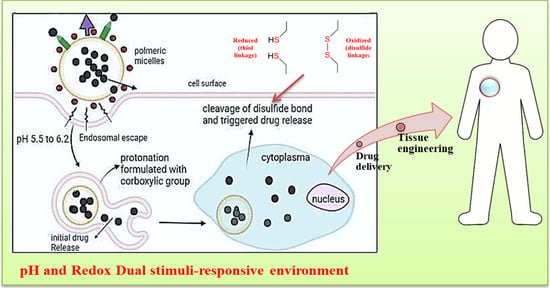
Recent Advances in pH and Redox Responsive Polymer Nanocomposites for Cancer Therapy
Abstract
:1. Introduction
1.1. Polymeric Nanomaterials
- (1)
- Remain stable in blood until they reach the TME.
- (2)
- Improve hydrophilic properties and delay recognition in the immune system, allowing it to enhance targets of desired cells/tissues after the reticuloendothelial system (RES) and mononuclear phagocyte system (MPS) surface activity.
- (3)
- These are gathered in the TMS while allowing them to pass through an irregular vasculature tumor condition.
- (4)
- Respond to stimuli-controlled drug release of loaded therapeutic contents.
- (5)
- The ability to modify surface functionalization.
- (6)
- Tumor interstitial fluid penetration occurs in the TMS.
- (7)
1.2. Biopolymer-Coated Nanocomposites
2. Role of Tumor Microenvironment
2.1. Tumor Redox Microenvironment
2.2. Tumor pH Microenvironment
2.3. pH–Redox Tumor Microenvironment
3. Design Principles and Fabrication Methods
3.1. Design Principles of pH- and Redox-Responsive Polymer Nanocomposites
3.2. Fabrication Methods
4. Biocompatibility and Safety
5. Future Perspectives and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puyol, M.; Seoane, J.; Aguilar, E.; Vozza, L.B.; Orbe, I.; Crawford, K.H.; Fernández, A.; Bray, F.; Johnson, S.E.; Gopal, S. WORLD CANCER RESEARCH DAY: A Call to Action for a Coordinated International Research Effort to Prevent, Diagnose, and Treat Cancer. Clin. Cancer Res. 2021, 27, 963–966. [Google Scholar] [CrossRef]
- Smith, L.; Stiller, C.A.; Aitken, J.F.; Hjalgrim, L.L.; Johannesen, T.; Lahteenmaki, P.; McCabe, M.G.; Phillips, R.; Pritchard-Jones, K.; Steliarova-Foucher, E.; et al. International variation in childhood cancer mortality rates from 2001 to 2015: Comparison of trends in the International Cancer Benchmarking Partnership countries. Int. J. Cancer 2021, 150, 28–37. [Google Scholar] [CrossRef]
- Parvanian, S.; Mostafavi, S.M.; Aghashiri, M. Multifunctional nanoparticle developments in cancer diagnosis and treatment. Sens. Bio-Sens. Res. 2017, 13, 81–87. [Google Scholar] [CrossRef]
- ReFaey, K.; Tripathi, S.; Grewal, S.S.; Bhargav, A.G.; Quinones, D.J.; Chaichana, K.L.; Antwi, S.O.; Cooper, L.T.; Meyer, F.B.; Dronca, R.S.; et al. Cancer Mortality Rates Increasing vs Cardiovascular Disease Mortality Decreasing in the World: Future Implications. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Moodley, T.; Singh, M. Current Stimuli-Responsive Mesoporous Silica Nanoparticles for Cancer. Pharmaceutics 2021, 13, 71. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2021, 11, 632079. [Google Scholar] [CrossRef]
- Li, W.Q.; Guo, H.F.; Li, L.Y.; Zhang, Y.F.; Cui, J.W. The promising role of antibody drug conjugate in cancer therapy: Combining targeting ability with cytotoxicity effectively. Cancer Med. 2021, 10, 4677–4696. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Jang, M.S.; Gao, G.H.; Lee, J.H.; Lee, D.S. Construction of redox/pH dual stimuli-responsive PEGylated polymeric micelles for intracellular doxorubicin delivery in liver cancer. Polym. Chem. 2016, 7, 1813–1825. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Al-Hibs, A.S.; Alzhrani, R.; Alrabighi, K.K.; Alqathama, A.; Alwithenani, A.; Almalki, A.H.; Althobaiti, Y.S. Nanomaterials for Antiangiogenic Therapies for Cancer: A Promising Tool for Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 1631. [Google Scholar] [CrossRef]
- Das Kurmi, B.; Patel, P.; Paliwal, R.; Paliwal, S.R. Molecular approaches for targeted drug delivery towards cancer: A concise review with respect to nanotechnology. J. Drug Deliv. Sci. Technol. 2020, 57, 101682. [Google Scholar] [CrossRef]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Solanki, R.; Rostamabadi, H.; Patel, S.; Jafari, S.M. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: A critical review. Int. J. Biol. Macromol. 2021, 193, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Lai, C.-H.; Peng, S.-L.; Hsu, C.-Y.; Hsu, P.-H.; Chu, P.-Y.; Feng, C.-L.; Lin, Y.-H. Targeting Tumor Cells with Nanoparticles for Enhanced Co-Drug Delivery in Cancer Treatment. Pharmaceutics 2021, 13, 1327. [Google Scholar] [CrossRef]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Stimuli-Responsive Polym. Nanoplatforms 2021, 9, 707319. [Google Scholar] [CrossRef] [PubMed]
- Shivalingayya; Preeti, R.K.; Ganiger, S.K.; Shashidhar, A.C.; Lagashetty, A. Multifunctional Nanoparticles for Biomedical Applications. J. Chem. Biol. Phys. Sci. 2022, 12, 410–422. [Google Scholar]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Rahman, N.A.; Liou, N.-S. Recent advances in celluloses and their hybrids for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2020, 158, 670–688. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Z.; Liu, B.; He, F.; Gai, S.; Yang, P.; Yang, D.; Li, C.; Lin, J. Recent advances on endogenous/exogenous stimuli-triggered nanoplatforms for enhanced chemodynamic therapy. Coord. Chem. Rev. 2021, 451, 214267. [Google Scholar] [CrossRef]
- Abdo, G.G.; Zagho, M.M.; Khalil, A. Recent advances in stimuli-responsive drug release and targeting concepts using mesoporous silica nanoparticles. Emergent Mater. 2020, 3, 407–425. [Google Scholar] [CrossRef]
- Chivere, V.T.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Nanotechnology-Based Biopolymeric Oral Delivery Platforms for Advanced Cancer Treatment. Cancers 2020, 12, 522. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Aflori, M. Smart Nanomaterials for Biomedical Applications—A Review. Nanomaterials 2021, 11, 396. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Cui, J.; Hao, J. Dual-Stimuli-Responsive Polypeptide Nanoparticles for Photothermal and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 3, 561–569. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer. Arch. Med. Res. 2021, 52, 131–142. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Singh, A.; Talekar, M.; Tran, T.-H.; Samanta, A.; Sundaram, R.; Amiji, M. Combinatorial Approach in the Design of Multifunctional Polymeric Nano-Delivery Systems for Cancer Therapy. J. Mater. Chem. B 2014, 2, 8069–8084. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Raposo, L.R.; Valente, R.; Fernandes, A.R.; Baptista, P.V. Combined cancer therapeutics—Tackling the complexity of the tumor microenvironment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1704. [Google Scholar] [CrossRef]
- Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; Santini, A.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar]
- Katifelis, H.; Gazouli, M. Cancer-Targeted Nanotheranostics: Recent Advances and Future Perspectives. Cancer Nanotheranostics 2021, 2, 97–115. [Google Scholar]
- Luo, C.; Sun, J.; Sun, B.; He, Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol. Sci. 2014, 35, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chen, X. Polymeric Nanostructured Materials for Biomedical Applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef]
- Tyrrell, Z.L.; Shen, Y.; Radosz, M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; An, J.; Son, S.-R.; Kim, S.; Park, J.; Park, C.B.; Lee, J.H. Rational design of surface-confined nanostructured self-assemblies based on functional comb-shaped copolymers for tunable molecular orientation. React. Funct. Polym. 2021, 168, 105042. [Google Scholar] [CrossRef]
- Bae, Y.; Kataoka, K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 2009, 61, 768–784. [Google Scholar] [CrossRef]
- Lallana, E.; Sousa-Herves, A.; Fernandez-Trillo, F.; Riguera, R.; Fernandez-Megia, E. Click chemistry for drug delivery nanosystems. Pharm. Res. 2012, 29, 1–34. [Google Scholar] [CrossRef]
- Deng, B.; Ma, P.; Xie, Y. Reduction-Sensitive Polymeric Nanocarriers in Cancer Therapy: A Comprehensive Review. Nanoscale 2015, 7, 12773–12795. [Google Scholar] [CrossRef]
- Date, T.; Nimbalkar, V.; Kamat, J.; Mittal, A.; Mahato, R.I.; Chitkara, D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J. Control. Release 2017, 271, 60–73. [Google Scholar] [CrossRef]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted Delivery of Drugs and Genes Using Polymer Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Lv, S.; Sylvestre, M.; Prossnitz, A.N.; Yang, L.F.; Pun, S.H. Design of Polymeric Carriers for Intracellular Peptide Delivery in Oncology Applications. Chem. Rev. 2021, 121, 11653–11698. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Feldman, D. Review Polymers and Polymer Nanocomposites for Cancer Therapy. Appl. Sci. 2019, 9, 3899. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol. Symp. 2018, 380, 1800114. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Zahraee, Z. Controlled release of metformin from chitosan-based nanocomposite films containing mesoporous MCM-41 nanoparticles as novel drug delivery systems. J. Colloid Interface Sci. 2017, 501, 60–76. [Google Scholar] [CrossRef]
- Luo, H.; Ao, H.; Li, G.; Li, W.; Xiong, G.; Zhu, Y.; Wan, Y. Bacterial cellulose/graphene oxide nanocomposite as a novel drug delivery system. Appl. Phys. 2017, 17, 249. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.; Kim, J.H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar] [CrossRef]
- Yang, H.Y.; Li, Y.; Lee, D.S. Multifunctional and Stimuli-Responsive Magnetic Nanoparticle-Based Delivery Systems for Biomedical Applications. Adv. Therap. 2018, 1, 1800011. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.L.; Li, J.; Wei, R.; Lin, H.; Xiong, L.X. Internal and external triggering mechanism of “smart” nanoparticle-based DDSs in targeted tumor therapy. Curr. Pharm. Des. 2018, 24, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Intracellular drug release nanosystems. Mater. Today 2012, 15, 436–442. [Google Scholar] [CrossRef]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-Responsive Nanoparticles for Targeting the Tumor Microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef]
- Van Vlerken, L.E.; Amiji, M.M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006, 3, 205–216. [Google Scholar] [CrossRef]
- Vaupel, P. Tumor Microenvironmental Physiology and Its Implications for Radiation Oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Note, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of the iLiNP Device: Fine Tuning the Lipid Nanoparticle Size within 10 nm for Drug Delivery. ACS Omega 2018, 3, 5044–5051. [Google Scholar] [CrossRef]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 57–64. [Google Scholar] [CrossRef]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.P.; Luddy, K.A.; Harmon, C.; O’farrelly, C. Hepatic tumor microenvironments and effects on NK cell phenotype and function. Int. J. Mol. Sci. 2019, 20, 4131. [Google Scholar] [CrossRef]
- Gu, F.X.; Karnik, R.; Wang, A.Z.; Alexis, F.; Levy-Nissenbaum, E.; Hong, S.; Langer, R.S.; Farokhzad, O.C. Targeted nanoparticles for cancer therapy. Nano Today 2007, 2, 14–21. [Google Scholar] [CrossRef]
- Lan, Q.; Xia, S.; Wang, Q.; Xu, W.; Huang, H.; Jiang, S.; Lu, L. Development of oncolytic virotherapy: From genetic modification to combination therapy. Front. Med. 2020, 14, 160–184. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent advances in the redox-responsive drug delivery nanoplatforms: A chemical structure and physical property perspective. Mater. Sci. Eng. C 2021, 118, 111536. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, L.; Breunig, M. Preventing obstructions of nanosized drug delivery systems by the extracellular matrix. Adv. Healthc. Mater. 2018, 7, 1700739. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Liu, M.; Wang, K.; Tao, L.; Wan, Q.; Wei, Y. Recent progress and advances in redox-responsive polymers as controlled delivery nanoplatforms. Mater. Chem. Front. 2017, 1, 807–822. [Google Scholar] [CrossRef]
- Chibh, S.; Kour, A.; Yadav, N.; Kumar, P.; Yadav, P.; Chauhan, V.S.; Panda, J.J. Redox-Responsive Dipeptide Nanostructures toward Targeted Cancer Therapy. ACS Omega 2020, 5, 3365–3375. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Chen, D.; Li, Y.; Wu, S.; Xu, C.; Su, L.; Zhang, Q. Tumor redox microenvironment modulating composite hydrogels for enhanced sonodynamic therapy of colorectal cancer. J. Mater. Chem. B 2022, 10, 1960–1968. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Du, X.; Wang, T. Redox-responsive micelles for triggered drug delivery and effective laryngopharyngeal cancer therapy. Int. J. Biol. Macromol. 2018, 112, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-J.; Chen, Y.-Y.; Wang, D.-R.; Wei, C.; Guo, J.; Lu, D.-R.; Chu, C.-C.; Wang, C.-C. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 2012, 33, 6570–6579. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Celebioglu, A.; Aytac, Z.; Uyar, T. pH-responsive nanofibers with controlled drug release properties. Polym. Chem. 2014, 5, 2050–2056. [Google Scholar] [CrossRef]
- Ratemi, E. pH-responsive polymers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 121–141. [Google Scholar]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 18009107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Kabanov, A.V.; Bronich, T.K. Polymer Micelles with Cross-Linked Polyanion Core for Delivery of a Cationic Drug Doxorubicin. J. Control. Release 2009, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, J.H.; Bae, S.M.; Shin, H.; Kim, M.S.; Park, S.; Lee, H.; Park, R.W.; Kim, I.S.; Kim, K.; et al. Tumoral acidic pH-responsive MPEG-poly (β-amino ester) polymeric micelles for cancer targeting therapy. J. Control. Release 2010, 144, 259–266. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Uthaman, S.; Huh, K.M.; Park, I.-K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater. Res. 2018, 22, 22. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Lee, D.S. Polymer-based and pH-sensitive nanobiosensors for imaging and therapy of acidic pathological areas. Pharm. Res. 2016, 33, 2358–2372. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Li, C.; Lu, W.; Ding, J. N-Boc-histidine-capped PLGA-PEG-PLGA as a smart polymer for drug delivery sensitive to tumor extracellular pH. Macromol. Biosci. 2010, 10, 1248–1256. [Google Scholar] [CrossRef]
- Hu, F.Q.; Zhang, Y.Y.; You, J.; Yuan, H.; Du, Y.Z. pH triggered doxorubicin delivery of PEGylated glycolipid conjugate micelles for tumor targeting therapy. Mol. Pharm. 2012, 9, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, X.; Qiu, L. The anti-tumor efficacy of curcumin when delivered by size/charge-changing multistage polymeric micelles based on amphiphilic poly(beta-amino ester) derivates. Biomaterials 2014, 35, 467–3479. [Google Scholar] [CrossRef]
- Shi, J.; Ren, Y.; Ma, J.; Luo, X.; Li, J.; Wu, Y.; Gu, H.; Fu, C.; Cao, Z.; Zhang, J. Novel CD44-targeting and pH/redox-dual-stimuli-responsive core–shell nanoparticles loading triptolide combats breast cancer growth and lung metastasis. J. Nanobiotechnol. 2021, 19, 188. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Cilingir, E.K.; Maklouf, H.; Seven, E.S.; Paudyal, S.; Vanni, S.; Graham, R.M.; Leblanc, R.M. pH and redox triggered doxorubicin release from covalently linked carbon dots conjugates. Nanoscale 2021, 13, 5507–5518. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Reduction and pH dual-bioresponsive crosslinked polymersomes for efficient intracellular delivery of proteins and potent induction of cancer cell apoptosis. Acta Biomater. 2014, 10, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, H.; Luo, S.; Liu, T.; Jiang, Y.; Liu, S. Thiol and pH dual-responsive dynamic covalent shell cross-linked micelles for triggered release of chemotherapeutic drugs. Polym. Chem. 2013, 4, 695–706. [Google Scholar] [CrossRef]
- Chiang, W.H.; Ho, V.T.; Huang, W.C.; Huang, Y.F.; Chern, C.S.; Chiu, H.C. Dual Stimuli-Responsive Polymeric Hollow Nanogels Designed as Carriers for Intracellular Triggered Drug Release. Langmuir 2012, 28, 15056–15064. [Google Scholar] [CrossRef]
- Luo, Y.; Yin, X.; Yin, X.; Chen, A.; Zhao, L.; Zhang, G.; Liao, W.; Huang, X.; Li, J.; Zhang, C.Y. Dual pH/Redox-Responsive Mixed Polymeric Micelles for Anticancer Drug Delivery and Controlled Release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef]
- Liang, K.; Such, G.K.; Zhu, Z.; Yan, Y.; Lomas, H.; Caruso, F. Charge-Shifting Click Capsules with Dual-Responsive Cargo Release Mechanisms. Adv. Mater. 2011, 23, H273–H277. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, K.C.R.; Thapa, B.; Xu, P. pH and Redox Dual Responsive Nanoparticle for Nuclear Targeted Drug Delivery. Mol. Pharm. 2012, 9, 2719–2729. [Google Scholar]
- Jing, X.; Zhi, Z.; Jin, L.; Wang, F.; Wu, Y.; Wang, D.; Yan, K.; Shao, Y.; Meng, L. pH/redox dual-stimuli responsive cross-linked polyphosphazene nanoparticles for multimodal imaging guided chemo-photodynamic therapy. Nanoscale 2012, 11, 9457–9467. [Google Scholar] [CrossRef]
- Curcio, M.; Paolì, A.; Cirillo, G.; Di Pietro, S.; Forestiero, M.; Giordano, F.; Mauro, L.; Amantea, D.; Di Bussolo, V.; Nicoletta, F.P.; et al. Combining Dextran Conjugates with Stimuli-Responsive and Folate-Targeting Activity: A New Class of Multifunctional Nanoparticles for Cancer Therapy. Nanomaterials 2021, 11, 1108. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, X.; Ouyang, J.; Kong, J.; Zhong, W.; Xing, M.M. pH and Reduction Dual-Sensitive Copolymeric Micelles for Intracellular Doxorubicin Delivery. Biomacromolecules 2011, 12, 3601–3611. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Yuan, Y.; Li, Q.; Chang, B.; Xu, L.; Cai, B.; Qi, C.; Li, C.; Jiang, X.; et al. Supramolecular Modular Approach towards Conveniently Constructing and Multifunctioning a pH/Redox Dual Responsive Drug Delivery Nanoplatform for Improved Cancer Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 26473–26484. [Google Scholar] [CrossRef]
- Yu, K.; Yang, X.; He, L.; Zheng, R.; Min, J.; Su, H.; Shan, S.; Jia, Q. Facile preparation of pH/reduction dual-stimuli responsive dextran nanogel as environment-sensitive carrier of doxorubicin. Polymer 2020, 200, 122585. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.A.; Zhong, Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Guo, X.; Wei, X.; Jing, Y.; Zhou, S. Size Changeable Nanocarriers with Nuclear Targeting for Effectively Overcoming Multidrug Resistance in Cancer Therapy. Adv. Mater. 2015, 27, 6450–6456. [Google Scholar] [CrossRef]
- Du, J.Z.; Du, X.J.; Mao, C.Q.; Wang, J. Tailor made Dual pH-Sensitive Polymer-Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef]
- Dai, L.; Li, X.; Duan, X.; Li, M.; Niu, P.; Xu, H.; Cai, K.; Yang, H. A pH/ROS Cascade-Responsive Charge-Reversal Nanosystem with Self-Amplified Drug Release for Synergistic Oxidation-Chemo-therapy. Adv. Sci. 2019, 6, 1801807. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv. Mater. 2017, 29, 1701170. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Zhang, X.; Peng, S.; Gu, H.; Zhang, L.J. Smart pH-sensitive micelles based on redox degradable polymers as DOX/GNPs carriers for controlled drug release and CT imaging. Colloid Surf. B 2018, 163, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xu, M.; Zhu, J.; He, Z.; Zhang, Y.; Xu, Q.; Niu, Y.; Liu, Y. Programmed co-delivery of platinum nanodrugs and gemcitabine by a clustered nanocarrier for precision chemotherapy for NSCLC tumors. J. Mater. Chem. B 2020, 8, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef] [PubMed]
- John, J.V.; Uthaman, S.; Augustine, R.; Chen, H.; Park, I.-K.; Kim, I. pH/Redox Dual Stimuli-Responsive Sheddable Nanodaisies for Efficient Intracellular Tumour-Triggered Drug Delivery. J. Mater. Chem. B 2013, 5, 5027–5036. [Google Scholar] [CrossRef] [PubMed]
- Malaki, M.; Xu, W.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced Metal Matrix Nanocomposites. Metals 2019, 9, 330. [Google Scholar] [CrossRef]
- Konopka, K. Particle-Reinforced Ceramic Matrix Composites—Selected Examples. J. Compos. Sci. 2022, 6, 178. [Google Scholar] [CrossRef]
- Popova, V.; Dmitrienko, E.; Chubarov, A. Magnetic Nanocomposites and Imprinted Polymers for Biomedical Applications of Nucleic Acids. Magnetochemistry 2023, 9, 12. [Google Scholar] [CrossRef]
- Ramesh, M.; Kumar, L.R.; Khan, A.; Asiri, A.M. 22-Self-healing polymer composites and its chemistry. In Self Healing Composite Marker; Woodhead Publishing: Sawston, UK, 2020; pp. 415–427. [Google Scholar]
- Chauhan, N.; Singh, Y. Engineered polymeric materials/nanomaterials for growth factor/drug delivery in bone tissue engineering applications. In Nanoscale Engineering of Biomaterials: Properties and Applications; Springer Nature: Singapore, 2022; pp. 349–396. [Google Scholar]
- Roy, A.; Manna, K.; Pal, S. Recent advances in various stimuli-responsive hydrogels: From synthetic designs to emerging healthcare applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Okamoto, M. Polymer Nanocomposites. Eng 2023, 4, 457–479. [Google Scholar] [CrossRef]







| Polymeric Nanoparticles | Cargo/Drug | Drug Release/Targeting | Therapy | Biological Evolution | References |
|---|---|---|---|---|---|
| poly (β-amino esters) | CD44 | Controlled drug release | Breast cancer; lung metastasis | In vitro | [98] |
| Conjugated (C-dots-HBA-dox) and (C-dots-S–S-dox) | Doxorubicin | Controlled drug release | Cancer chemotherapy | In vitro | [99] |
| PEG–PAA(SH)–PDEA | FITC-BSA/CC | Controlled drug release | Cancer therapy | In vitro | [100] |
| PCL-b-P(OEGMA-co-MAEBA) | Camptothecin or Doxorubicin | Accelerated drug release | Cancer chemotherapy | In vitro | [101] |
| acrylic acid (AAc) and 2-methacryloylethyl acrylate (MEA) | Doxorubicin | Rapid drug release | Anticancer treatment | In vitro | [102] |
| (PAE-ss-mPEG) | Doxorubicin | Controlled drug release | Anticancer treatment | In vitro | [103] |
| (PDPA) capsules | Rhodamine B isothiocyanate- labeled OVA | Cargo release | -- | In vitro | [104] |
| Poly (2-(pyridin-2-yldisulfanyl) ethyl acrylate) | RPDSG/DOX | Controlled drug release | Cancer therapy | In vitro | [105] |
| cross-linked polyphosphazene | Curcumin and Ce6 | Controlled drug release | Cancer therapy | In vivo and in vitro | [106] |
| DEXssPEGCOOH | Doxorubicin | Targeted release | Cancer therapy | In vitro | [107] |
| RPAE-PEG | Doxorubicin | Controlled drug release | Cancer therapy | In vitro | [108] |
| MSNs (DOX@PRMSNs) | Doxorubicin | Targeting ligands | Cancer therapy | In vitro | [86] |
| DOX@Dex-SS nanogel | Doxorubicin | Cumulative amount of drug release | Cancer chemotherapy | In vitro | [87] |
| Polymeric Nanoparticles | Cargo/Drug | Therapy | Biological Evolution | References |
|---|---|---|---|---|
| FHCPCe NPs | Curcumin and Ce6 | Chemotherapy/ photodynamic therapy | In vivo | [106] |
| Triblock copolymer | Doxorubin | Anticancer treatment | In vivo | [97] |
| P(CPT-MAA) nanogel | Camptothecin | Chemotherapy | In vivo | [109] |
| mPEG-SS-PNLG | Doxorubicin | Anticancer treatment | In vivo | [110] |
| mPEG-b-PAE-ss-DOX | Doxorubin | Chemotherapy | In vivo | [111] |
| Therapeutic Agents | Before Therapy | During Therapy | Fold Changes | References |
|---|---|---|---|---|
| pH-causing species (H+, OH−, H3O+) | Slightly acidic (6.5–7.0) | More acidic (5.0–6.0) | Increased twice | [93,96] |
| Redox-causing species (ROS, GSH, etc.) | High ROS: damage cells | Low ROS: reduce cell damage | Reduced to half | [86,93,94,95,96,97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaddimath, S.; Payamalle, S.; Channabasavana Hundi Puttaningaiah, K.P.; Hur, J. Recent Advances in pH and Redox Responsive Polymer Nanocomposites for Cancer Therapy. J. Compos. Sci. 2024, 8, 28. https://doi.org/10.3390/jcs8010028
Gaddimath S, Payamalle S, Channabasavana Hundi Puttaningaiah KP, Hur J. Recent Advances in pH and Redox Responsive Polymer Nanocomposites for Cancer Therapy. Journal of Composites Science. 2024; 8(1):28. https://doi.org/10.3390/jcs8010028
Chicago/Turabian StyleGaddimath, Shivalingayya, Shivanand Payamalle, Keshavananada Prabhu Channabasavana Hundi Puttaningaiah, and Jaehyun Hur. 2024. "Recent Advances in pH and Redox Responsive Polymer Nanocomposites for Cancer Therapy" Journal of Composites Science 8, no. 1: 28. https://doi.org/10.3390/jcs8010028