Mussel-Inspired Construction of Silica-Decorated Ceramic Membranes for Oil–Water Separation
Abstract
:1. Introduction
2. Experiment
2.1. Materials
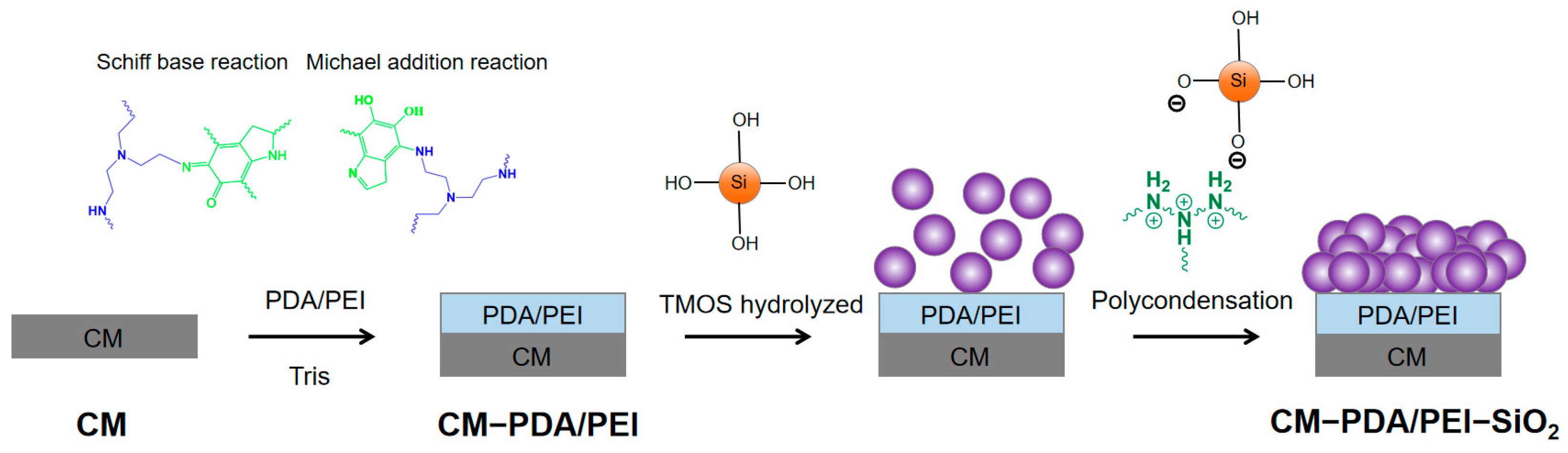
2.2. Preparation of Modified Ceramic Membranes
2.3. Characterization of the Membrane
2.4. Membrane Separation Performance
3. Results and Discussion
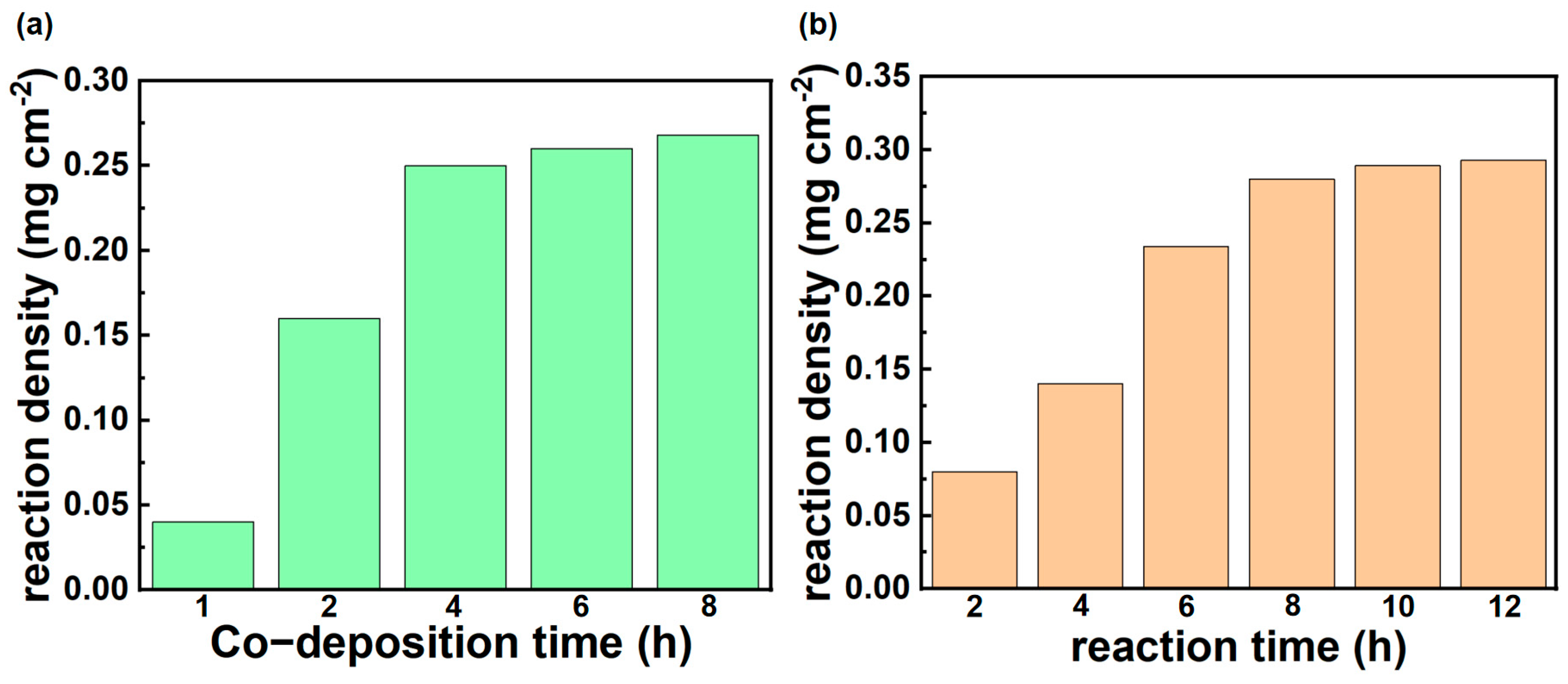
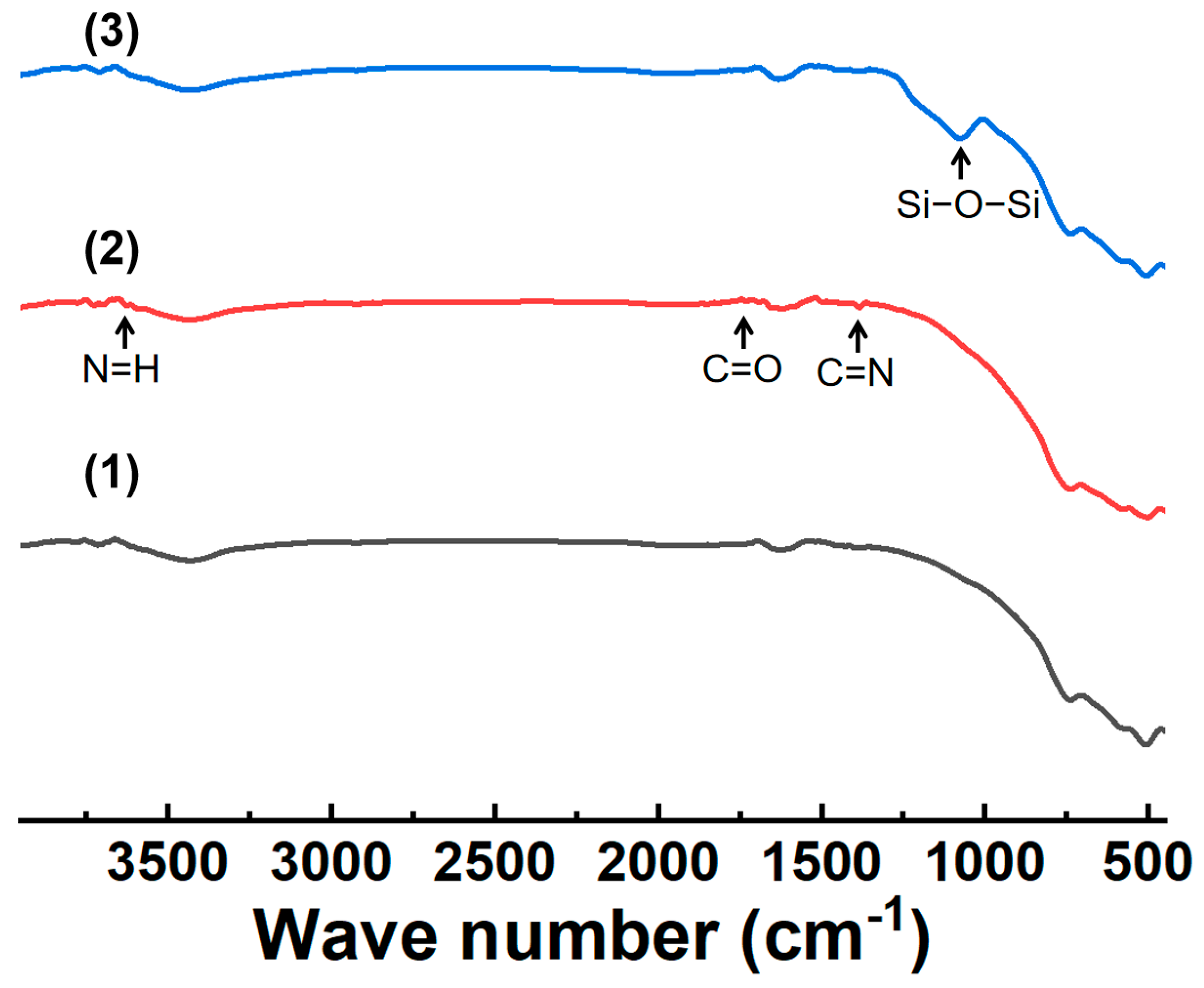
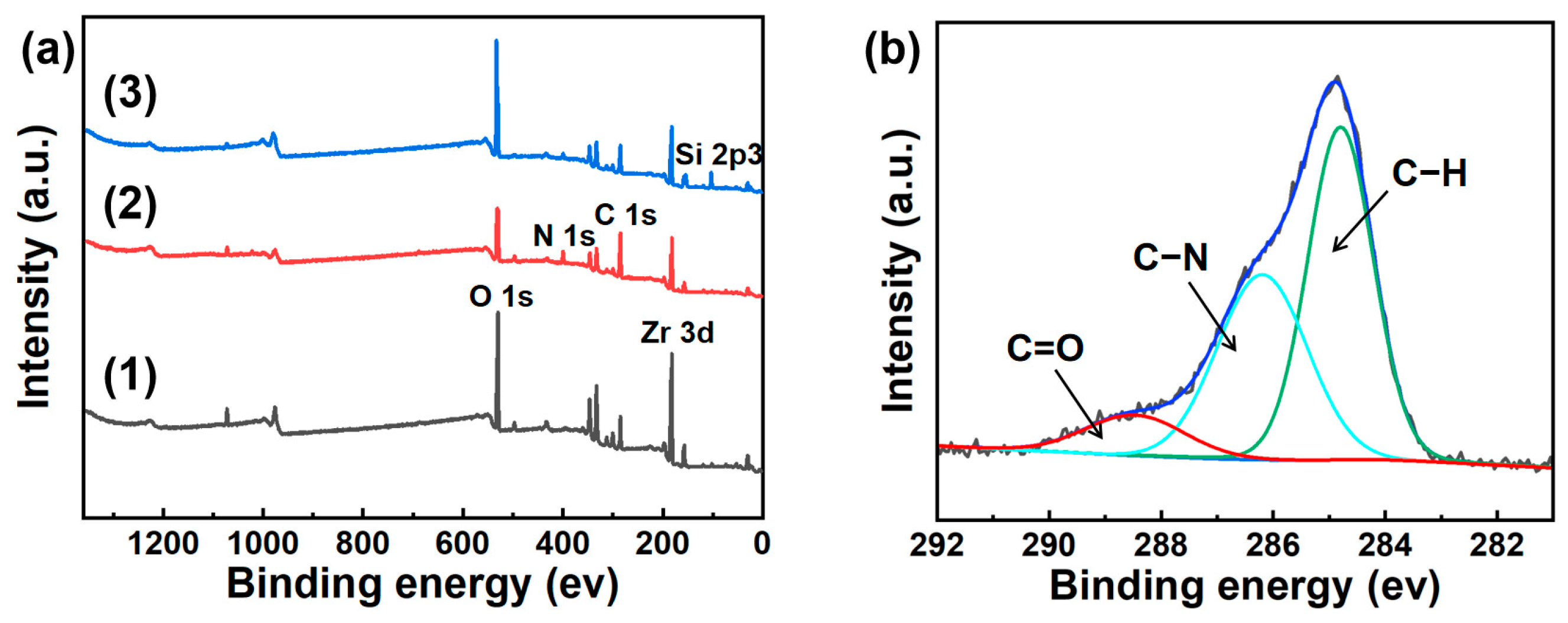
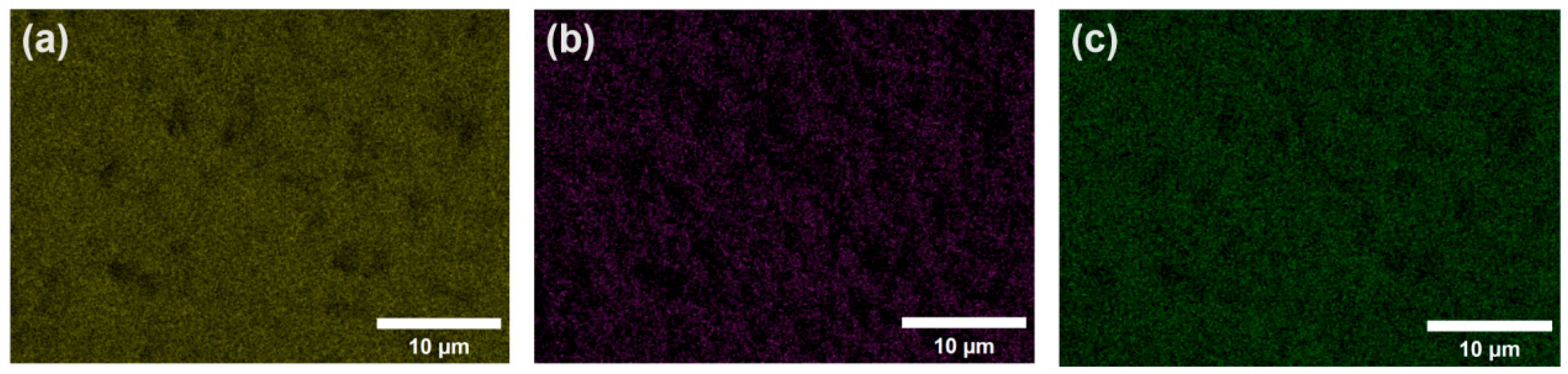
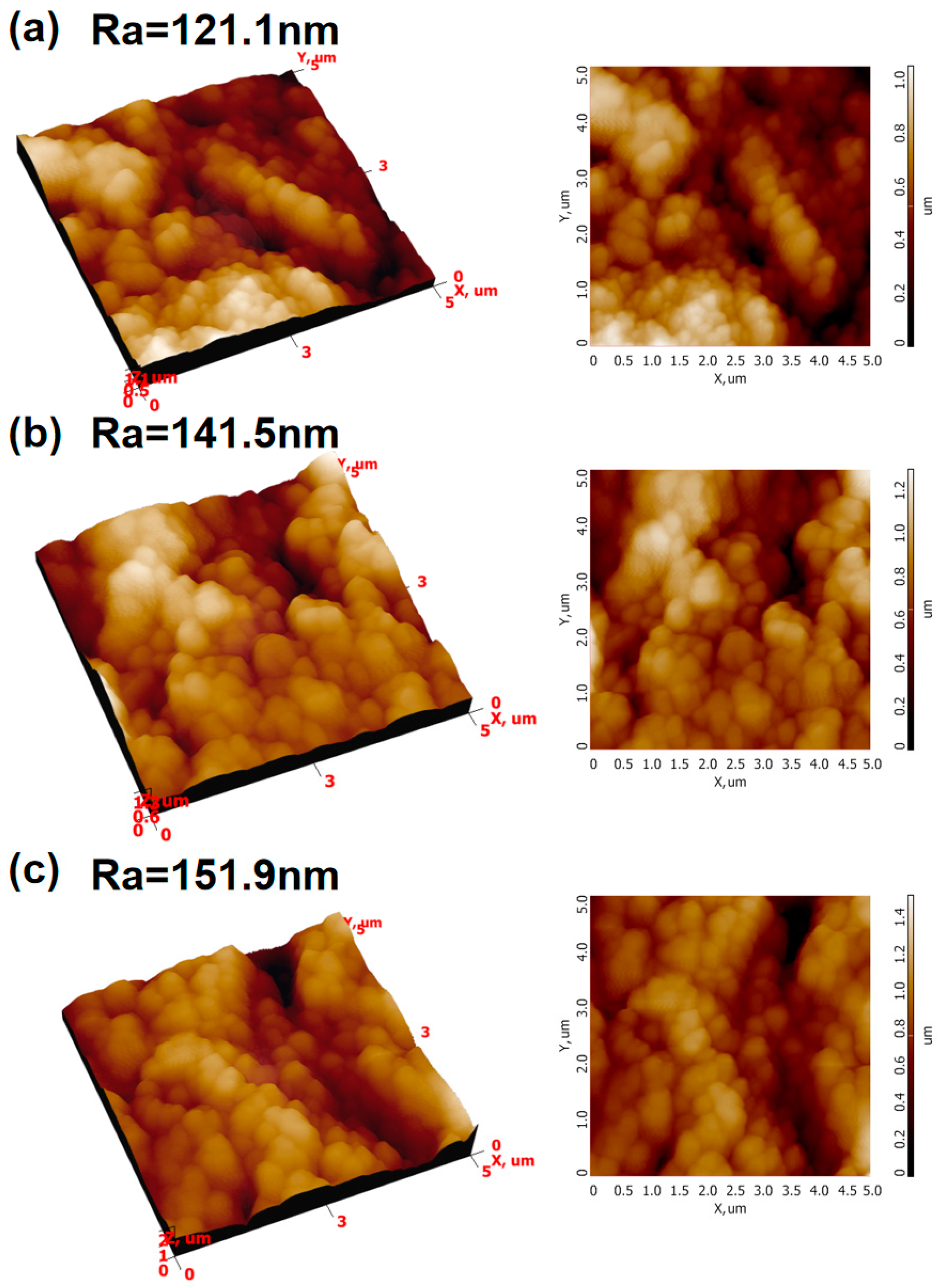
3.1. Microstructure and Chemical Composition of Ceramic Membrane Surface
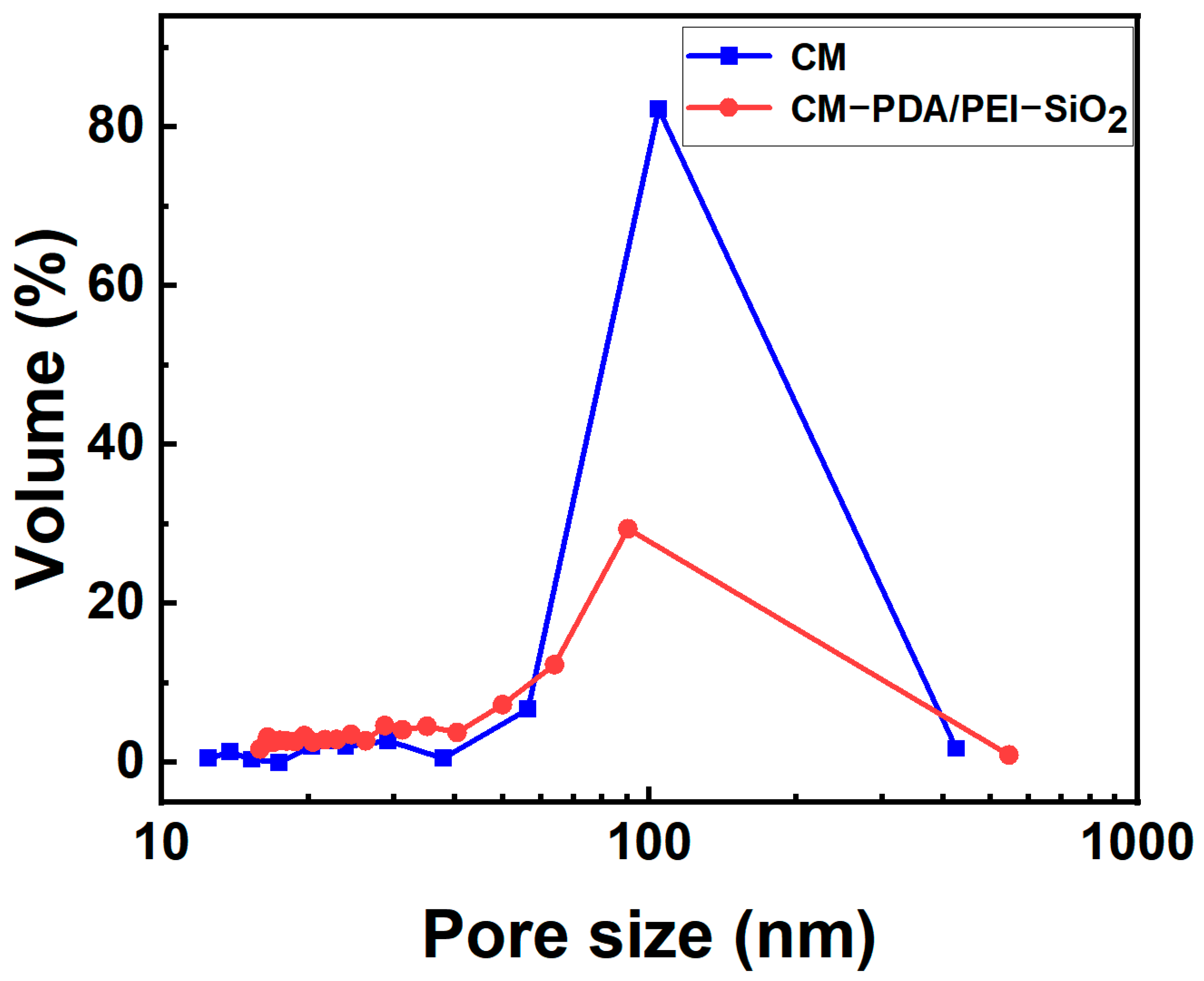
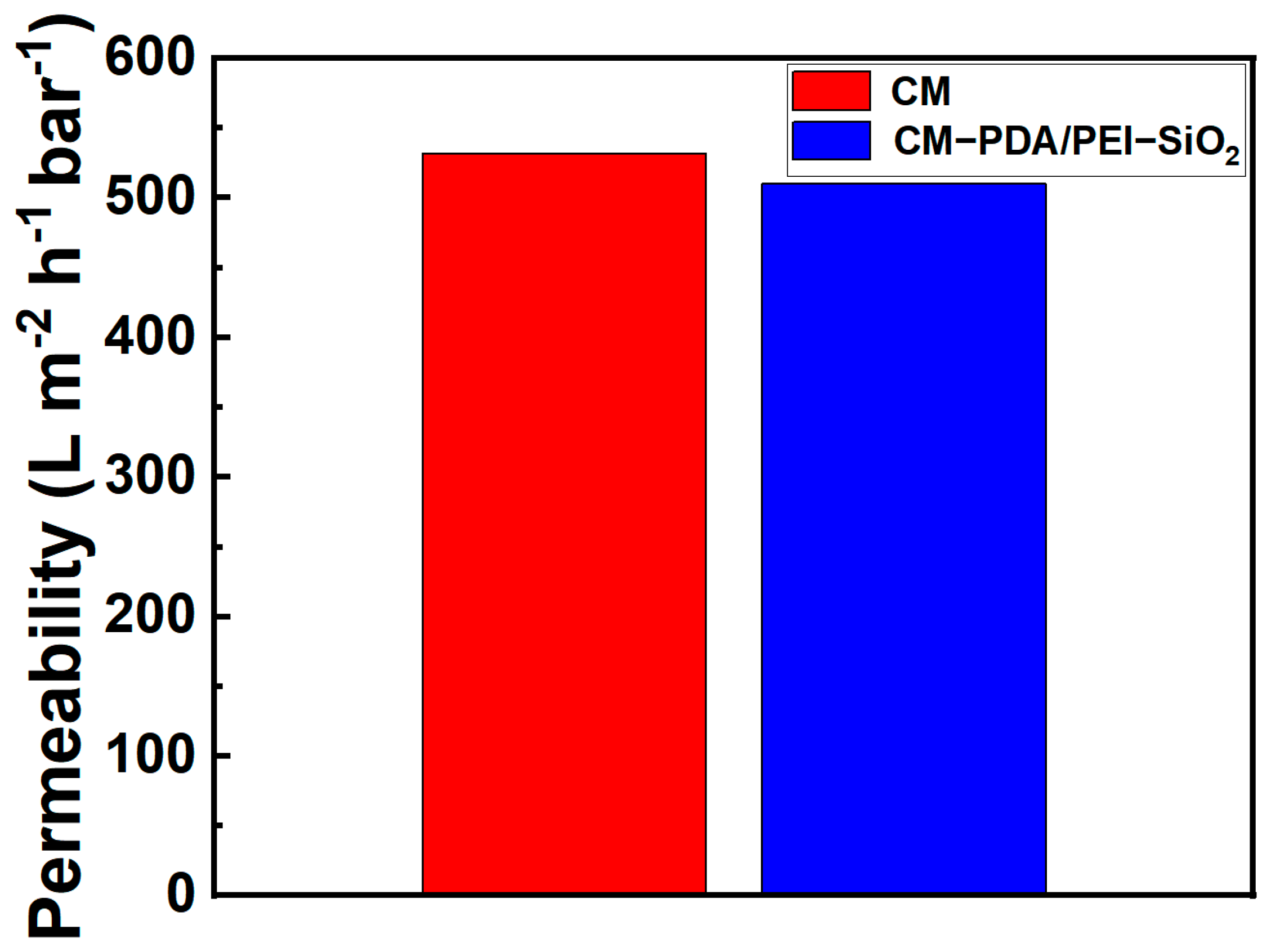
3.2. Contamination Resistance of Ceramic Membrane Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shukla, A.K.; Alam, J.; Mishra, U.; Kesari, K.K. Investigating the efficiency of a ceramic-based thin-film composite nanofiltration membrane for dyes removal. Ceram. Int. 2023, 49, 37670–37679. [Google Scholar] [CrossRef]
- Li, C.; Li, S.; Zhang, J.; Yang, C.; Su, B.; Han, L.; Gao, X. Emerging sandwich-like reverse osmosis membrane with interfacial assembled covalent organic frameworks interlayer for highly-efficient desalination. J. Membr. Sci. 2020, 604, 118065. [Google Scholar] [CrossRef]
- Ding, J.; Mao, Z.; Chen, H.; Zhang, X.; Fu, H. Fabrication of ZnO/PDA/GO composite membrane for high efficiency oil–water separation. Appl. Phys. A 2023, 129, 369. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Kurnia, K.A.; Siagian, U.W.R.; Ismadji, S.; Wenten, I.G. Membrane fouling and fouling mitigation in oil–water separation: A review. J. Environ. Chem. Eng. 2022, 10, 107532. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z. Biomimetic materials in oil/water separation: Focusing on switchable wettabilities and applications. Adv. Colloid Interface Sci. 2023, 320, 103003. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Z.; Qiao, C.; Yang, W.; Huang, Y.; McKay, P.; Yang, D.; Liu, Q.; Zeng, H. Techniques for treating slop oil in oil and gas industry: A short review. Fuel 2020, 279, 118482. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, W.; Zhang, F.; Liu, X.; Wang, D.; Jin, J.; Jiang, L. Ultrafast separation of emulsified oil/water mixtures by ultrathin free-standing single-walled carbon nanotube network films. Adv. Mater. 2013, 25, 2422–2427. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Mu, S.; Jiang, C.; Song, M.; Fang, Q.; Xue, M.; Qiu, S.; Chen, B. UiO-66-Coated Mesh Membrane with Underwater Superoleophobicity for High-Efficiency Oil-Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 17301–17308. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, K.; Xu, Z.K.; Xiong, Y.; Liu, J.; Cai, C.; Huang, X. A comprehensive review on the behavior and evolution of oil droplets during oil/water separation by membranes. Adv. Colloid Interface Sci. 2023, 319, 102971. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Park, C.B. In situ oils/organic solvents cleanup and recovery using advanced oil-water separation system. Chemosphere 2020, 260, 127586. [Google Scholar] [CrossRef] [PubMed]
- Križan Milić, J.; Murić, A.; Petrinić, I.; Simonič, M. Recent Developments in Membrane Treatment of Spent Cutting-Oils: A Review. Ind. Eng. Chem. Res. 2013, 52, 7603–7616. [Google Scholar] [CrossRef]
- Roy, B.; Dey, S.; Sahoo, G.C.; Roy, S.N.; Bandyopadhyay, S. Degumming, Dewaxing and Deacidification of Rice Bran Oil-Hexane Miscella Using Ceramic Membrane: Pilot Plant Study. J. Am. Oil Chem. Soc. 2014, 91, 1453–1460. [Google Scholar] [CrossRef]
- Jia, H.; Feng, F.; Wang, J.; Ngo, H.-H.; Guo, W.; Zhang, H. On line monitoring local fouling behavior of membrane filtration process by in situ hydrodynamic and electrical measurements. J. Membr. Sci. 2019, 589, 117245. [Google Scholar] [CrossRef]
- Maddela, N.R.; Torres, R.O.V. The presence of low fouling-causing bacteria can lead to decreased membrane fouling potentials of mixed cultures. J. Environ. Chem. Eng. 2021, 9, 105131. [Google Scholar] [CrossRef]
- Marzouk, S.S.; Naddeo, V.; Banat, F.; Hasan, S.W. Preparation of TiO2/SiO2 ceramic membranes via dip coating for the treatment of produced water. Chemosphere 2021, 273, 129684. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Wang, Q.; Li, G. Further modification of oil–water separation membrane based on chitosan and titanium dioxide. J. Mater. Sci. Mater. Electron. 2021, 32, 4823–4832. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, J.-E.; Wang, Y.; Liang, J.; Zhang, X.; Cerneaux, S.; Wang, X.; Zhu, Z.; Dong, Y. Application of ceramic microfiltration membrane modified by nano-TiO2 coating in separation of a stable oil-in-water emulsion. J. Membr. Sci. 2014, 456, 128–133. [Google Scholar] [CrossRef]
- Meng, T.; Xie, R.; Ju, X.-J.; Cheng, C.-J.; Wang, S.; Li, P.-F.; Liang, B.; Chu, L.-Y. Nano-structure construction of porous membranes by depositing nanoparticles for enhanced surface wettability. J. Membr. Sci. 2013, 427, 63–72. [Google Scholar] [CrossRef]
- Suresh, K.; Srinu, T.; Ghoshal, A.K.; Pugazhenthi, G. Preparation and characterization of TiO2 and γ-Al2O3 composite membranes for the separation of oil-in-water emulsions. RSC Adv. 2016, 6, 4877–4888. [Google Scholar] [CrossRef]
- Raji, Y.O.; Othman, M.H.D.; Sapiaa Md Nordin, N.A.H.; Adam, M.R.; Mohd Said, K.A.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Farag, T.M.; Alftessi, S.A. Synthesis and characterization of superoleophobic fumed alumina nanocomposite coated via the sol-gel process onto ceramic-based hollow fibre membrane for oil-water separation. Ceram. Int. 2021, 47, 25883–25894. [Google Scholar] [CrossRef]
- Usman, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Raji, Y.O.; Gbadamosi, A.O.; El Badawy, T.H.; Said, K.A.M. An overview of superhydrophobic ceramic membrane surface modification for oil-water separation. J. Mater. Res. Technol. 2021, 12, 643–667. [Google Scholar] [CrossRef]
- Barati, N.; Husein, M.M.; Azaiez, J. Modifying ceramic membranes with in situ grown iron oxide nanoparticles and their use for oily water treatment. J. Membr. Sci. 2021, 617, 118641. [Google Scholar] [CrossRef]
- Kaur, H.; Bulasara, V.K.; Gupta, R.K. Influence of pH and temperature of dip-coating solution on the properties of cellulose acetate-ceramic composite membrane for ultrafiltration. Carbohydr. Polym. 2018, 195, 613–621. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, L.; Wang, X.; Huo, H.; Lin, H.; Wang, Q.; Yang, X.; Vogel, F.; Li, W.; Lin, Z.; et al. MOF@Polydopamine-incorporated membrane with high permeability and mechanical property for efficient fouling-resistant and oil/water separation. Environ. Res. 2023, 236 Pt 2, 116685. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, W.; Zhang, M.; Zhang, Q.; Xiong, R.; Huang, C. Fabrication of superhydrophobic electrospun polyimide nanofibers modified with polydopamine and polytetrafluoroethylene nanoparticles for oil–water separation. J. Appl. Polym. Sci. 2019, 136, 47638. [Google Scholar] [CrossRef]
- Gao, N.; Wang, L.; Zhang, Y.; Liang, F.; Fan, Y. Modified ceramic membrane with pH/ethanol induced switchable superwettability for antifouling separation of oil-in-acidic water emulsions. Sep. Purif. Technol. 2022, 293, 121022. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Zhuang, J.; Shi, H. An intelligent natural fibrous membrane anchored with ZnO for switchable oil/water separation and water purification. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 128041. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.; Malde, C.; Wang, R. Superoleophobic surface modification for robust membrane distillation performance. J. Membr. Sci. 2017, 541, 162–173. [Google Scholar] [CrossRef]
- Tian, Y.; Cao, Y.; Wang, Y.; Yang, W.; Feng, J. Realizing ultrahigh modulus and high strength of macroscopic graphene oxide papers through crosslinking of mussel-inspired polymers. Adv. Mater. 2013, 25, 2980–2983. [Google Scholar] [CrossRef]
- Yang, H.-C.; Liao, K.-J.; Huang, H.; Wu, Q.-Y.; Wan, L.-S.; Xu, Z.-K. Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation. J. Mater. Chem. A 2014, 2, 10225–10230. [Google Scholar] [CrossRef]
- Luan, W.; Nie, C.; Chen, X.; Tang, Z.; Qiu, M.; Fan, Y. Effective construction of anti-fouling zwitterion-functionalized ceramic membranes for separation of oil-in-water emulsion based on PDA/PEI co-deposition. J. Environ. Chem. Eng. 2022, 10, 108396. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, X.; Da, X.; Qiu, M.; Chen, X.; Fan, Y. Fabrication of a charged PDA/PEI/Al2O3 composite nanofiltration membrane for desalination at high temperatures. Sep. Purif. Technol. 2021, 263, 118388. [Google Scholar] [CrossRef]
- Song, H.-M.; Chen, C.; Shui, X.-X.; Yang, H.; Zhu, L.-J.; Zeng, Z.-X.; Xue, Q.-J. Asymmetric Janus membranes based on in situ mussel-inspired chemistry for efficient oil/water separation. J. Membr. Sci. 2019, 573, 126–134. [Google Scholar] [CrossRef]
- Naik, R.R.; Whitlock, P.W.; Rodriguez, F.; Brott, L.L.; Glawe, D.D.; Clarson, S.J.; Stone, M.O. Controlled formation of biosilica structures in vitro. Chem. Commun. 2003, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Pi, J.K.; Liao, K.J.; Huang, H.; Wu, Q.Y.; Huang, X.J.; Xu, Z.K. Silica-decorated polypropylene microfiltration membranes with a mussel-inspired intermediate layer for oil-in-water emulsion separation. ACS Appl. Mater. Interfaces 2014, 6, 12566–12572. [Google Scholar] [CrossRef]
- Gao, N.; Fan, W.; Xu, Z.-K. Ceramic membrane with protein-resistant surface via dopamine/diglycolamine co-deposition. Sep. Purif. Technol. 2020, 234, 116135. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Y.; Liu, H.; Wang, Q.; Wang, Y.; Zhou, J.-E.; Chang, Q. Preparation of disc ceramic membrane by a printing and dip-coating method for oil-water separation. Sep. Purif. Technol. 2023, 315, 123552. [Google Scholar] [CrossRef]
- Qu, Y.; Qin, L.; Guo, M.; Liu, X.; Yang, Y. Multilayered molecularly imprinted composite membrane based on porous carbon nanospheres/pDA cooperative structure for selective adsorption and separation of phenol. Sep. Purif. Technol. 2022, 280, 119915. [Google Scholar] [CrossRef]
- Liang, Q.; Jiang, B.; Yang, N.; Zhang, L.; Sun, Y.; Zhang, L. Superhydrophilic Modification of Polyvinylidene Fluoride Membrane via a Highly Compatible Covalent Organic Framework-COOH/Dopamine-Integrated Hierarchical Assembly Strategy for Oil-Water Separation. ACS Appl. Mater. Interfaces 2022, 14, 45880–45892. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Omura, T.; Asai, T.; Kanezashi, M.; Tsuru, T. Filtration of surfactant-stabilized oil-in-water emulsions with porous ceramic membranes: Effects of membrane pore size and surface charge on fouling behavior. J. Membr. Sci. 2020, 610, 118210. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Chang, Q.; Lu, Y.; Sun, J. Mussel-Inspired Construction of Silica-Decorated Ceramic Membranes for Oil–Water Separation. Ceramics 2024, 7, 250-263. https://doi.org/10.3390/ceramics7010016
Zhou Q, Chang Q, Lu Y, Sun J. Mussel-Inspired Construction of Silica-Decorated Ceramic Membranes for Oil–Water Separation. Ceramics. 2024; 7(1):250-263. https://doi.org/10.3390/ceramics7010016
Chicago/Turabian StyleZhou, Qibo, Qibing Chang, Yao Lu, and Jing Sun. 2024. "Mussel-Inspired Construction of Silica-Decorated Ceramic Membranes for Oil–Water Separation" Ceramics 7, no. 1: 250-263. https://doi.org/10.3390/ceramics7010016
APA StyleZhou, Q., Chang, Q., Lu, Y., & Sun, J. (2024). Mussel-Inspired Construction of Silica-Decorated Ceramic Membranes for Oil–Water Separation. Ceramics, 7(1), 250-263. https://doi.org/10.3390/ceramics7010016