Optical and Spectroscopic Properties of Ho:Lu2O3 Transparent Ceramics Elaborated by Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ho:Lu2O3 Nanopowders
2.2. Densification by Spark Plasma Sintering
2.3. Characterization of Sintered Samples
3. Results
3.1. Sample Microstructure and Optical Transmittance
3.2. Photoluminescence and Fluorescence Decay Time
- (1)
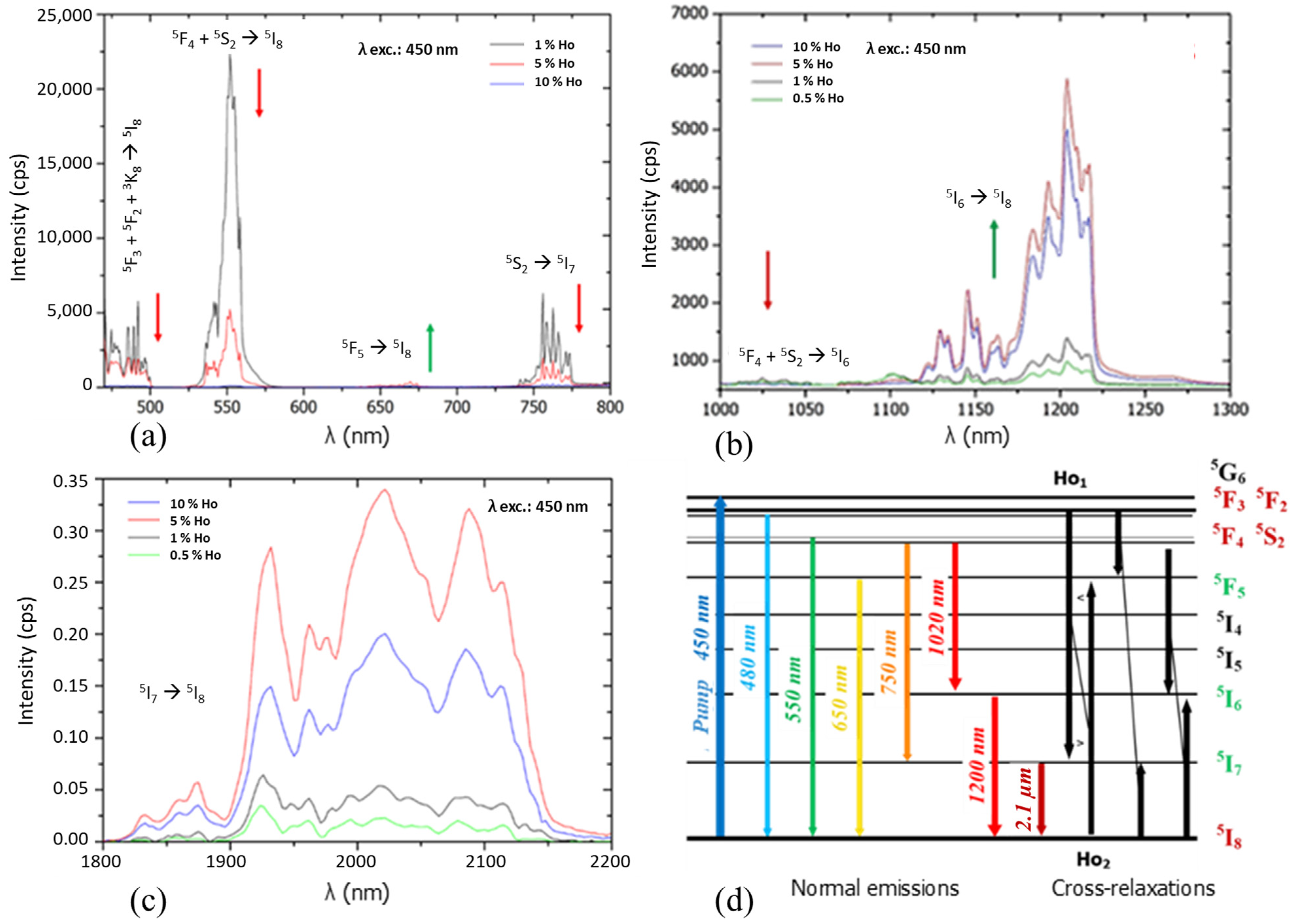
- At a low doping rate (<1%), and after excitation according to the 450 nm transition 5I8→5G6, a cascade of transitions occurs. First, 5F3 + 5F2 + 3K8→5I8 radiative transition at 480 nm happens, as well as a non-radiative 5G6→5F4 + 5S2 transition. From this electronic level, two optical emissions occur at 550 nm and 750 nm corresponding, respectively, to the 5F4 + 5S2→5I8 and 5F4 + 5S2→5I7 transitions. In a second step, the transition 5I7→5I8 at 2100 nm occurs. From level 5F4, 5S2, a second transition 5F4 + 5S2→5I6 at 1020 nm, as well as a non-radiative transition 5F4 + 5S2→5F5, is observed. Then, from the 5F5 electronic level, an emission is present at 650 nm, linked to the 5F5→5I8 transition.
- (2)
- When the doping increases, due to the increase in the volume concentration of Ho3+ ions in the matrix, Ho1-Ho2 non-radiative Cross-Relaxation interactions are increasingly favored. These interactions, which induce modification of electronic level populations, lead to the overcrowding of the 5I7 and 5F5 levels according to the 5I8→5I7 or 5F3 + 5F2→5I7 transitions of Ho1 and 5F3 + 5F2→5F5 or 5I8→5F5 of Ho2. Thus, the intensity of the 5I7→5I8 transition in the Mid-IR at 2100 nm increases at the expense of Visible transitions up to 5% in Ho3+ ions (Figure 7a,c). Finally, a last relaxation takes place and induces the overcrowding of level 5I6 according to the transitions 5F4 + 5S2→5I6 and 5I8→5I6. Hence, the transition in the Near-IR 5I6→5I8 at 1200 μm is favored (Figure 7b).
- (3)
- Above 5%, the phenomenon of fluorescence quenching by concentration is observed for all transitions [38]. For the highest concentration tested, namely 10% (2.84 × 1021 ions·cm−3), the fluorescence intensity in the Near-IR and Mid-IR is reduced by 40% compared to 5% (1.42 × 1021 ions·cm−3) (Figure 8b,c).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikesue, A.; Aung, Y.L. Ceramic laser materials. Nat. Photon. 2008, 2, 721–727. [Google Scholar] [CrossRef]
- Sanghera, J.; Bayya, S.; Villalobos, G.; Kim, W.; Frantz, J.; Shaw, B.; Sadowski, B.; Miklos, R.; Baker, C.; Hunt, M.; et al. Transparent ceramics for high-energy laser systems. Opt. Mater. 2011, 33, 511–518. [Google Scholar] [CrossRef]
- Kim, W.; Villalobos, G.; Baker, C.; Frantz, J.; Shaw, B.; Bayya, S.; Bowman, S.; Sadowski, B.; Hunt, M.; Rock, B.; et al. Overview of transparent optical ceramics for high-energy lasers at NRL. Appl. Opt. 2015, 54, F210–F221. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Z.-Z.; Song, Y.-J.; Yuan, L.; Lin, Y.-Y.; Zhang, L.-N.; Cui, D.-F.; Bo, Y.; Peng, Q.-J.; Xu, Z.-Y.; et al. High Power (~10 kW) Yb:YAG Ceramic Slab Laser Operating at 1030 nm. IEEE Photon. Technol. Lett. 2023, 35, 789–792. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhou, J.; Liu, W.B.; Li, J.; Wang, L.; Jiang, B.X.; Pan, Y.B.; Cheng, X.J.; Xu, J.Q. Fabrication, properties and laser per-formance of Ho:YAG transparent ceramic. J. Alloys Compd. 2010, 506, 745–748. [Google Scholar] [CrossRef]
- Zhang, W.X.; Pan, Y.B.; Zhou, J.; Liu, W.B.; Li, J.; Jiang, B.X.; Cheng, X.J.; Xu, J.Q. Diode-Pumped Tm:YAG Ceramic Laser. J. Am. Ceram. Soc. 2009, 92, 2434–2437. [Google Scholar] [CrossRef]
- Scholle, K.; Lamrini, S.; Koopmann, P.; Fuhrberg, P. 2 µm Laser Sources and Their Possible Applications. In Frontiers in Guided Wave Optics and Optoelectronics; Pal, B., Ed.; IntechOpen: London, UK, 2010. [Google Scholar]
- Yang, H.; Zhang, J.; Qin, X.P.; Luo, D.W.; Ma, J.; Tang, D.Y.; Zhang, Q.T. Fabrication and Properties of High Quality Transparent Ho:YAG Ceramics. Solid State Phenom. 2012, 185, 51–54. [Google Scholar] [CrossRef]
- Payne, S.A.; Chase, L.L.; Smith, L.K.; Kway, W.L.; Krupke, W.F. Infrared cross section measurements for crystals doped with Er3+, Tm3+, and Ho3+. IEEE J. Quantum Electron. 1992, 28, 2619–2630. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, R.; Mateos, X.; Li, J.; Hu, C.; Li, C.; Suomalainen, S.; Härkönen, A.; Guina, M.; Petrov, V.; et al. Broadly tunable mode-locked Ho:YAG ceramic laser around 21 µm. Opt. Express 2016, 24, 18003–18012. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, D.; Zhang, J.; Yang, H.; Tang, D.; Zhao, T.; Yang, X. In-band pumped highly efficient Ho:YAG ceramic laser with 21 W output power at 2097 nm. Opt. Lett. 2011, 36, 1575–1577. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, J.; Wang, M.; Jiang, B.; Zhang, W.; Pan, Y. Ho:YAG ceramic laser pumped by Tm:YLF lasers at room temperature. Laser Phys. Lett. 2010, 7, 351–354. [Google Scholar] [CrossRef]
- Antipov, O.L.; Novikov, A.A.; Zakharov, N.G.; Zinoviev, A.P. Optical properties and efficient laser oscillation at 2066 nm of novel Tm:Lu2O3 ceramics. Opt. Mater. Express 2012, 2, 183–189. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L.; Lupei, V. Ceramic Lasers; Cambridge University Press: Cambridge, UK, 2013; p. 165. [Google Scholar]
- Salek, G.; Devoti, A.; Garcia, A.; Gaudon, M.; Jubera, V.; Demourgues, A. Tuning the composition of rare earth sesquioxides Gd2-xLaxO3:Eu3+ to control phase transitions at a high temperature to design new highly sensitive luminescence-based thermal sensors. R. Soc. Chem. 2016, 6, 298–306. [Google Scholar] [CrossRef]
- Permin, D.; Kurashkin, S.; Novikova, A.; Savikin, A.; Gavrishchuk, E.; Balabanov, S.; Khamaletdinova, N. Synthesis and luminescence properties of Yb-doped Y2O3, Sc2O3 and Lu2O3 solid solutions nanopowders. Opt. Mater. 2018, 77, 240–245. [Google Scholar] [CrossRef]
- Tang, F.; Huang, J.; Guo, W.; Wang, W.; Fei, B.; Cao, Y. Photoluminescence and laser behavior of Yb:YAG ceramic. Opt. Mater. 2012, 34, 757–760. [Google Scholar] [CrossRef]
- Unal, F.; Kaya, F. Kazmanli, KEffects of dopant rate and calcination parameters on photoluminescence emission of Y2O3:Eu3+ phosphors: A statistical approach. Ceram. Int. 2019, 45, 17818–17825. [Google Scholar] [CrossRef]
- Malinowski, M.; Kaczkan, M.; Wnuk, A.; Szufliñska, M. Emission from the high lying excited states of Ho3+ ions in YAP and YAG crystals. J. Lumin. 2004, 106, 269–279. [Google Scholar] [CrossRef]
- Liu, Z.; Ikesue, A.; Li, J. Research progress and prospects of rare-earth doped sesquioxide laser ceramics. J. Eur. Ceram. Soc. 2021, 41, 3895–3910. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, N.; Long, H.; Hou, Z. Fabrication of fine-grained and high thermal properties Y2O3 transparent ceramics without sintering aids. Opt. Mater. 2024, 147, 114618. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, Y.; Toci, G.; Pirri, A.; Patrizi, B.; Vannini, M.; Li, J. Influence of annealing on microstructures and properties of Yb:Lu2O3 transparent ceramics. J. Am. Ceram. Soc. 2023, 107, 1974–1984. [Google Scholar] [CrossRef]
- Liu, Z.; Toci, G.; Pirri, A.; Patrizi, B.; Feng, Y.; Hu, D.; Chen, H.; Hreniak, D.; Vannini, M.; Li, J. Fabrication and characterizations of Tm:Lu2O3 transparent ceramics for 2 μm laser applications. Opt. Mater. 2022, 131, 112705. [Google Scholar] [CrossRef]
- Maksimov, R.; Shitov, V.; Osipov, V.; Samatov, O.; Vakalov, D.; Malyavin, F.; Basyrova, L.; Loiko, P.; Camy, P. Fabrication, microstructure and mid-infrared luminescence of Er:(ScxY1-x)2O3 transparent ceramics. Opt. Mater. 2023, 137, 113542. [Google Scholar] [CrossRef]
- Chaim, R.; Levin, M.; Shlayer, A.; Estournes, C. Sintering and densification of nanocrystalline ceramic oxide powders: A review. Adv. Appl. Ceram. 2008, 107, 159–169. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.-N.; Yoshida, H.; Hiraga, K.; Sakka, Y. Distribution of carbon contamination in oxide ceramics occurring during spark-plasma-sintering (SPS) processing: II Effect of SPS and loading temperatures. J. Eur. Ceram. Soc. 2018, 38, 2596–2604. [Google Scholar] [CrossRef]
- Alombert-Goget, G.; Guyot, Y.; Guzik, M.; Boulon, G.; Ito, A.; Goto, T.; Yoshikawa, A.; Kikuchi, M. Nd3+-doped Lu2O3 transparent sesquioxide ceramics elaborated by the Spark Plasma Sintering (SPS) method. Part 1: Structural, thermal conductivity and spectroscopic characterization. Opt. Mater. 2015, 41, 3–11. [Google Scholar] [CrossRef]
- Boulesteix, R.; Epherre, R.; Noyau, S.; Vandenhende, M.; Maître, A.; Sallé, C.; Alombert-Goget, G.; Guyot, Y.; Brenier, A. Highly transparent Nd:Lu2O3 ceramics obtained by coupling slip-casting and spark plasma sintering. Scr. Mater. 2014, 75, 54–57. [Google Scholar] [CrossRef]
- Lupei, V.; Lupei, A.; Boulon, G.; Jouini, A.; Ikesue, A. Assessment of the distribution of the Yb3+ ions in Sc2O3 ceramics from cooperative absorption and emission. J. Alloys Compd. 2008, 451, 179–181. [Google Scholar] [CrossRef]
- Liu, Z.; Toci, G.; Pirri, A.; Patrizi, B.; Li, J.; Hu, Z.; Wei, J.; Pan, H.; Xie, T.; Vannini, M.; et al. Fabrication and laser operation of Yb:Lu2O3 transparent ceramics from co-precipitated nano-powders. J. Am. Ceram. Soc. 2019, 102, 7491–7499. [Google Scholar] [CrossRef]
- Thoř, T.; Rubešová, K.; Jakeš, V.; Mikolášová, D.; Cajzl, J.; Havlíček, J.; Nádherný, L.; Průša, F.; Kučerková, R.; Nikl, M. Dense ceramics of lanthanide-doped Lu2O3 prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2021, 41, 741–751. [Google Scholar] [CrossRef]
- Viers, L.; Delaunay, F.; Boulesteix, R.; Vandenhende, M.; Antou, G.; Maître, A. Study of densification mechanisms during Spark Plasma Sintering of co-precipitated Ho:Lu2O3 nanopowders: Application to transparent ceramics for lasers. J. Eur. Ceram. Soc. 2021, 41, 7199–7207. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Akchurin, M.S.; Becker, P.; Ueda, K.; Bohatý, L.; Shirakawa, A.; Tokurakawa, M.; Takaichi, K.; Yagi, H.; Dong, J. Mechanical and optical properties of Lu2O3 host-ceramics for Ln3+ lasants. Laser Phys. Lett. 2008, 5, 300–303. [Google Scholar] [CrossRef]
- Boulesteix, R.; Maître, A.; Baumard, J.-F.; Rabinovitch, Y.; Reynaud, F. Reynaud, Light scattering by pores in transparent Nd:YAG ceramics for lasers: Correlations between microstructure and optical properties. Opt. Express 2010, 18, 14992–15002. [Google Scholar] [CrossRef]
- Brown, D.C.; Envid, V.; Zembek, J. Ho:YAG absorption cross sections from 1700 to 2200 nm at 83, 175, and 295 K. Appl. Opt. 2012, 51, 8147–8158. [Google Scholar] [CrossRef]
- Silver, J.; Barrett, E.; Marsh, P.J.; Withnall, R. Yttrium Oxide Upconverting Phosphors. 5. Upconversion Luminescent Emission from Holmium-Doped Yttrium Oxide under 632.8 nm Light Excitation. J. Phys. Chem. B 2003, 107, 9236–9242. [Google Scholar] [CrossRef]
- Capobianco, J.A.; Boyer, J.C.; Vetrone, F.; Speghini, A.; Bettinelli, M. Optical Spectroscopy and Upconversion Studies of Ho3+-Doped Bulk and Nanocrystalline Y2O3. Chem. Mater. 2002, 14, 2915–2921. [Google Scholar] [CrossRef]
- Auzel, F.; Baldacchini, G.; Laversenne, L.; Boulon, G. Radiation trapping and self-quenching analysis in Yb3+, Er3+, and Ho3+ doped Y2O3. Opt. Mater. 2003, 24, 103–109. [Google Scholar] [CrossRef]
- Kim, W.; Baker, C.; Bowman, S.; Florea, C.; Villalobos, G.; Shaw, B.; Sadowski, B.; Hunt, M.; Aggarwal, I.; Sanghera, J. Laser oscillation from Ho3+ doped Lu2O3 ceramics. Opt. Mater. Express 2013, 3, 913–919. [Google Scholar] [CrossRef]
- Kijko, V.; Maksimov, R.N.; Shitov, V.; Demakov, S. Yurovskikh, ASintering of transparent Yb-doped Lu2O3 ceramics using nanopowder produced by laser ablation method. J. Alloys Compd. 2015, 643, 207–211. [Google Scholar] [CrossRef]
- Toci, G.; Vannini, M.; Ciofini, M.; Lapucci, A.; Pirri, A.; Ito, A.; Goto, T.; Yoshikawa, A.; Ikesue, A.; Alombert-Goget, G.; et al. Nd3+-doped Lu2O3 transparent sesquioxide ceramics elaborated by the Spark Plasma Sintering (SPS) method. Part 2: First laser output results and comparison with Nd3+-doped Lu2O3 and Nd3+-Y2O3 ceramics elaborated by a conventional method. Opt. Mater. 2014, 41, 12–16. [Google Scholar] [CrossRef]
- Xu, C.; Yang, C.; Zhang, H.; Duan, Y.; Zhu, H.; Tang, D.; Huang, H.; Zhang, J. Efficient laser operation based on transparent Nd:Lu2O3 ceramic fabricated by Spark Plasma Sintering. Opt. Express 2016, 24, 20571–20579. [Google Scholar] [CrossRef] [PubMed]
- Boulon, G.; Epicier, T.; Zhao, W.; Guzik, M.; Pan, Y.; Jiang, B. Is There Segregation of Rare Earth Ions in Garnet Optical Ceramics? Bartolo, B.D., Collins, J., Baldassare, D.B., John, C., Eds.; Nano-Optics for enhancing light-matter interactions on a molecular scale. NATO Science for Space and Security Series B: Physics and Biophysics; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Dong, J.; Wang, W.; Xue, Y.; Hou, W.; Cao, X.; Xu, X.; Wu, F.; Luo, P.; Wang, Q.; Li, D.; et al. Crystal growth and spectroscopic analysis of Ho:Lu2O3 crystal for mid-infrared emission. J. Lumin. 2022, 251, 119192. [Google Scholar] [CrossRef]
- Koopmann, P.; Lamrini, S.; Scholle, K.; Schäfer, M.; Fuhrberg, P.; Huber, G. Multi-watt laser operation and laser parameters of Ho-doped Lu2O3 at 2.12 μm. Opt. Mater. Express 2011, 1, 1447–1456. [Google Scholar] [CrossRef]

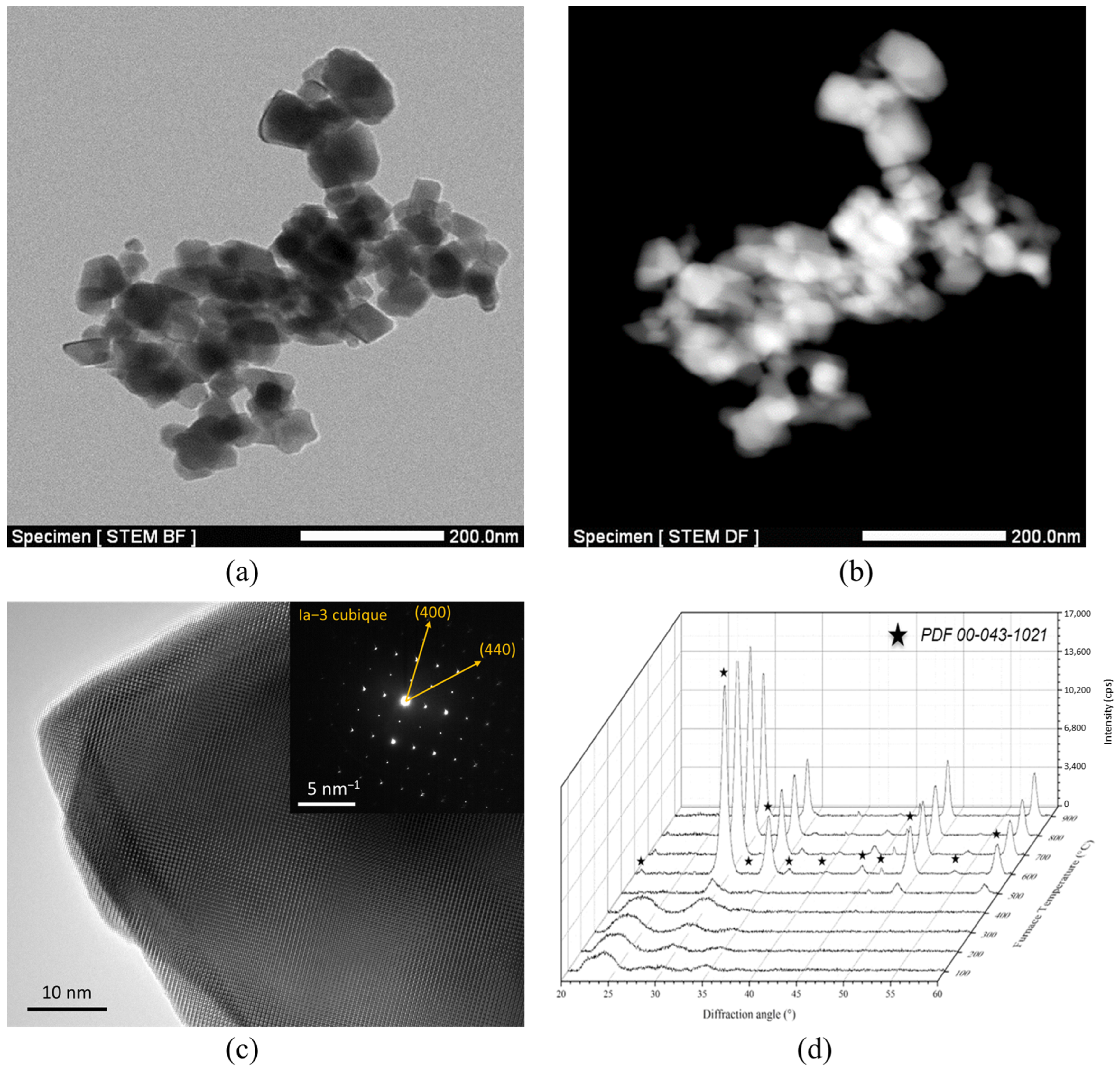

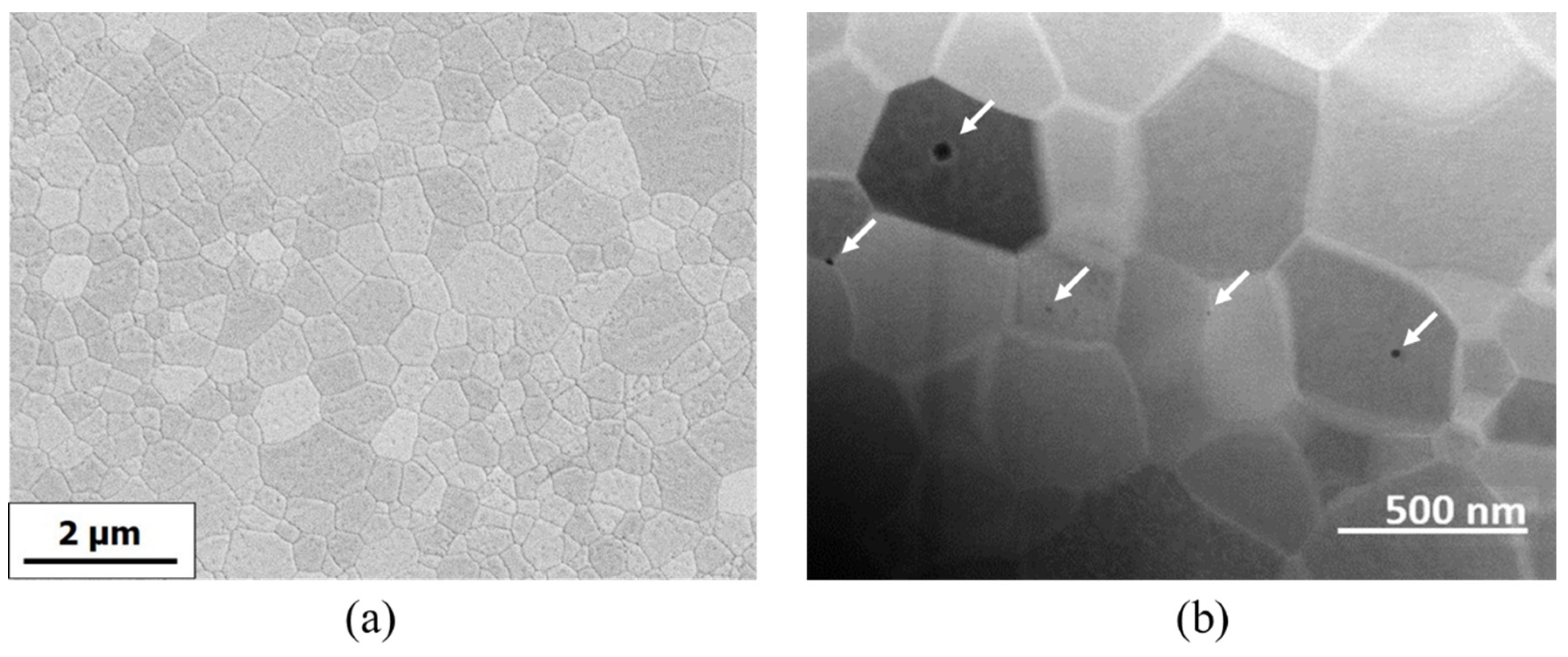
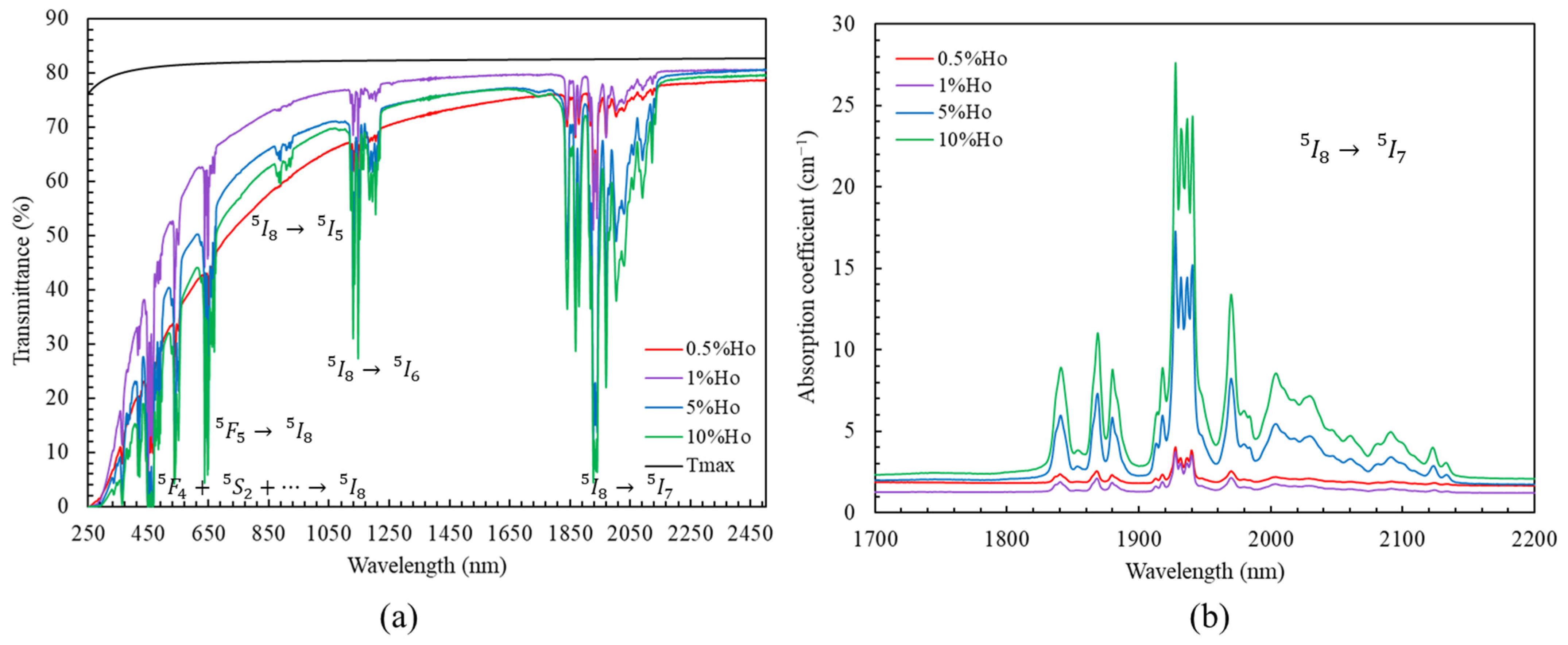
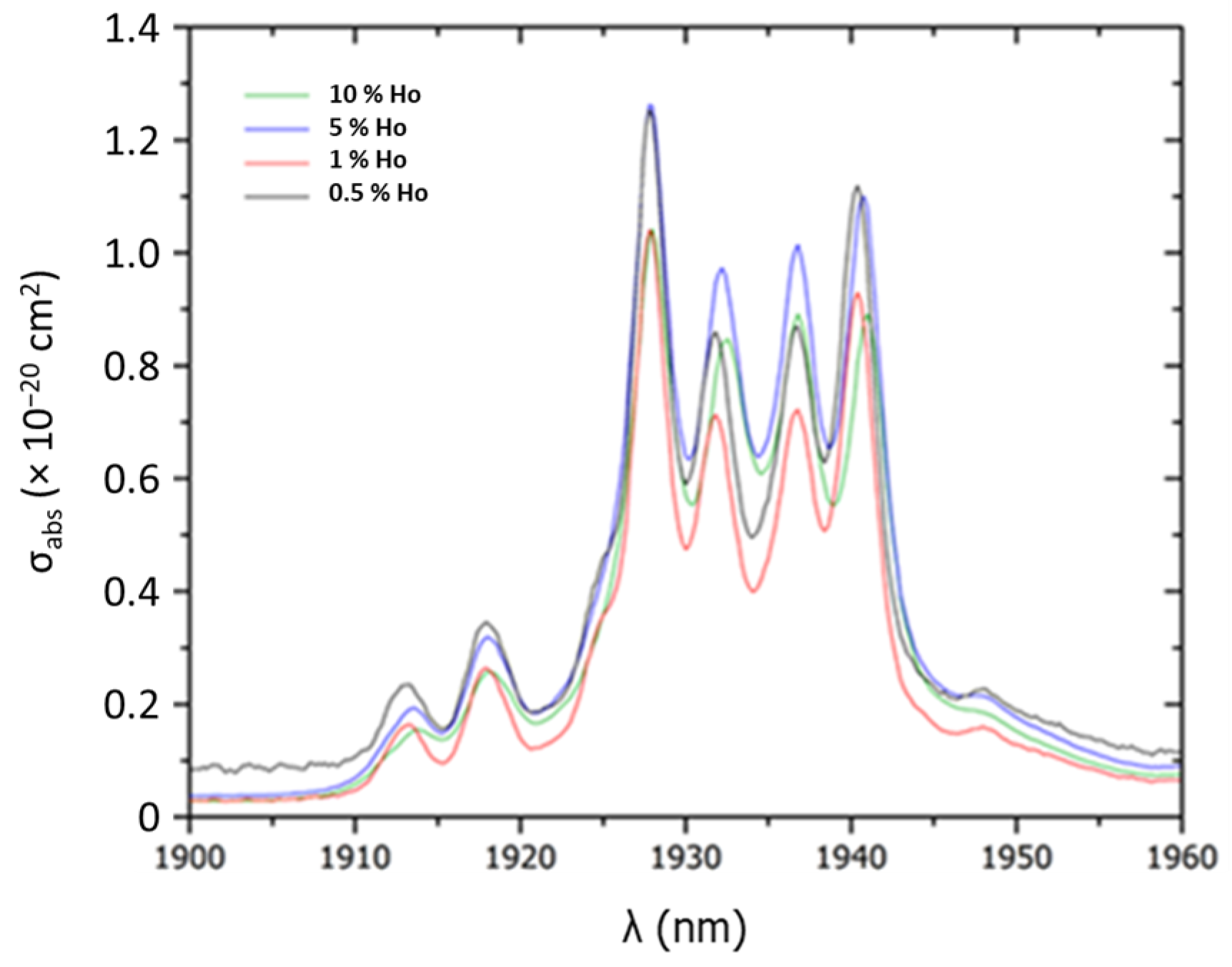


| %at. Ho | 0.5% | 1% | 5% | 10% |
|---|---|---|---|---|
| CHo (cm−3) | 1.43 × 1020 | 2.85 × 1020 | 1.42 × 1021 | 2.84 × 1021 |
| Absorption cross section at 1927 nm (cm2) | 1.26 × 10−20 | 1.03 × 10−20 | 1.26 × 10−20 | 1.03 × 10−20 |
| Fluorescence lifetime at 2030 nm (ms) | - | 11.5 | - | 4.92 |
| Reference | Composition | Material | Sintering | Gmean (µm) | T% at 2 µm | λabs (nm) | σabs (cm2) | λem (nm) | σem (cm2) | τrad (ms) |
|---|---|---|---|---|---|---|---|---|---|---|
| This work | 1%Ho:Lu2O3 | Ceramic | SPS + air annealing | <1 µm | 80% | 1927 | 1 × 10−20 | 2030 | 11.5 | |
| Kim et al. [39] | 2%Ho:Lu2O3 | Ceramic | Post-HIP + air annealing | 50 µm | >80% | 1942 | - | 2140 | - | 10 |
| Dong et al. [44] | 1%Ho:Lu2O3 | Single-crystal | - | - | >80% | 1929 | 1.1 × 10−20 | 2027 | 3.3 × 10−21 | 9.5 |
| Koopmann et al. [45] | 0.3%Ho:Lu2O3 | Single-crystal | - | - | >80% | 1928 | 1.2 × 10−20 | 2124 | 4.5 × 10−21 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viers, L.; Guené-Girard, S.; Dalla-Barba, G.; Jubéra, V.; Cormier, É.; Boulesteix, R.; Maître, A. Optical and Spectroscopic Properties of Ho:Lu2O3 Transparent Ceramics Elaborated by Spark Plasma Sintering. Ceramics 2024, 7, 208-221. https://doi.org/10.3390/ceramics7010013
Viers L, Guené-Girard S, Dalla-Barba G, Jubéra V, Cormier É, Boulesteix R, Maître A. Optical and Spectroscopic Properties of Ho:Lu2O3 Transparent Ceramics Elaborated by Spark Plasma Sintering. Ceramics. 2024; 7(1):208-221. https://doi.org/10.3390/ceramics7010013
Chicago/Turabian StyleViers, Lucas, Simon Guené-Girard, Gilles Dalla-Barba, Véronique Jubéra, Éric Cormier, Rémy Boulesteix, and Alexandre Maître. 2024. "Optical and Spectroscopic Properties of Ho:Lu2O3 Transparent Ceramics Elaborated by Spark Plasma Sintering" Ceramics 7, no. 1: 208-221. https://doi.org/10.3390/ceramics7010013







