Quantification of Irgafos P-168 and Degradative Profile in Samples of a Polypropylene/Polyethylene Composite Using Microwave, Ultrasound and Soxhlet Extraction Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. GC-MS Analysis
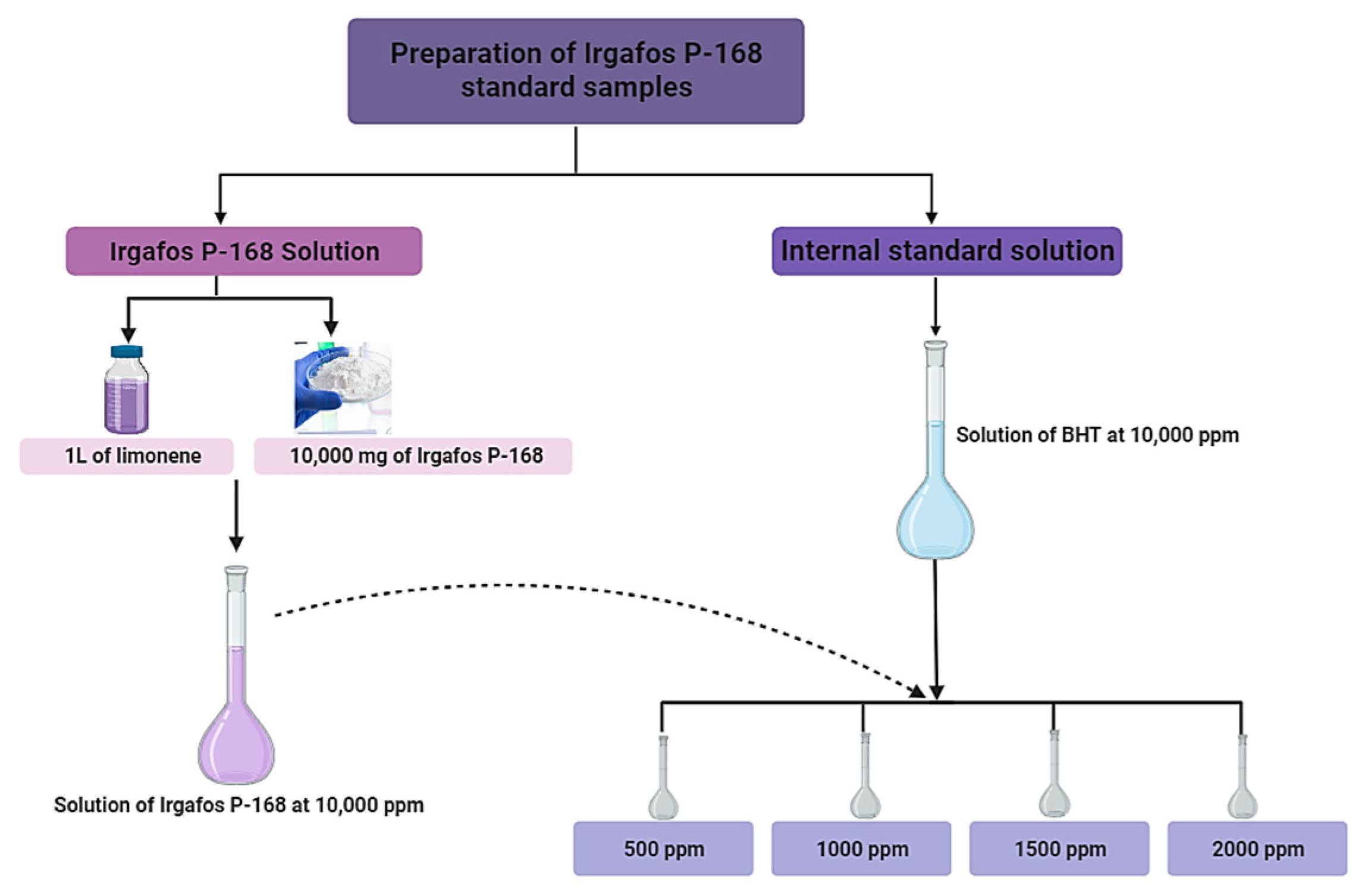
2.2.1. Prepare Irgafos 168 Calibration Standards and C-PP/PE Samples with Irgafos P-168
Preparation of the Curve for Calibration of the Chromatograph
Preparation of the C-PP/PE Sample with Different Concentrations of Irgafos P-168
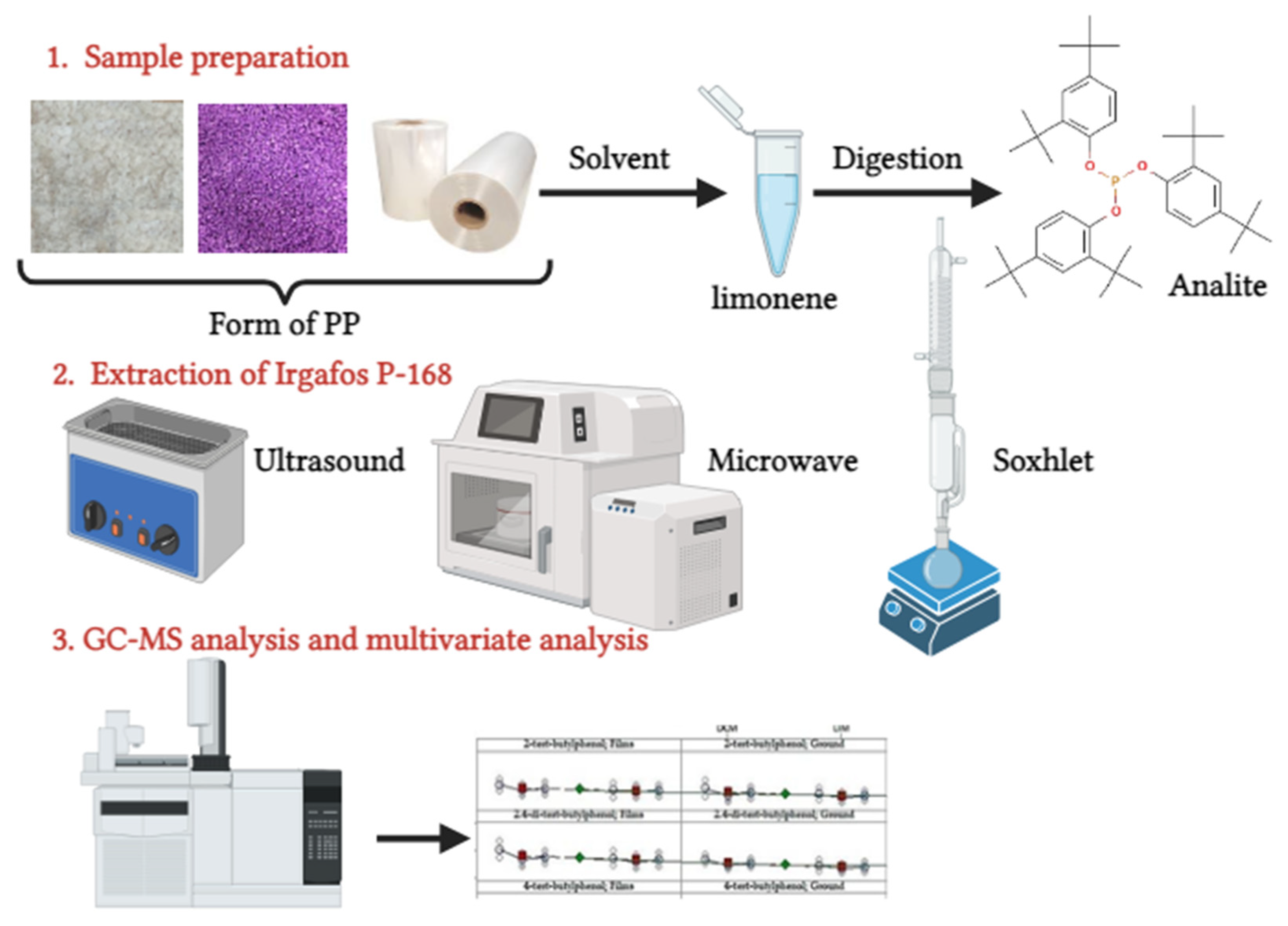
Extraction of Irgafos P-168 in C-PP/PE Samples
2.3. Multivariate Graphical Analysis
3. Results and Discussion
3.1. Quantification and Recovery of the Additive Irgafos P-168 by GC
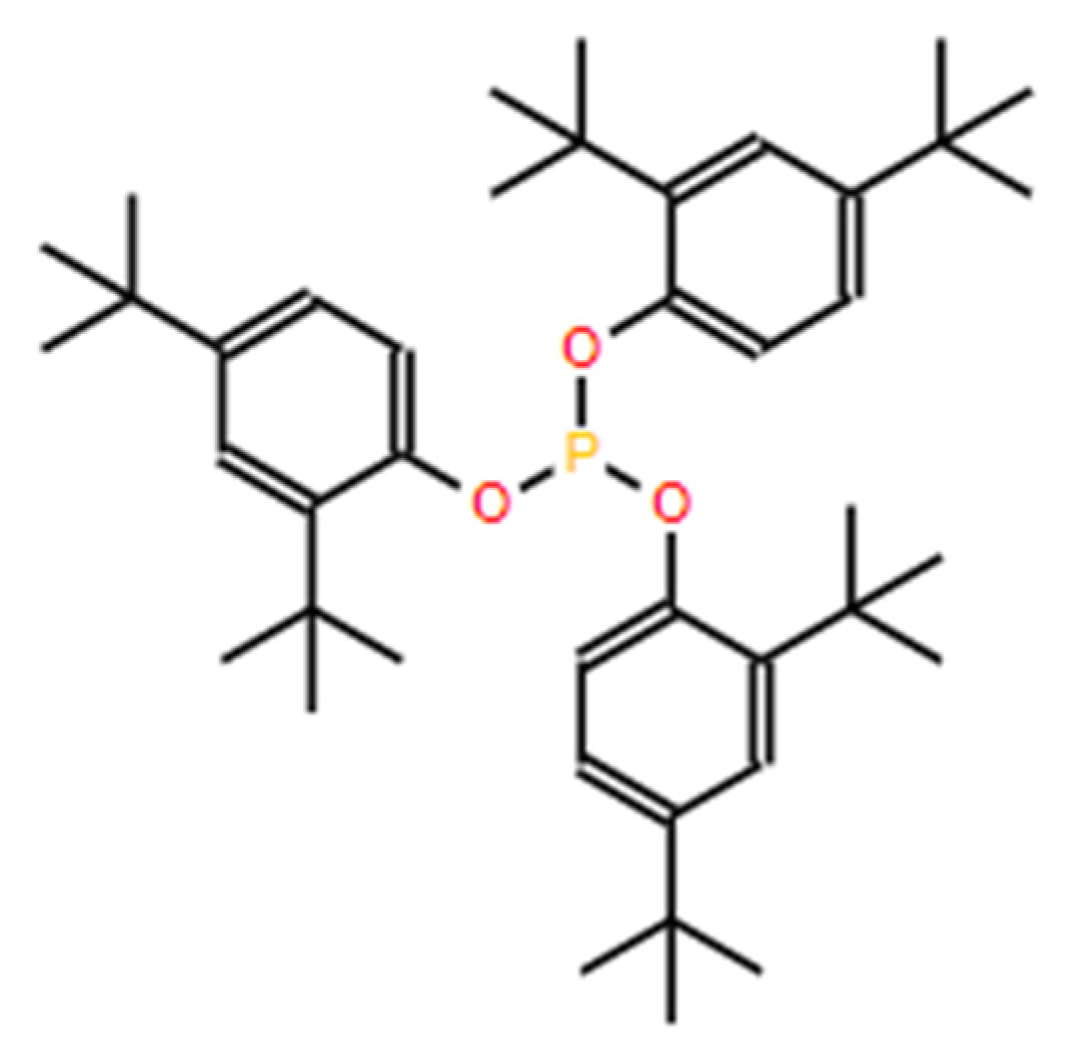
3.2. Identification of Irgafos P-168 by Gas Chromatography Coupled to Mass Spectrometry (GC-MS) Analysis
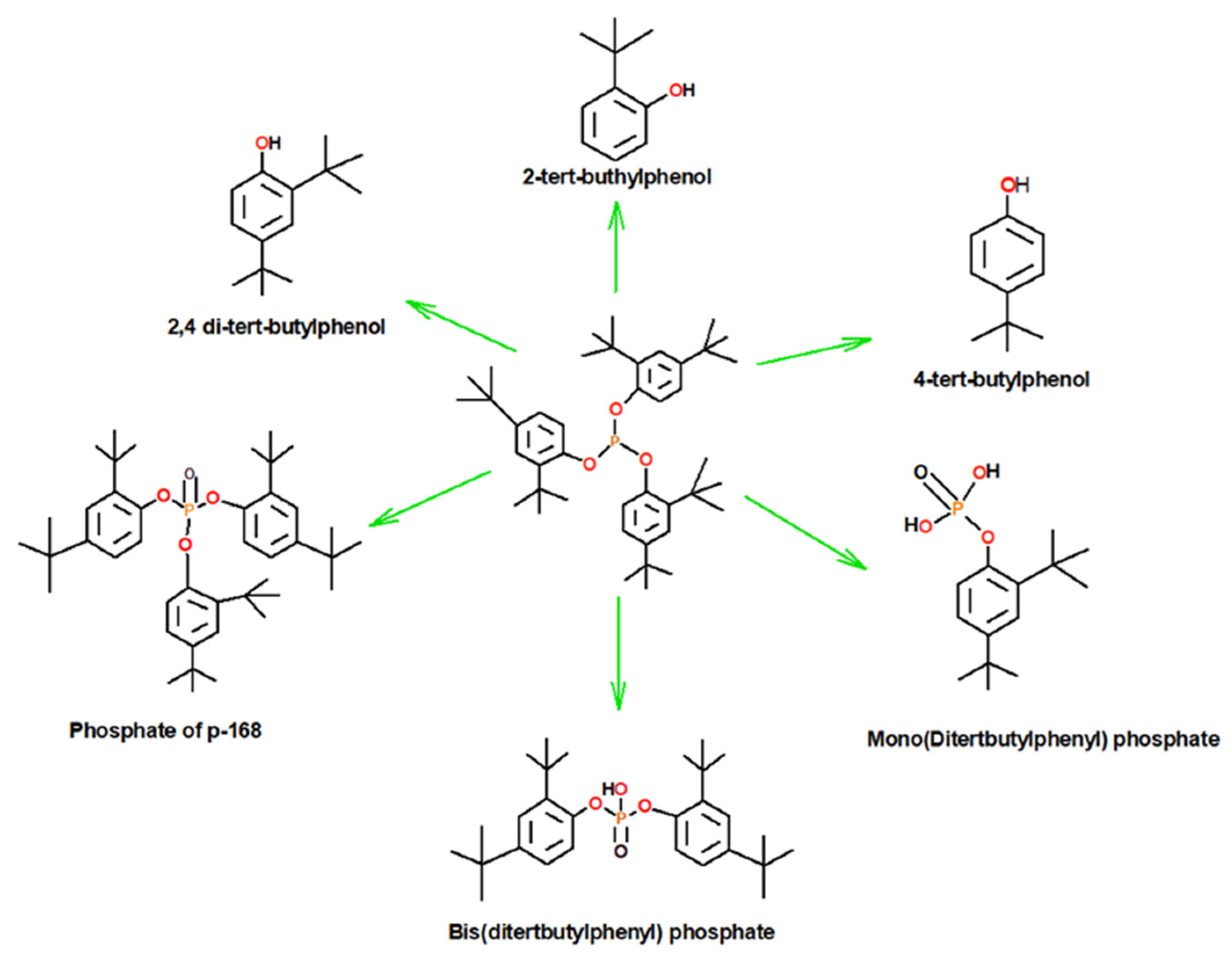
3.3. Determination of the Thermo-Oxidative Degradation Products of Irgafos P-168
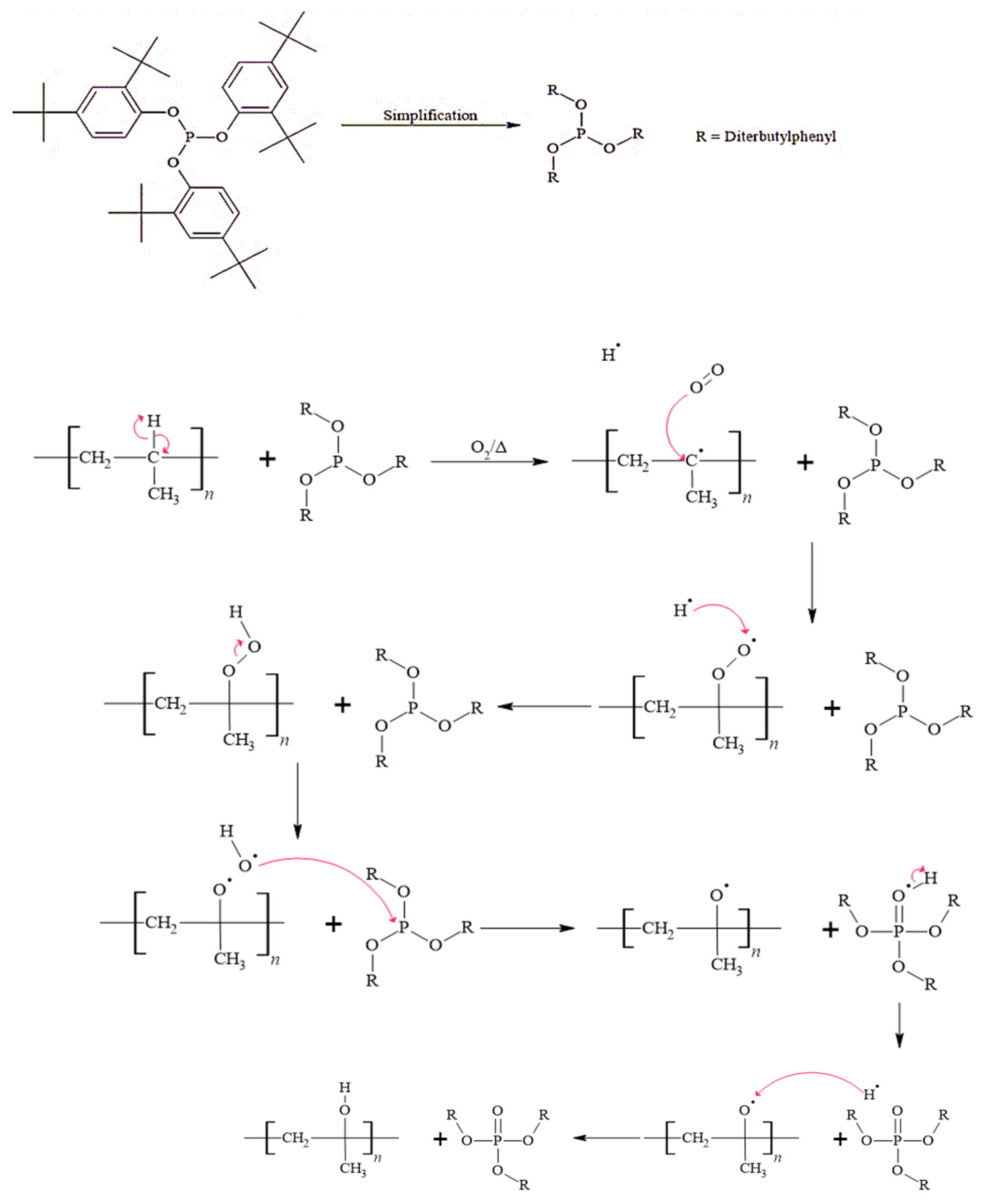
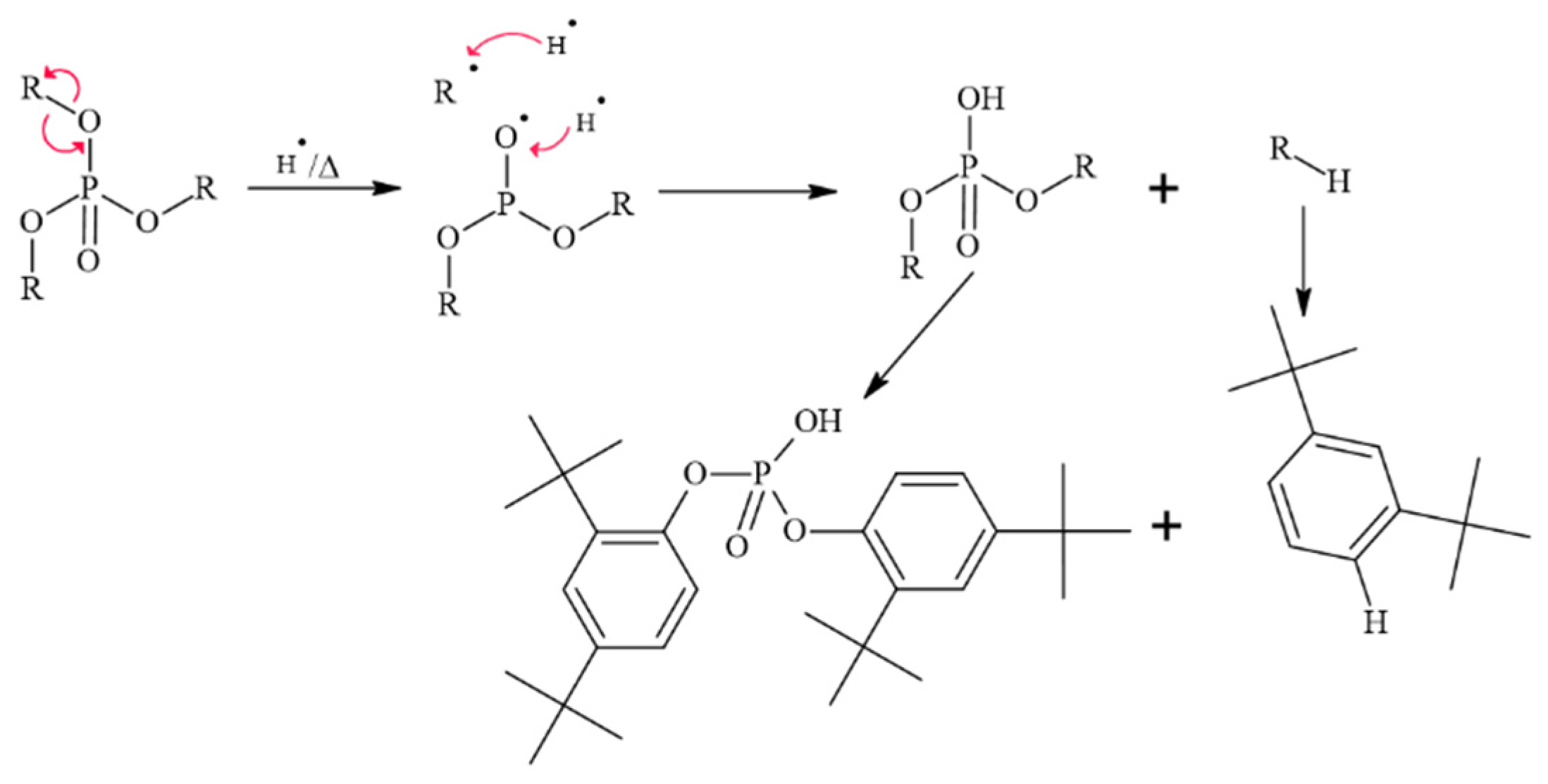
3.3.1. Mechanism of the Phosphate Product of Irgafos P-168
3.3.2. Mechanism of Formation of 2,4-Di-tert-butylphenol
3.3.3. Mechanism of Formation of the Bis(di-tert-butylphenyl) Phosphate Product
3.3.4. Mechanism of Formation of the Mono(di-tert-butylphenyl) Phosphate Product
3.3.5. Mechanism of 2-Tert-butylphenol Product Formation
3.3.6. Mechanism for Obtaining the Product 4-Tert-butylphenol
3.4. Validation of Proposed Mechanisms
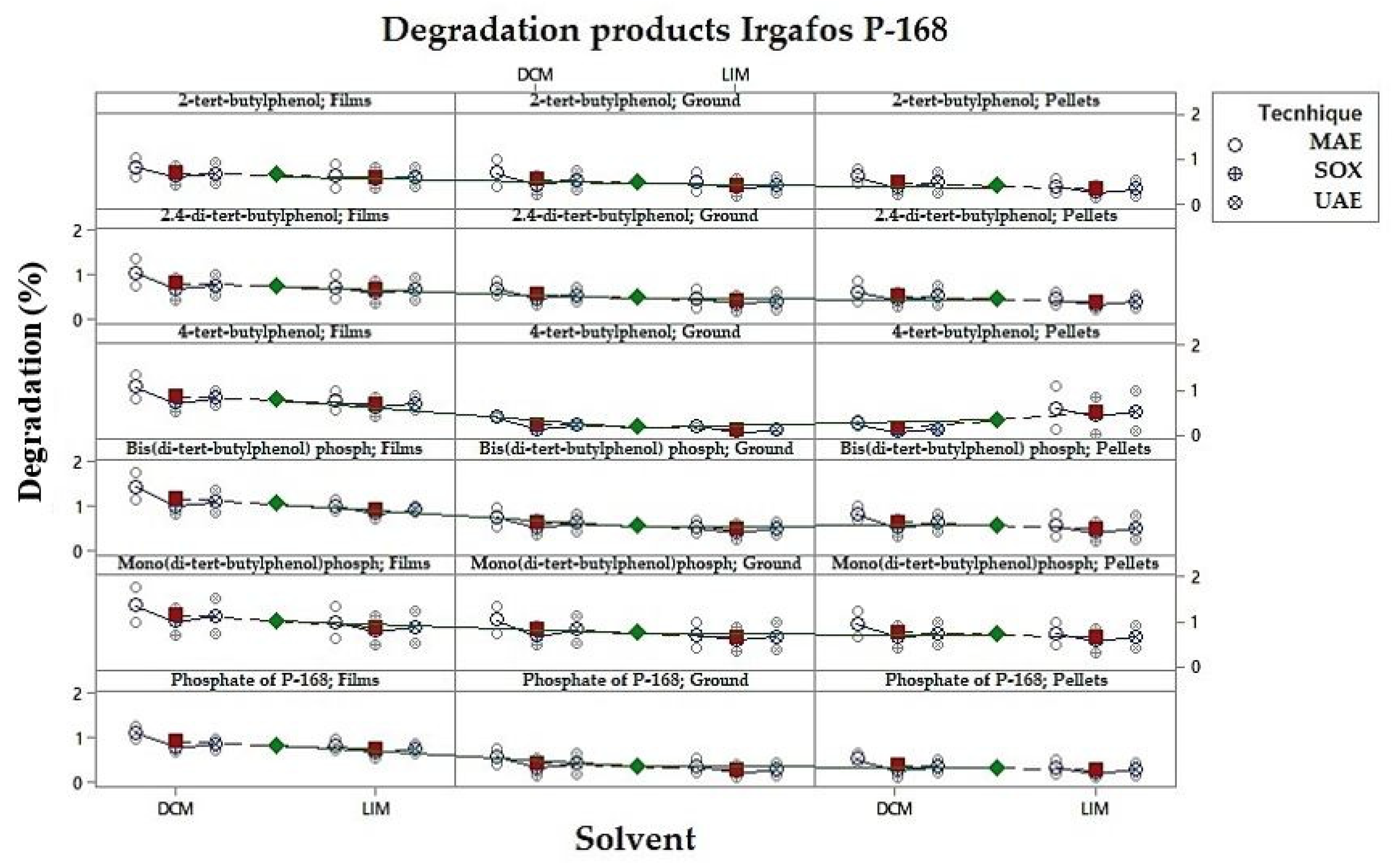
3.5. Percentage Analysis of the Degradation Products of Irgafos P-168
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfaendner, R. How will additives shape the future of plastics? Polym. Degrad. Stab. 2006, 91, 2249–2256. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, H.; Lu, L.; Lv, X.; Ju, G.; Zhao, J.; Sun, F.; Wang, Y.; Yu, W. Simultaneous Determination and Exposure Assessment of Antioxidants in Food-Contact Plastic Materials by HPLC-MS/MS. J. Food Prot. 2023, 86, 100121. [Google Scholar] [CrossRef] [PubMed]
- Pavon, C.; Aldas, M.; Hernández-Fernández, J.; López-Martínez, J. Comparative characterization of gum rosins for their use as sustainable additives in polymeric matrices. J. Appl. Polym. Sci. 2022, 139, 51734. [Google Scholar] [CrossRef]
- Ambrogi, V.; Carfagna, C.; Cerruti, P.; Marturano, V. Additives in Polymers. Modif. Polym. Prop. 2017, 87–108. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Castro-Suarez, J.R.; Toloza, C.A.T. Iron Oxide Powder as Responsible for the Generation of Industrial Polypropylene Waste and as a Co-Catalyst for the Pyrolysis of Non-Additive Resins. Int. J. Mol. Sci. 2022, 23, 11708. [Google Scholar] [CrossRef] [PubMed]
- Chacon, H.; Cano, H.; Fernández, J.H.; Guerra, Y.; Puello-Polo, E.; Ríos-Rojas, J.F.; Ruiz, Y. Effect of Addition of Polyurea as an Aggregate in Mortars: Analysis of Microstructure and Strength. Polymers 2022, 14, 1753. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Cano, H.; Aldas, M. Impact of Traces of Hydrogen Sulfide on the Efficiency of Ziegler–Natta Catalyst on the Final Properties of Polypropylene. Polymers 2022, 14, 3910. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Bai, S.; Cheatham, M.; Cong, R.; Yau, W. Determination of antioxidants in polyolefins using total dissolution methodology followed by RPLC. J. Sep. Sci. 2010, 33, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Spikes, H. Friction Modifier Additives. Tribol. Lett. 2015, 60, 5. [Google Scholar] [CrossRef]
- Muller, B. Colorants for Thermoplastic Polymers. In Applied Plastics Engineering Handbook: Processing and Materials; William Andrew Publishing: Norwich, NY, USA, 2011; pp. 435–440. [Google Scholar] [CrossRef]
- Moura, J.C.V.P.; Oliveira-Campos, A.M.F.; Griffiths, J. The effect of additives on the photostability of dyed polymers. Dye. Pigment. 1997, 33, 173–196. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M. Perspectives on additives for polymers. 1. Aspects of stabilization. J. Vinyl Addit. Technol. 2021, 27, 5–27. [Google Scholar] [CrossRef]
- Müller, W.W.; Jakob, I.; Li, C.; Tatzky-Gerth, R. Antioxidant depletion and oit values of high impact pp strands. Chin. J. Polym. Sci. (Engl. Ed.) 2009, 27, 435–445. [Google Scholar] [CrossRef]
- Yan, Y.; Hu, C.Y.; Wang, Z.W.; Jiang, Z.W. Degradation of Irgafos 168 and migration of its degradation products from PP-R composite films. Packag. Technol. Sci. 2018, 31, 679–688. [Google Scholar] [CrossRef]
- Singh, N.; Mann, B.; Sharma, R.; Verma, A.; Panjagari, N.R.; Gandhi, K. Identification of polymer additives from multilayer milk packaging materials by liquid-solid extraction coupled with GC-MS. Food Packag. Shelf Life 2022, 34, 100975. [Google Scholar] [CrossRef]
- Vandenburg, H.J. Critical Review: Analytical Extraction of Additives from Polymers. Analyst 1997, 122, 101R–116R. [Google Scholar] [CrossRef]
- Cano, J.M.; Marín, M.L.; Sánchez, A.; Hernandez, V. Determination of adipate plasticizers in poly(vinyl chloride) by microwave-assisted extraction. J. Chromatogr. A 2002, 963, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y. Development of a screening method for phthalate esters in polymers using a quantitative database in combination with pyrolyzer /thermal desorption gas chromatography-mass spectrometry. J. Chromatogr. A 2019, 1602, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Grape (Vitis vinifera) Seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Camacho, W.; Karlsson, S. Quality-determination of recycled plastic packaging waste by identification of contaminants by GC–MS after microwave-assisted extraction (MAE). Polym. Degrad. Stab. 2000, 71, 123–134. [Google Scholar] [CrossRef]
- López-Bascón, M.A.; De Castro, M.L. Soxhlet Extraction. Liq. Phase Extr. 2020, 327–354. [Google Scholar] [CrossRef]
- Romdhane, M.; Gourdon, C. Investigation in solid–liquid extraction: Influence of ultrasound. Chem. Eng. J. 2002, 87, 11–19. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano, H.; Reyes, A.F. Valuation of the Synthetic Antioxidant Tris-(Diterbutyl-Phenol)-Phosphite (Irgafos P-168) from Industrial Wastewater and Application in Polypropylene Matrices to Minimize Its Thermal Degradation. Molecules 2023, 28, 3163. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Espinosa, E. Development and Application of a Principal Component Analysis Model to Quantify the Green Ethylene Content in Virgin Impact Copolymer Resins During Their Synthesis on an Industrial Scale. J. Polym. Environ. 2022, 30, 4800–4808. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, M.; Sillanpää, M. Removal of dichloromethane from soil and wastewater: A review. Chemosphere 2013, 93, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.; Giraudet, S.; Vilmain, J.B.; Le Cloirec, P. Intensification of the temperature-swing adsorption process with a heat pump for the recovery of dichloromethane. J. Environ. Chem. Eng. 2015, 3, 734–743. [Google Scholar] [CrossRef]
- Zhao, Y.-B.; Lv, X.-D.; Ni, H.-G. Solvent-based separation and recycling of waste plastics: A review. Chemosphere 2018, 209, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Jongedijk, E.; Cankar, K.; Buchhaupt, M.; Schrader, J.; Bouwmeester, H.; Beekwilder, J. Biotechnological production of limonene in microorganisms. Appl. Microbiol. Biotechnol. 2016, 100, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Mahdavi, S. Nanodelivery systems for d-limonene; techniques and applications. Food Chem. 2022, 384, 132479. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Development and validation of a methodology for quantifying partsper-billion levels of arsine and phosphine in nitrogen, hydrogen and liquefied petroleum gas using a variable pressure sampler coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 2021, 1637, 461833. [Google Scholar] [CrossRef] [PubMed]
- Cui, G. Potential Use of Limonene as an Alternative Solvent for Extraction of Gutta-Percha from Eucommia ulmoides. ACS Sustain. Chem. Eng. 2022, 10, 11057–11068. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total Lipid Extraction of Food Using d-Limonene as an Alternative to n-Hexane. Chromatographia 2008, 68, 311–313. [Google Scholar] [CrossRef]
- Haunschmidt, M.; Klampfl, C.W.; Buchberger, W.; Hertsens, R. Rapid identification of stabilisers in polypropylene using time-of-flight mass spectrometry and DART as ion source. Analyst 2009, 135, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kato, L.S.; Conte-Junior, C.A. Safety of Plastic Food Packaging: The Challenges about Non-Intentionally Added Substances (NIAS) Discovery, Identification and Risk Assessment. Polymers 2021, 13, 2077. [Google Scholar] [CrossRef] [PubMed]








| Technique | Time (min) | Temperature | Solvent | Form of C-PP/PE | Irgafos 168 Recovery Rate (%) | |||
|---|---|---|---|---|---|---|---|---|
| C-PP/PE 2 | C-PP/PE 3 | C-PP/PE 4 | C-PP/PE 5 | |||||
| 500 | 1000 | 1500 | 2000 | |||||
| Soxhlet | 1440 | 90 | Dichloromethane | Pellets | 69.98 | 71.76 | 73.56 | 73.83 |
| Soxhlet | 1440 | 90 | Dichloromethane | Film | 72.44 | 76.38 | 76.04 | 77.42 |
| Soxhlet | 1440 | 90 | Dichloromethane | Ground | 77.4 | 79.92 | 77.35 | 79.88 |
| Soxhlet | 720 | 90 | Dichloromethane | Pellets | 47.52 | 61.14 | 61.15 | 59.75 |
| Soxhlet | 720 | 90 | Dichloromethane | Film | 54.68 | 64.42 | 64.67 | 62.81 |
| Soxhlet | 720 | 90 | Dichloromethane | Ground | 59.96 | 69.36 | 67.53 | 67.46 |
| Soxhlet | 1440 | 90 | Limonene | Pellets | 68.04 | 69.9 | 72.47 | 72.21 |
| Soxhlet | 1440 | 90 | Limonene | Film | 69.68 | 74.5 | 74.71 | 76.87 |
| Soxhlet | 1440 | 90 | Limonene | Ground | 74.44 | 77.92 | 75.13 | 79.14 |
| Soxhlet | 720 | 90 | Limonene | Pellets | 45.36 | 59.52 | 59.4 | 58.76 |
| Soxhlet | 720 | 90 | Limonene | Film | 51.84 | 61.96 | 63.41 | 61.15 |
| Soxhlet | 720 | 90 | Limonene | Ground | 56.56 | 67.76 | 65.59 | 66.09 |
| Ultrasound | 90 | 50 | Dichloromethane | Pellets | 73.84 | 76.2 | 79.25 | 77.83 |
| Ultrasound | 90 | 50 | Dichloromethane | Film | 75.84 | 79.62 | 84.72 | 82.36 |
| Ultrasound | 90 | 50 | Dichloromethane | Ground | 92.04 | 88.9 | 93.24 | 92.76 |
| Ultrasound | 60 | 50 | Dichloromethane | Pellets | 65.48 | 66.3 | 67.77 | 66.09 |
| Ultrasound | 60 | 50 | Dichloromethane | Film | 67.92 | 71.8 | 73.8 | 70.41 |
| Ultrasound | 60 | 50 | Dichloromethane | Ground | 78.84 | 75.46 | 78.89 | 78.96 |
| Ultrasound | 90 | 50 | Limonene | Pellets | 70 | 74.94 | 77.11 | 76.24 |
| Ultrasound | 90 | 50 | Limonene | Film | 72.84 | 78.24 | 82.65 | 80.23 |
| Ultrasound | 90 | 50 | Limonene | Ground | 89.56 | 87 | 92.05 | 90.23 |
| Ultrasound | 60 | 50 | Limonene | Pellets | 62.6 | 64.3 | 66.25 | 64.31 |
| Ultrasound | 60 | 50 | Limonene | Film | 65.56 | 69.86 | 71.88 | 69.87 |
| Ultrasound | 60 | 50 | Limonene | Ground | 75.88 | 73.08 | 76.73 | 76.25 |
| Microwave | 45 | 117 | Dichloromethane | Pellets | 77.4 | 78.32 | 82.73 | 79.74 |
| Microwave | 45 | 117 | Dichloromethane | Film | 83.6 | 83.66 | 88.8 | 83.91 |
| Microwave | 45 | 117 | Dichloromethane | Ground | 96.2 | 94.34 | 97.01 | 96.71 |
| Microwave | 25 | 117 | Dichloromethane | Pellets | 70.32 | 69.7 | 72.07 | 68.25 |
| Microwave | 25 | 117 | Dichloromethane | Film | 73.88 | 74.04 | 77.35 | 73.19 |
| Microwave | 25 | 117 | Dichloromethane | Ground | 84.72 | 78.54 | 80.64 | 81.86 |
| Microwave | 45 | 117 | Limonene | Pellets | 74.4 | 76.48 | 81.36 | 78.58 |
| Microwave | 45 | 117 | Limonene | Film | 80.32 | 81.84 | 87.09 | 82.48 |
| Microwave | 45 | 117 | Limonene | Ground | 91.88 | 89.78 | 94.72 | 94.94 |
| Microwave | 25 | 117 | Limonene | Pellets | 65.12 | 66.98 | 70.57 | 65.52 |
| Microwave | 25 | 117 | Limonene | Film | 68.4 | 71.48 | 75.32 | 71.23 |
| Microwave | 25 | 117 | Limonene | Ground | 78.44 | 75.44 | 78.91 | 80.09 |
| % Area under the Degradation by-Product Curve P-168 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tecnhique | Time (min) | Temperature | Solvent | Form of C-PP/PE | 2.4-Di-tertbutylphenol | 2-Tert-butylphenol | 4-Tert-butylphenol | Phosphate of P-168 | Bis(di-tertbutylphenyl) Phosphate | Mono(di-tertbutylphenyl) Phosphate | P-168 |
| Soxhlet | 1440 | 90 | Dichloromethane | Pellets | 0.6 | 0.5 | 0.1 | 0.4 | 0.7 | 0.9 | 72.28 |
| Soxhlet | 1440 | 90 | Dichloromethane | Film | 0.9 | 0.8 | 0.9 | 0.9 | 1.2 | 1.3 | 75.57 |
| Soxhlet | 1440 | 90 | Dichloromethane | Ground | 0.6 | 0.6 | 0.15 | 0.51 | 0.7 | 0.9 | 78.64 |
| Soxhlet | 720 | 90 | Dichloromethane | Pellets | 0.25 | 0.15 | 0.06 | 0.1 | 0.31 | 0.42 | 57.39 |
| Soxhlet | 720 | 90 | Dichloromethane | Film | 0.41 | 0.38 | 0.52 | 0.67 | 0.82 | 0.69 | 61.64 |
| Soxhlet | 720 | 90 | Dichloromethane | Ground | 0.28 | 0.17 | 0.1 | 0.11 | 0.33 | 0.46 | 66.08 |
| Soxhlet | 1440 | 90 | Limonene | Pellets | 0.42 | 0.38 | 0.84 | 0.31 | 0.62 | 0.82 | 70.65 |
| Soxhlet | 1440 | 90 | Limonene | Film | 0.82 | 0.76 | 0.84 | 0.79 | 0.95 | 1.12 | 73.94 |
| Soxhlet | 1440 | 90 | Limonene | Ground | 0.52 | 0.53 | 0.09 | 0.37 | 0.58 | 0.86 | 76.66 |
| Soxhlet | 720 | 90 | Limonene | Pellets | 0.18 | 0.07 | 0.01 | 0.08 | 0.19 | 0.31 | 55.76 |
| Soxhlet | 720 | 90 | Limonene | Film | 0.32 | 0.29 | 0.41 | 0.53 | 0.71 | 0.46 | 59.59 |
| Soxhlet | 720 | 90 | Limonene | Ground | 0.15 | 0.12 | 0.06 | 0.08 | 0.24 | 0.34 | 64.00 |
| Ultrasound | 90 | 50 | Dichloromethane | Pellets | 0.72 | 0.68 | 0.15 | 0.47 | 0.82 | 0.97 | 76.78 |
| Ultrasound | 90 | 50 | Dichloromethane | Film | 0.96 | 0.87 | 0.98 | 0.96 | 1.34 | 1.52 | 80.64 |
| Ultrasound | 90 | 50 | Dichloromethane | Ground | 0.68 | 0.71 | 0.24 | 0.64 | 0.82 | 1.12 | 91.74 |
| Ultrasound | 60 | 50 | Dichloromethane | Pellets | 0.29 | 0.19 | 0.12 | 0.18 | 0.41 | 0.46 | 66.41 |
| Ultrasound | 60 | 50 | Dichloromethane | Film | 0.52 | 0.42 | 0.67 | 0.72 | 0.86 | 0.72 | 70.98 |
| Ultrasound | 60 | 50 | Dichloromethane | Ground | 0.37 | 0.27 | 0.18 | 0.16 | 0.42 | 0.52 | 78.04 |
| Ultrasound | 90 | 50 | Limonene | Pellets | 0.52 | 0.47 | 0.96 | 0.41 | 0.76 | 0.92 | 74.57 |
| Ultrasound | 90 | 50 | Limonene | Film | 0.92 | 0.78 | 0.86 | 0.85 | 0.99 | 1.24 | 78.49 |
| Ultrasound | 90 | 50 | Limonene | Ground | 0.57 | 0.55 | 0.12 | 0.41 | 0.64 | 0.96 | 89.71 |
| Ultrasound | 60 | 50 | Limonene | Pellets | 0.23 | 0.12 | 0.08 | 0.14 | 0.23 | 0.41 | 64.37 |
| Ultrasound | 60 | 50 | Limonene | Film | 0.42 | 0.34 | 0.53 | 0.64 | 0.83 | 0.52 | 69.29 |
| Ultrasound | 60 | 50 | Limonene | Ground | 0.19 | 0.18 | 0.11 | 0.12 | 0.34 | 0.38 | 75.49 |
| Microwave | 45 | 117 | Dichloromethane | Pellets | 0.82 | 0.75 | 0.19 | 0.62 | 0.99 | 1.24 | 79.55 |
| Microwave | 45 | 117 | Dichloromethane | Film | 1.34 | 0.99 | 1.34 | 1.25 | 1.76 | 1.75 | 84.99 |
| Microwave | 45 | 117 | Dichloromethane | Ground | 0.82 | 0.96 | 0.37 | 0.75 | 0.96 | 1.34 | 96.07 |
| Microwave | 25 | 117 | Dichloromethane | Pellets | 0.38 | 0.41 | 0.33 | 0.42 | 0.66 | 0.67 | 70.08 |
| Microwave | 25 | 117 | Dichloromethane | Film | 0.71 | 0.57 | 0.81 | 0.96 | 1.12 | 0.96 | 74.61 |
| Microwave | 25 | 117 | Dichloromethane | Ground | 0.51 | 0.34 | 0.41 | 0.38 | 0.52 | 0.74 | 81.44 |
| Microwave | 45 | 117 | Limonene | Pellets | 0.57 | 0.51 | 1.08 | 0.48 | 0.82 | 0.99 | 77.71 |
| Microwave | 45 | 117 | Limonene | Film | 0.96 | 0.86 | 0.97 | 0.96 | 1.12 | 1.34 | 82.93 |
| Microwave | 45 | 117 | Limonene | Ground | 0.66 | 0.67 | 0.24 | 0.51 | 0.66 | 0.98 | 92.83 |
| Microwave | 25 | 117 | Limonene | Pellets | 0.29 | 0.18 | 0.11 | 0.16 | 0.29 | 0.47 | 67.05 |
| Microwave | 25 | 117 | Limonene | Film | 0.45 | 0.31 | 0.55 | 0.69 | 0.89 | 0.61 | 71.61 |
| Microwave | 25 | 117 | Limonene | Ground | 0.23 | 0.22 | 0.16 | 0.19 | 0.38 | 0.41 | 78.22 |
| Mechanism | Structure | Gibbs Free Energy (Hartree) | Enthalpy (Hartree) | ∆G of the Reaction (kcal/mol) | ∆H of the Reaction (kcal/mol) |
|---|---|---|---|---|---|
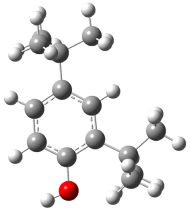
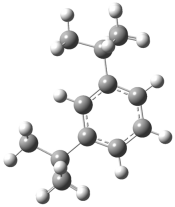
| Mechanism of formation of 2,4-di-tert-butylphenol. |  | −2279.760 | −2279.614 | −744.23 | −732.30 |
 | −1659.277 | −1659.171 | |||
 | −621.669 | −621.610 | |||
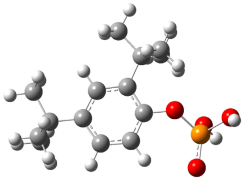
| Mechanism of formation of the Bis(di-tert-butylphenyl) phosphate product |  | −2279.760 | −2279.614 | −761.17 | −747.99 |
 | −1734.514 | −1734.404 | |||
 | −546.459 | −546.402 | |||
| Mechanism of formation of the Mono(di-tert-butylphenyl) phosphate product | 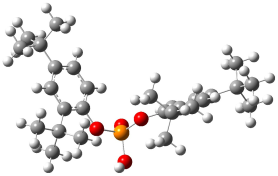 | −1734.514 | −1734.404 | −760.54 | −747.99 |
 | −1189.267 | −1189.194 | |||
 | −546.459 | −546.402 | |||
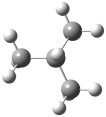
| Mechanism for obtaining the product 4-tert-buthylphenol |  | −621.669 | −621.610 | −878.51 | −863.45 |
 | −464.665 | −464.618 | |||
 | −158.403 | −158.368 | |||
| Mechanism for obtaining the product of 2-tert-buthylphenol |  | −621.669 | −621.610 | −792.54 | −778.11 |
 | −464.529 | −464.482 | |||
 | −158.403 | −158.368 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Fernández, J.; Pérez-Mendoza, J.; Ortega-Toro, R. Quantification of Irgafos P-168 and Degradative Profile in Samples of a Polypropylene/Polyethylene Composite Using Microwave, Ultrasound and Soxhlet Extraction Techniques. J. Compos. Sci. 2024, 8, 156. https://doi.org/10.3390/jcs8040156
Hernández-Fernández J, Pérez-Mendoza J, Ortega-Toro R. Quantification of Irgafos P-168 and Degradative Profile in Samples of a Polypropylene/Polyethylene Composite Using Microwave, Ultrasound and Soxhlet Extraction Techniques. Journal of Composites Science. 2024; 8(4):156. https://doi.org/10.3390/jcs8040156
Chicago/Turabian StyleHernández-Fernández, Joaquín, Jaime Pérez-Mendoza, and Rodrigo Ortega-Toro. 2024. "Quantification of Irgafos P-168 and Degradative Profile in Samples of a Polypropylene/Polyethylene Composite Using Microwave, Ultrasound and Soxhlet Extraction Techniques" Journal of Composites Science 8, no. 4: 156. https://doi.org/10.3390/jcs8040156







