting the coherence between its components.
- The SEM-EDX analysis performed on the obtained optimum composite film highlighted a uniform and smooth surface at 1 kx magnification, indicating strong interactions within the components of the matrix and kaolin. The EDX analysis confirmed the major elemental groups representing the proper formation of the composite, with clear percentages of carbon (35.50%), oxygen (49.57%), aluminum (6.38%), and silica (8.55%). The TGA analysis revealed the excellent thermal stability of the optimum composite film, as indicated by the maximum residual of 18.68%.
- An ANOVA and 3D graphs were employed to analyze the relationship between the parameters and responses. The coefficient of determination (R2) was utilized to assess the goodness of fit of the model to the data. Based on the model statistical analysis, all the models were significant at a p-value lower than 0.05, the F-statistic was not significant, Adj R2 values were close to Pred R2 values, and the CV% values were also below 10%. This suggests that there is a strong correlation between the process factors and the relevant responses, and all the models are reproducible.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, S. Major factors affecting the characteristics of starch-based biopolymer films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Aretoulaki, E.; Ponis, S.; Plakas, G.; Agalianos, K. A systematic meta-review analysis of review papers in the marine plastic pollution literature. Mar. Pollut. Bull. 2020, 161, 111690. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Hakkarainen, M.; Varma, I.K.; Albertsson, A.C. Degradable polyethylene: Fantasy or reality. Environ. Sci. Technol. 2021, 45, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- Chidambarampadmavathy, K.; Karthikeyan, O.P.; Heimann, K. Sustainable bioplastic production through landfill methane recycling. Renew. Sustain. Energy Rev. 2017, 71, 555–562. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Green, D.S.; Boots, B.; Blockley, D.J.; Rocha, C.; Thompson, R. Impacts of Discarded Plastic Bags on Marine Assemblages and Ecosystem Functioning. Environ. Sci. Technol. 2015, 49, 5380–5389. [Google Scholar] [CrossRef]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- Zhang, C.; Show, P.L.; Ho, S.H. Progress and perspective on algal plastics–a critical review. Bioresour. Technol. 2019, 289, 121700. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Bajpai, S. Thermal decomposition kinetics and properties of grafted barley husk reinforced PVA/starch composite films for packaging applications. Carbohydr. Polym. 2020, 240, 116225. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and water resistant poly (vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Farrag, Y.; Malmir, S.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Starch edible films loaded with donut-shaped starch microparticles. LWT 2018, 98, 62–68. [Google Scholar] [CrossRef]
- Ross, G.; Ross, S.; Tighe, B.J. Bioplastics: New routes, new products. Brydson’s Plast. Mater. 2017, 2017, 631–652. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahman WA, W.A.; Rahmat, A.R.; Khan, M.I. Detection of synergistic interactions of polyvinyl alcohol–cassava starch blends through DSC. Carbohydr. Polym. 2010, 79, 224–226. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. To study the effect of boric acid modification on starch–polyvinyl alcohol blend wood adhesive. J. Indian Acad. Wood Sci. 2018, 15, 190–198. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly (lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Jin, R.; Li, D. Preparation and characterization of nanocomposite films containing starch and cellulose nano-fibers. Ind. Crops Prod. 2018, 123, 654–660. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; López-Córdoba, A.; Avalos-Belmontes, F. Epoxidised sesame oil as a biobased coupling agent and plasticiser in polylactic acid/thermoplastic yam starch blends. Heliyon 2021, 7, e06176. [Google Scholar] [CrossRef]
- Souza, A.C.D.; Benze, R.F.E.S.; Ferrão, E.S.; Ditchfield, C.; Coelho, A.C.V.; Tadini, C.C. Cassava starch biodegradable films: Influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT-Food Sci. Technol. 2012, 46, 110–117. [Google Scholar] [CrossRef]
- Gierszewska, M.; Jakubowska, E.; Olewnik-Kruszkowska, E. Effect of chemical crosslinking on properties of chitosan-montmorillonite composites. Polym. Test. 2019, 77, 105872. [Google Scholar] [CrossRef]
- Montes, M.L.; Barraqué, F.; Bursztyn Fuentes, A.L.; Taylor, M.A.; Mercader, R.C.; Miehé-Brendlé, J.; Torres Sánchez, R.M. Effect of synthetic beidellite structural characteristics on the properties of beidellite/Fe oxides magnetic composites as Sr and Cs adsorbent materials. Mater. Chem. Phys. 2020, 245, 122760. [Google Scholar] [CrossRef]
- Gardolinski, J.E.; Carrera LC, M.; Cantao, M.P.; Wypych, F. Layered polymer-kaolinite nanocomposites. J. Mater. Sci. 2000, 35, 3113–3119. [Google Scholar] [CrossRef]
- Ma, J.; Jia, Y.; Jing, Y.; Sun, J.; Yao, Y. Synthesis and photocatalytic activity of TiO2-hectorite composites. Appl. Clay Sci. 2009, 46, 114–116. [Google Scholar] [CrossRef]
- Mbey, J.A.; Hoppe, S.; Thomas, F. Cassava starch–kaolinite composite film. Effect of clay content and clay modification on film properties. Carbohydr. Polym. 2012, 88, 213–222. [Google Scholar] [CrossRef]
- Behnezhad, M.; Goodarzi, M.; Baniasadi, H. Fabrication and characterization of polyvinyl alcohol/carboxymethyl cellulose/titanium dioxide degradable composite films: An RSM study. Mater. Res. Express 2020, 6, 125548. [Google Scholar] [CrossRef]
- Yari, S.; Mohammadi-Rovshandeh, J.; Shahrousvand, M. Preparation and optimization of Starch/Poly vinyl alcohol/ZnO nanocomposite films applicable for food packaging. J. Polym. Environ. 2022, 30, 1502–1517. [Google Scholar] [CrossRef]
- Arikan, E.B.; Bilgen, H.D. Production of bioplastic from potato peel waste and investigation of its biodegradability. Int. Adv. Res. Eng. J. 2019, 3, 93–97. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Bajpai, S. Fabrication and characteristics of poly (vinyl alcohol)-starch-cellulosic material based biodegradable composite film for packaging application. Mater. Today Proc. 2020, 21, 1577–1582. [Google Scholar] [CrossRef]
- Admase, A.T.; Sendekie, Z.B.; Alene, A.N. Biodegradable Film from Mango Seed Kernel Starch Using Pottery Clay as Filler. J. Polym. Environ. 2022, 30, 3431–3446. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of glutaraldehyde on thermal and mechanical properties of starch and polyvinyl alcohol blends. Des. Monomers Polym. 2019, 22, 164–170. [Google Scholar] [CrossRef]
- Shen, X.L.; Wu, J.M.; Chen, Y.; Zhao, G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Ge, X.; Chang, M.; Jiang, W.; Zhang, B.; Xing, R.; Bulin, C. Selective location of kaolin and effects of maleic anhydride in kaolin/poly (ε-caprolactone)/poly (lactic acid) composites. Appl. Clay Sci. 2020, 189, 105524. [Google Scholar] [CrossRef]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Zhang, Y.; Lu, P. Novel active starch films incorporating tea polyphenols-loaded porous starch as food packaging materials. Int. J. Biol. Macromol. 2021, 192, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Mollick MM, R.; Bhowmick, B.; Maity, D.; Bain, M.K.; Rana, D.; Chattopadhyay, D. Effect of poly (vinyl pyrrolidone) on the morphology and physical properties of poly (vinyl alcohol)/sodium montmorillonite nanocomposite films. Prog. Nat. Sci. Mater. Int. 2013, 23, 579–587. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Lutfi, Z.; Hasnain, A. Mango kernel starch-gum composite films: Physical, mechanical and barrier properties. Int. J. Biol. Macromol. 2017, 98, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Chowdhury, M.A.; Kowser, M.A. Characterization and performance analysis of composite bioplastics synthesized using titanium dioxide nanoparticles with corn starch. Heliyon 2019, 5, e02009. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, Z.; Li, J.; Li, X.; Kan, Y.; Gao, Z. Effect of kaolin content on the performances of kaolin-hybridized soybean meal-based adhesives for wood composites. Compos. Part B Eng. 2019, 173, 106919. [Google Scholar] [CrossRef]
- Fahma, F.; Sunarti, T.C.; Indriyani, S.M.; Lisdayana, N. Thermoplastic cassava starch-PVA composite films with cellulose nanofibers from oil palm empty fruit bunches as reinforcement agent. Int. J. Polym. Sci. 2017, 2017, 2745721. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S. Physico-mechanical and microstructural properties of semolina flour films as influenced by different sorbitol/glycerol concentrations. Int. J. Food Prop. 2018, 21, 983–995. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, P.; Li, Y.; Wang, S. Durable thermal fluid super-repellency of elastic fluorine-modified SiO2@sponge composite aerogel. Chem. Eng. J. 2023, 454, 140247. [Google Scholar] [CrossRef]
- Shanmathy, M.; Mohanta, M.; Thirugnanam, A. Development of biodegradable bioplastic films from Taro starch reinforced with bentonite. Carbohydr. Polym. Technol. Appl. 2021, 2, 100173. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Tanrattanakul, V.; Phetrat, W. Preparation and characterization of kaolin/starch foam. Appl. Clay Sci. 2013, 80, 413–416. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E.K. Poly (vinyl alcohol): Montmorillonite: Boiled rice water (starch) blend film reinforced with silver nanoparticles; characterization and antibacterial properties. Appl. Clay Sci. 2018, 161, 464–473. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef]
- Behera, L.; Mohanta, M.; Thirugnanam, A. Intensification of yam-starch based biodegradable bioplastic film with bentonite for food packaging application. Environ. Technol. Innov. 2022, 25, 102180. [Google Scholar] [CrossRef]
- Shafqat, A.; Al-Zaqri, N.; Tahir, A.; Alsalme, A. Synthesis and characterization of starch based bioplatics using varying plant-based ingredients, plasticizers and natural fillers. Saudi J. Biol. Sci. 2021, 28, 1739–1749. [Google Scholar] [CrossRef]
- Suderman, N.; Sarbon, N.M. Optimization of chicken skin gelatin film production with different glycerol concentrations by response surface methodology (RSM) approach. J. Food Sci. Technol. 2020, 57, 463–472. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Response surface methodology (RSM) of chicken skin gelatin based composite films with rice starch and curcumin incorporation. Polym. Test. 2020, 81, 106161. [Google Scholar] [CrossRef]
- Thakur, R.; Saberi, B.; Pristijono, P.; Stathopoulos, C.E.; Golding, J.B.; Scarlett, C.J.; Vuong, Q.V. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J. Food Sci. Technol. 2017, 54, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Hassani, F.; Nafchi, A.M. Preparation and characterization of bionanocomposite films based on potato starch/halloysite nanoclay. Int. J. Biol. Macromol. 2014, 67, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Rahman, M.A.; Islam, J.M.; Khan, M.A.; Saadat, A.H.M. Preparation and characterization of starch/PVA blend for biodegradable packaging material. Adv. Mater. Res. 2010, 123, 351–354. [Google Scholar] [CrossRef]
- Rammak, T.; Boonsuk, P.; Kaewtatip, K. Mechanical and barrier properties of starch blend films enhanced with kaolin for application in food packaging. Int. J. Biol. Macromol. 2021, 192, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking reaction of poly (vinyl alcohol) with glyoxal. J. Polym. Res. 2010, 17, 725–730. [Google Scholar] [CrossRef]
- Sharma, M.; Beniwal, P.; Toor, A.P. The effect of rice straw derived microfibrillated cellulose as a reinforcing agent in starch/polyvinyl alcohol/polyethylene glycol biocompatible films. Mater. Chem. Phys. 2022, 291, 126652. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Zhang, L.; Cao, W.; Wang, H.; Chen, G.; Wang, S. Preparation and properties of biodegradable films made of cationic potato-peel starch and loaded with curcumin. Food Hydrocoll. 2022, 130, 107690. [Google Scholar] [CrossRef]
- Heydari, A.; Alemzadeh, I.; Vossoughi, M. Functional properties of biodegradable corn starch nanocomposites for food packaging applications. Mater. Des. 2013, 50, 954–961. [Google Scholar] [CrossRef]
- Rahman WA, W.A.; Sin, L.T.; Rahmat, A.R.; Samad, A.A. Thermal behaviour and interactions of cassava starch filled with glycerol plasticized polyvinyl alcohol blends. Carbohydr. Polym. 2010, 81, 805–810. [Google Scholar] [CrossRef]
- Nandi, S.; Guha, P. Modelling the effect of guar gum on physical, optical, barrier and mechanical properties of potato starch based composite film. Carbohydr. Polym. 2018, 200, 498–507. [Google Scholar] [CrossRef]
- Sayyahi, Z.; Beigmohammadi, F.; Shoaiee, S. Optimization of starch biopolymer enriched with chitosan containing rosemary essential oil and its application in packaging of peanuts. Nutr. Food Sci. Res. 2017, 4, 19–28. [Google Scholar] [CrossRef]





| Symbols | Independent Variables | Minimum Level | Mean | Maximum Level |
|---|---|---|---|---|
| A | Starch (g) | 0 | 2.50 | 5.02 |
| B | PVA (g) | 0.98 | 3.50 | 6.02 |
| C | Kaolin (%) | 0 | 5.62 | 13.07 |
| Parameters | Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Std | Expt | Starch (g) | PVA (g) | Kaolin (%) | Tensile Strength (Mpa) | Elongation (%) | Water Absorption (%) | Moisture Content (%) | Degradability (%) |
| 7 | 1 | 1.00 | 5.00 | 10.00 | 15 | 84 | 8 | 5 | 34 |
| 14 | 2 | 2.50 | 3.50 | 13.07 | 10 | 79 | 6 | 4 | 30 |
| 6 | 3 | 4.00 | 2.00 | 10.00 | 14 | 80 | 9 | 4 | 29 |
| 10 | 4 | 5.02 | 3.50 | 5.50 | 17 | 84 | 14 | 6 | 26 |
| 1 | 5 | 1.00 | 2.00 | 1.00 | 7 | 38 | 32 | 20 | 25 |
| 2 | 6 | 4.00 | 2.00 | 1.00 | 9 | 50 | 38 | 28 | 16 |
| 16 | 7 | 2.50 | 3.50 | 5.50 | 20 | 89 | 15 | 3 | 39 |
| 5 | 8 | 1.00 | 2.00 | 10.00 | 10 | 75 | 10 | 5 | 29 |
| 12 | 9 | 2.50 | 6.02 | 5.50 | 16 | 89 | 20 | 8 | 36 |
| 11 | 10 | 2.50 | 0.98 | 5.50 | 9.9 | 50 | 14 | 6 | 29 |
| 4 | 11 | 4.00 | 5.00 | 1.00 | 11 | 76 | 45 | 32 | 18 |
| 15 | 12 | 2.50 | 3.50 | 5.50 | 19 | 88 | 18 | 4 | 38 |
| 3 | 13 | 1.00 | 5.00 | 1.00 | 9.8 | 74 | 44 | 40 | 17 |
| 13 | 14 | 2.50 | 3.50 | 0.00 | 13 | 75 | 51 | 33 | 19 |
| 9 | 15 | 0.00 | 3.50 | 5.50 | 10.8 | 70 | 12 | 15 | 34 |
| 17 | 16 | 2.50 | 3.50 | 5.50 | 20.5 | 94 | 22 | 3 | 45 |
| 8 | 17 | 4.00 | 5.00 | 10.00 | 17 | 90 | 13 | 5 | 40 |
| TS(MPa) | E (%) | WA (%) | MC (%) | D (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value |
| Model | 17.27 | 0.0005 | 17.19 | 0.0006 | 24.18 | 0.0002 | 24.99 | 0.0002 | 23.88 | 0.0002 |
| A | 17.76 | 0.0040 | 6.86 | 0.0345 | 1.32 | 0.0482 | 2.60 | 0.1511 | 3.77 | 0.0934 |
| B | 24.14 | 0.0015 | 63.42 | <0.0001 | 4.29 | 0.0772 | 3.92 | 0.0481 | 4.76 | 0.0454 |
| C | 25.48 | 0.0015 | 39.94 | 0.0004 | 207.35 | <0.0001 | 185.99 | <0.0001 | 118.39 | <0.0001 |
| AB | 0.96 | 0.0403 | 0.74 | 0.4178 | 0.21 | 0.0437 | 1.10 | 0.0298 | 6.64 | 0.0366 |
| AC | 0.32 | 0.0472 | 0.23 | 0.0487 | 8.383 | 0.037 | 0.11 | 0.0450 | 4.82 | 0.042 |
| BC | 0.33 | 0.5851 | 12.04 | 0.0104 | 1.25 | 0.0005 | 2.75 | 0.0411 | 10.41 | 0.0145 |
| A2 | 26.09 | 0.0014 | 9.99 | 0.0159 | 2.20 | 0.1817 | 15.68 | 0.0055 | 25.54 | 0.0015 |
| B2 | 25.81 | 0.0006 | 26.65 | 0.0013 | 4.458 | 0.0486 | 6.05 | 0.0434 | 14.40 | 0.0068 |
| C2 | 77.53 | <0.0001 | 19.86 | 0.0029 | 12.78 | 0.0090 | 58.13 | 0.0001 | 93.41 | <0.0001 |
| TS (MPa) | E (%) | WA (%) | MC (%) | D (%) | |
|---|---|---|---|---|---|
| R2 | 0.9569 | 0.9567 | 0.9688 | 0.9698 | 0.9685 |
| Adj R2 | 0.9015 | 0.9010 | 0.9288 | 0.9310 | 0.9279 |
| Pred R2 | 0.7187 | 0.7260 | 0.7830 | 0.7363 | 0.8252 |
| Adequate Precision | 13.384 | 13.588 | 14.038 | 14.086 | 14.279 |
| C.V% | 4.95 | 4.24 | 8.88 | 4.99 | 4.11 |
| Samples | Functional Groups | Peaks Observed (cm−1) | References |
|---|---|---|---|
| Kaolin | O-H | 3587 | [45,46,47,48] |
| O-H | 1654 | ||
| Si-O | 962 | ||
| Si-O | 804 | ||
| Al-O | 848 | ||
| Si-O-Al | 671 | ||
| PVA/PS | O-H | 3431 | [25,32,47,49] |
| C=O | 2343 | ||
| C-O | 2125 | ||
| C=C | 1660 | ||
| O-H | 1259 | ||
| C-O-C | 1010 | ||
| C-H | 736 | ||
| Composite | O-H | 3469 | [46,47,48,49,50,51,52] |
| C=O | 2538 | ||
| Si-O | 2100 | ||
| C=C | 1888 | ||
| H-O-H | 1650 | ||
| C-O | 1265 | ||
| Si-O-Si | 1018 |
| Sample | 1st Peak (°C) | 2nd Peak (°C) | 3rd Peak (°C) | Residue Left (%) |
|---|---|---|---|---|
| Kaolin | 110 | 250 | 450 | 9.9 |
| PVA/PS | 150 | 268 | 475 | 5.5 |
| Composite | 180 | 275 | 499 | 18.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabassum, N.; Rafique, U.; Qayyum, M.; Mohammed, A.A.A.; Asif, S.; Bokhari, A. Kaolin–Polyvinyl Alcohol–Potato Starch Composite Films for Environmentally Friendly Packaging: Optimization and Characterization. J. Compos. Sci. 2024, 8, 29. https://doi.org/10.3390/jcs8010029
Tabassum N, Rafique U, Qayyum M, Mohammed AAA, Asif S, Bokhari A. Kaolin–Polyvinyl Alcohol–Potato Starch Composite Films for Environmentally Friendly Packaging: Optimization and Characterization. Journal of Composites Science. 2024; 8(1):29. https://doi.org/10.3390/jcs8010029
Chicago/Turabian StyleTabassum, Noshabah, Uzaira Rafique, Maria Qayyum, Abdallah A. A. Mohammed, Saira Asif, and Awais Bokhari. 2024. "Kaolin–Polyvinyl Alcohol–Potato Starch Composite Films for Environmentally Friendly Packaging: Optimization and Characterization" Journal of Composites Science 8, no. 1: 29. https://doi.org/10.3390/jcs8010029





