Short-Interval, High-Severity Wildfire Depletes Diversity of Both Extant Vegetation and Soil Seed Banks in Fire-Tolerant Eucalypt Forests
Abstract
:1. Introduction
- Does the soil seed store respond to fire-related germination cues, and are these cues related to fire history and other plant traits? We hypothesize that the soil seed store will demonstrate a positive response to fire-related germination cues and that these cues will be more prevalent in areas more frequently burnt by fire. We expect seeders, ant-dispersed species, and short-lived species to demonstrate stronger responses to fire-related germination cues than other traits.
- Do the soil seed bank and extant vegetation demonstrate similar responses to fire frequency? We expect a decline in the soil seed bank with more frequent fires and a reduction in the capacity of the soil seed bank to buffer species losses in extant vegetation with an increasing fire frequency. For both the soil seed bank and extant vegetation, we hypothesize that an increase in fire frequency will benefit species with fast reproductive maturity but filter out longer-lived woody species with a greater age to reproductive maturity.
2. Materials and Methods
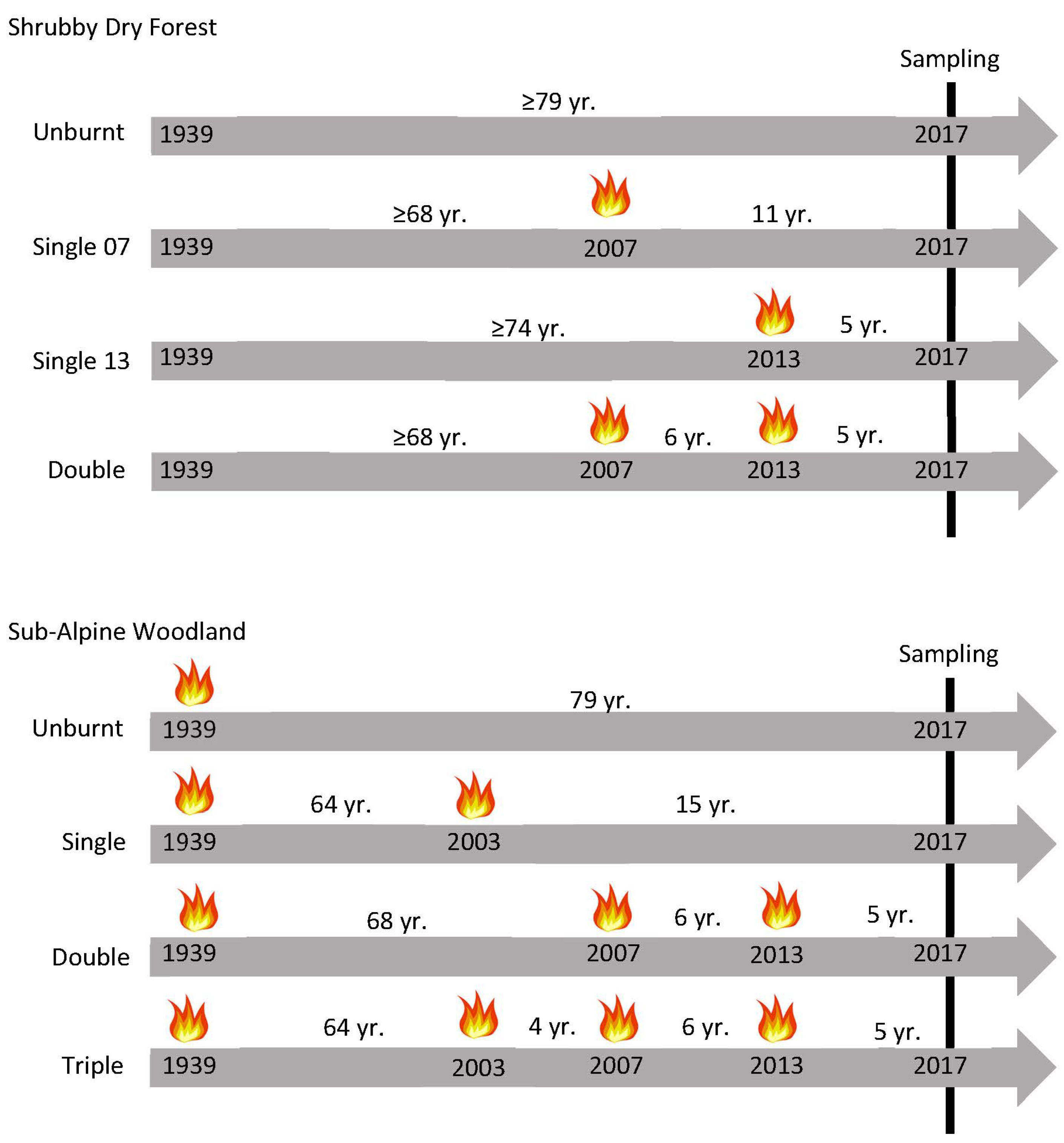
2.1. Glenmaggie Study Area and Site Selection
2.2. Mount Hotham Study Area and Site Selection
2.3. Vegetation Survey and Treatment
2.4. Soil Seed Bank Treatment
2.5. Functional Types
2.6. Data Screening
2.7. Statistical Analysis—Soil Seed Bank Treatment and Fire Frequency
2.8. Statistical Analysis—Vegetation Type and Fire Frequency
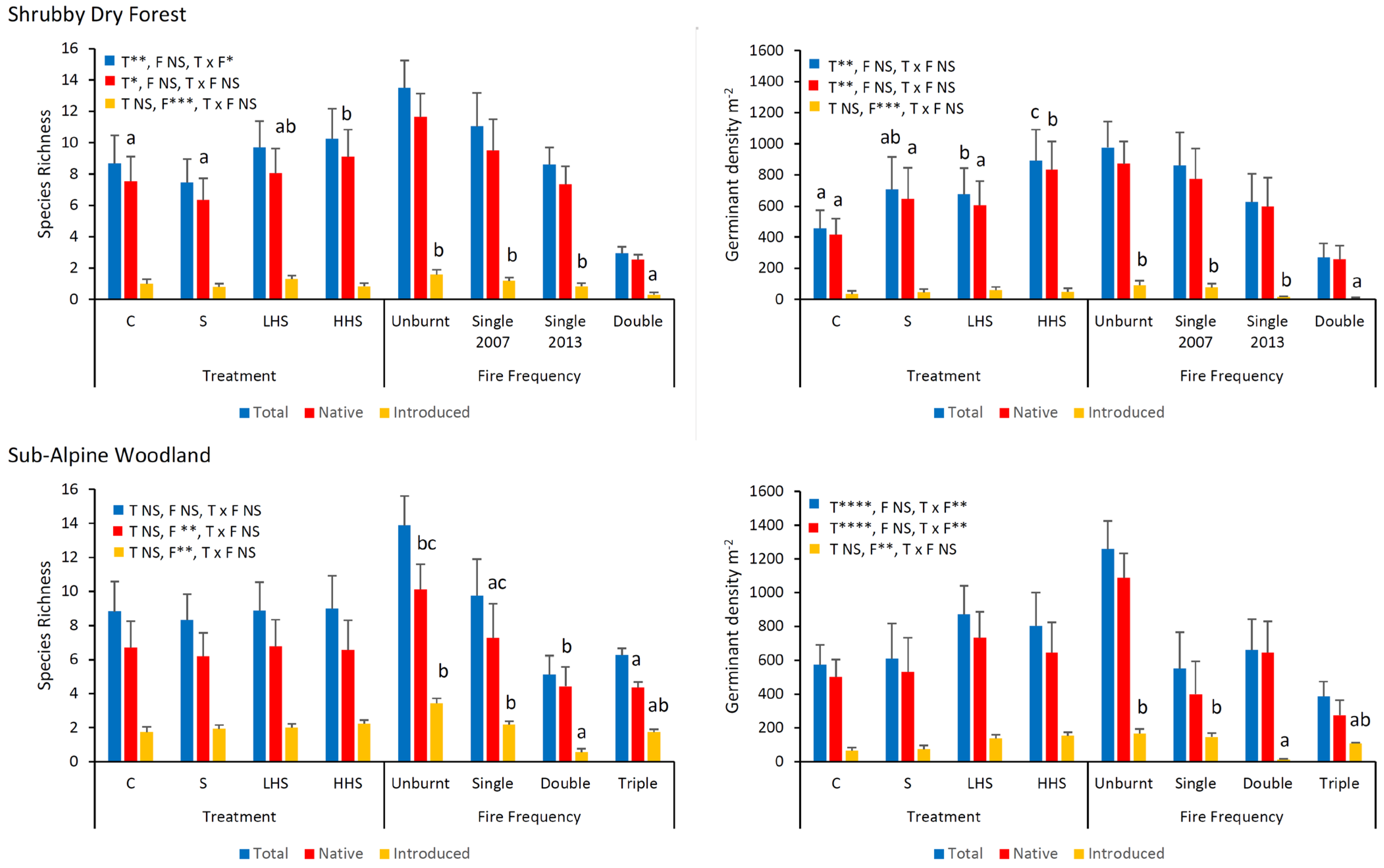
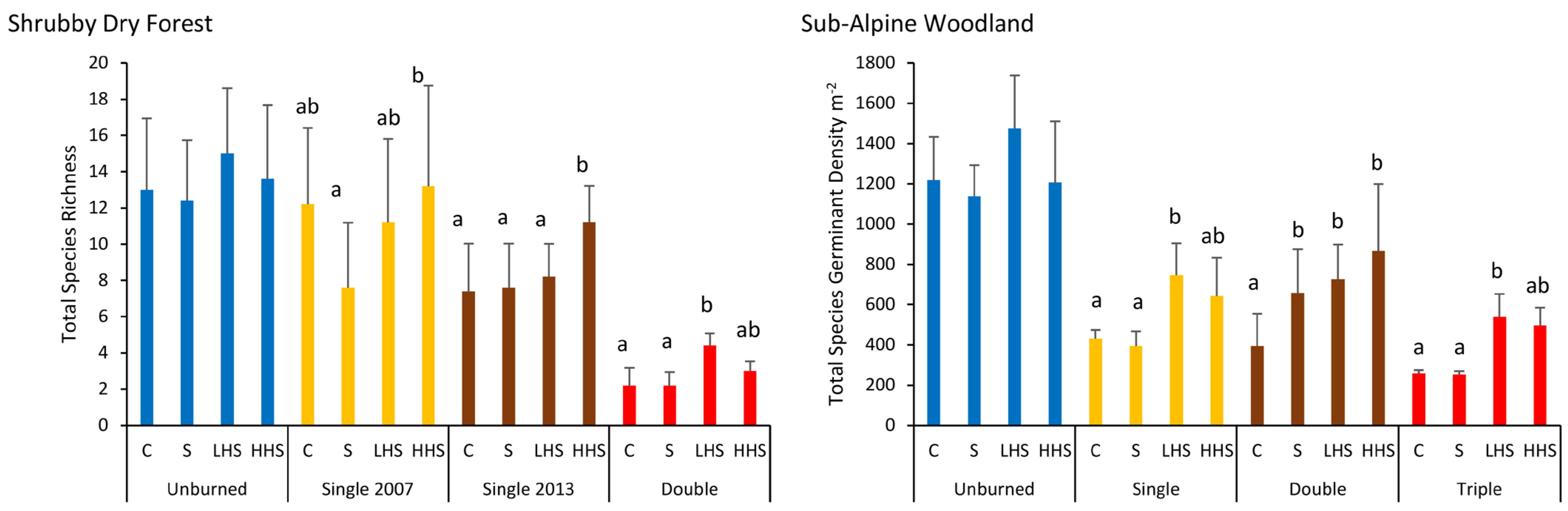
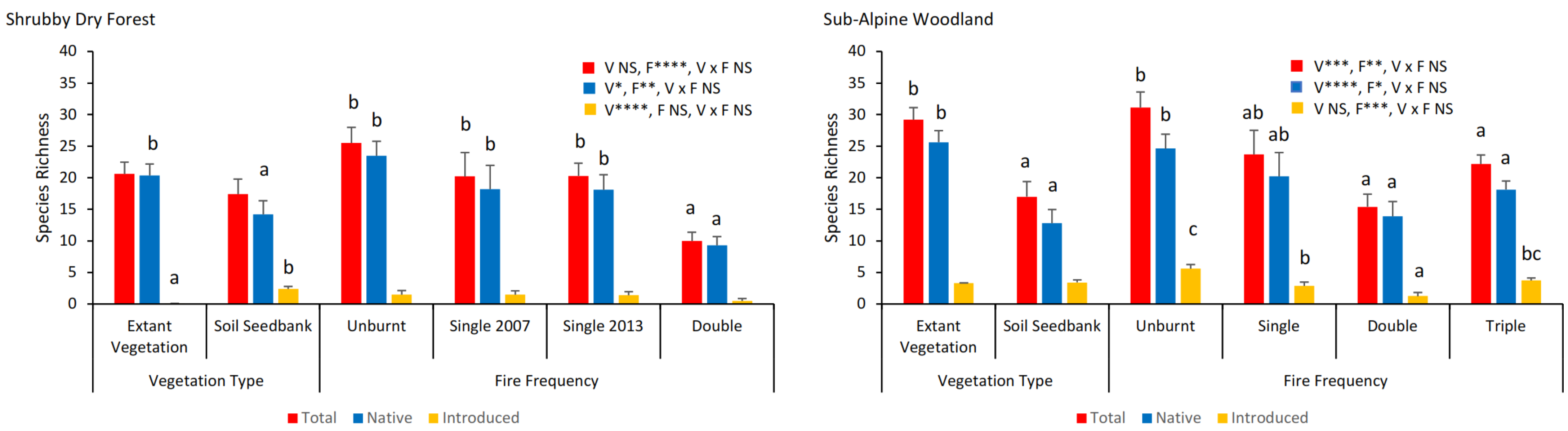
3. Results
3.1. Soil Seed Bank—Individual Species
3.2. Soil Seed Bank—All Species
3.3. Soil Seed Bank—Functional Types
3.4. Vegetation Type and Fire Frequency Effects—All Species
3.5. Vegetation Type and Fire Frequency Effects—Functional Types
4. Discussion
4.1. Fire-Related Germination Cues
4.2. Fire Frequency Effects on the Soil Seed Bank and Extant Vegetation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowman, D.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth System. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.M. Fire and The Australian Flora: A Review. Aust. For. 1975, 38, 4–25. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. A Burning Story: The Role of Fire in the History of Life. Bioscience 2009, 59, 593–601. [Google Scholar] [CrossRef]
- Bradstock, R.A. A biogeographic model of fire regimes in Australia: Current and future implications. Glob. Ecol. Biogeogr. 2010, 19, 145–158. [Google Scholar] [CrossRef]
- Clarke, H.; Evans, J.P. Exploring the future change space for fire weather in southeast Australia. Theor. Appl. Climatol. 2019, 136, 513–527. [Google Scholar] [CrossRef]
- Flannigan, M.; Cantin, A.S.; de Groot, W.J.; Wotton, M.; Newbery, A.; Gowman, L.M. Global wildland fire season severity in the 21st century. For. Ecol. Manag. 2013, 294, 54–61. [Google Scholar] [CrossRef]
- Stephens, S.L.; Burrows, N.; Buyantuyev, A.; Gray, R.W.; Keane, R.E.; Kubian, R.; Liu, S.; Seijo, F.; Shu, L.; Tolhurst, K.G.; et al. Temperate and boreal forest mega-fires: Characteristics and challenges. Front. Ecol. Environ. 2014, 12, 115–122. [Google Scholar] [CrossRef]
- Nolan, R.H.; Bowman, D.M.J.S.; Clarke, H.; Haynes, K.; Ooi, M.K.J.; Price, O.F.; Williamson, G.J.; Whittaker, J.; Bedward, M.; Boer, M.M.; et al. What Do the Australian Black Summer Fires Signify for the Global Fire Crisis? Fire 2021, 4, 97. [Google Scholar] [CrossRef]
- Collins, L.; Clarke, H.; Clarke, M.F.; McColl Gausden, S.C.; Nolan, R.H.; Penman, T.; Bradstock, R. Warmer and drier conditions have increased the potential for large and severe fire seasons across south-eastern Australia. Glob. Ecol. Biogeogr. 2022, 31, 1933–1948. [Google Scholar] [CrossRef]
- Enright, N.J.; Fontaine, J.B.; Bowman, D.; Bradstock, R.A.; Williams, R.J. Interval squeeze: Altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Front. Ecol. Environ. 2015, 13, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Fairman, T.A.; Nitschke, C.R.; Bennett, L.T. Too much, too soon? A review of the effects of increasing wildfire frequency on tree mortality and regeneration in temperate eucalypt forests. Int. J. Wildland Fire 2016, 25, 831–848. [Google Scholar] [CrossRef]
- McColl-Gausden, S.C.; Bennett, L.T.; Ababei, D.A.; Clarke, H.G.; Penman, T.D. Future fire regimes increase risks to obligate-seeder forests. Divers. Distrib. 2022, 18, 542–558. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.J. The Ecology of Fire; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Keeley, J.F. Resilience of Mediterranean shrub communities to fires. In Resilience in Mediterranean-Type Ecosystems; Dell, B., Hopkins, J.J.M., Lamont, B.B., Eds.; Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1986; pp. 95–112. [Google Scholar]
- Auld, T.D.; Denham, A.J. How much seed remains in the soil after a fire? Plant Ecol. 2006, 187, 15–24. [Google Scholar] [CrossRef]
- Auld, T.D.; O’Connell, M.A. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Aust. J. Ecol. 1991, 16, 53–70. [Google Scholar] [CrossRef]
- Carthey, A.J.R.; Tims, A.; Geedicke, I.; Leishman, M.R. Broad-scale patterns in smoke-responsive germination from the south-eastern Australian flora. J. Veg. Sci. 2018, 29, 737–745. [Google Scholar] [CrossRef]
- Enright, N.J.; Kintrup, A. Effects of smoke, heat and charred wood on the germination of dormant soil-stored seeds from a Eucalyptus baxteri heathy-woodland in Victoria, SE Australia. Austral Ecol. 2001, 26, 132–141. [Google Scholar] [CrossRef]
- Noble, I.R.; Slatyer, R.O. The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbances. Vegetatio 1980, 43, 5–21. [Google Scholar] [CrossRef]
- Fenner, M. The effects of the parent environment on seed germinability. Seed Sci. Res. 1991, 1, 75–84. [Google Scholar] [CrossRef]
- Bradbury, D.; Tapper, S.L.; Coates, D.; McArthur, S.; Hankinson, M.; Byrne, M. The role of fire and a long-lived soil seed bank in maintaining persistence, genetic diversity and connectivity in a fire-prone landscape. J. Biogeogr. 2016, 43, 70–84. [Google Scholar] [CrossRef]
- Manela, N.; Dagon, E.; Semesh, H.; Ovadia, O. Smoke interacts with fire history to stimulate soil seed bank germination in Mediterranean woodlands. J. Plant Ecol. 2019, 12, 419–427. [Google Scholar] [CrossRef]
- Zaki, E.; Abedi, M.; Naqinezhad, A. How fire history affects germination cues of three perennial grasses from the mountain steppes of Golestan National Park. Flora 2021, 280, 151835. [Google Scholar] [CrossRef]
- Maikano, G.N.; Cohn, J.; Di Stefano, J. Are germination cues for soil-stored seed banks different in structurally different fire-prone communities? Austral Ecol. 2018, 43, 89–101. [Google Scholar] [CrossRef]
- Paula, S.; Pausas, J.G. Burning seeds: Germinative response to heat treatments in relation to resprouting ability. J. Ecol. 2008, 96, 543–552. [Google Scholar] [CrossRef]
- Tormo, J.; Moreira, B.; Pausas, J.G.; Vandvik, V. Field evidence of smoke-stimulated seedling emergence and establishment in Mediterranean Basin flora. J. Veg. Sci. 2014, 25, 771–777. [Google Scholar] [CrossRef]
- Keeley, J.E.; Bond, W.J. Convergent seed germination in South African fynbos and Californian chaparral. Plant Ecol. 1997, 133, 153–167. [Google Scholar] [CrossRef]
- Read, T.R.; Bellairs, S.M.; Mulligan, D.R.; Lamb, D. Smoke and heat effects on soil seed bank germination for the re-establishment of a native forest community in New South Wales. Austral Ecol. 2000, 25, 48–57. [Google Scholar] [CrossRef]
- Santana, V.M.; Alday, J.G.; Baeza, M.J. Effects of fire regime shift in Mediterranean Basin ecosystems: Changes in soil seed bank composition among functional types. Plant Ecol. 2014, 215, 555–566. [Google Scholar] [CrossRef]
- Ne’eman, G.; Ne’eman, R.; Keith, D.A.; Whelan, R.J. Does post-fire plant regeneration mode affect the germination response to fire-related cues? Oecologia 2009, 159, 483–492. [Google Scholar] [CrossRef]
- Merritt, D.J.; Turner, S.R.; Clarke, S.; Dixon, K.W. Seed dormancy and germination stimulation syndromes for Australian temperate species. Aust. J. Bot. 2007, 55, 336–344. [Google Scholar] [CrossRef]
- Kasel, S.; Nitschke, C.R.; Baker, S.C.; Pryde, E.C. Concurrent assessment of functional types in extant vegetation and soil seed banks informs environmental constraints and mechanisms of plant community turnover in temperate forests of south-eastern Australia. For. Ecol. Manag. 2022, 519, 120321. [Google Scholar] [CrossRef]
- Walck, J.L.; Baskin, J.M.; Baskin, C.C.; Hidayati, S.N. Defining transient and persistent seed banks in species with pronounced seasonal dormancy and germination patterns. Seed Sci. Res. 2005, 15, 189–196. [Google Scholar] [CrossRef]
- Berg, R.Y. Myrmecochorous plants in Australia and their dispersal by ants. Aust. J. Bot. 1975, 23, 475–508. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Bradstock, R.A.; Auld, T.D. Soil Temperatures During Experimental Bushfires in Relation to Fire Intensity: Consequences for Legume Germination and Fire Management in South-Eastern Australia. J. Appl. Ecol. 1995, 32, 76–84. [Google Scholar] [CrossRef]
- Bradstock, R.A.; Tozer, M.G.; Keith, D.A. Effects of high frequency fire on floristic composition and abundance in a fire-prone heathland near Sydney. Aust. J. Bot. 1997, 45, 641–655. [Google Scholar] [CrossRef]
- Pausas, J.G. Response of plant functional types to changes in the fire regime in Mediterranean ecosystems: A simulation approach. J. Veg. Sci. 1999, 10, 717–722. [Google Scholar] [CrossRef]
- Bond, W.J.; van Wilgen, B.W. Fire and Plants: Populationand Community Biology Series 14; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Collins, L. Eucalypt forests dominated by epicormic resprouters are resilient to repeated canopy fires. J. Ecol. 2020, 108, 310–324. [Google Scholar] [CrossRef]
- Fairman, T.A.; Bennett, L.T.; Tupper, S.; Nitschke, C.R. Frequent wildfires erode tree persistence and alter stand structure and initial composition of a fire-tolerant sub-alpine forest. J. Veg. Sci. 2017, 28, 1151–1165. [Google Scholar] [CrossRef]
- Buma, B.; Brown, C.D.; Donato, D.C.; Fontaine, J.B.; Johnstone, J.F. The Impacts of Changing Disturbance Regimes on Serotinous Plant Populations and Communities. Bioscience 2013, 63, 866–876. [Google Scholar] [CrossRef]
- Vila-Cabrera, A.; Saura-Mas, S.; Lloret, F. Effects of fire frequency on species composition in a Mediterranean shrubland. Ecoscience 2008, 15, 519–528. [Google Scholar] [CrossRef]
- Zedler, P.H.; Gautier, C.R.; McMaster, G.S. Vegetation Change in Response to Extreme Events: The Effect of a Short Interval between Fires in California Chaparral and Coastal Scrub. Ecology 1983, 64, 809–818. [Google Scholar] [CrossRef]
- Keeley, J.E.; Brennan, T.J. Fire-driven alien invasion in a fire-adapted ecosystem. Oecologia 2012, 169, 1043–1052. [Google Scholar] [CrossRef]
- Santana, V.M.; Baeza, M.J.; Marrs, R.H.; Vallejo, V.R. Old-field secondary succession in SE Spain: Can fire divert it? Plant Ecol. 2010, 211, 337–349. [Google Scholar] [CrossRef]
- Syphard, A.D.; Brennan, T.J.; Keeley, J.E. Drivers of chaparral type conversion to herbaceous vegetation in coastal Southern California. Divers. Distrib. 2019, 25, 90–101. [Google Scholar] [CrossRef]
- Chick, M.P.; Cohn, J.S.; Nitschke, C.R.; York, A. Lack of soil seedbank change with time since fire: Relevance to seed supply after prescribed burns. Int. J. Wildland Fire 2016, 25, 849–860. [Google Scholar] [CrossRef]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, Ç.; Lloret, F.; Buhk, C.; Ojeda, F.; Luna, B.; Moreno, J.M.; Rodrigo, A.; et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef]
- Lewis, T.; Reif, M.; Prendergast, E.; Tran, C. The effect of long-term repeated burning and fire exclusion on above- and below-ground Blackbutt (Eucalyptus pilularis) forest vegetation assemblages. Austral Ecol. 2012, 37, 767–778. [Google Scholar] [CrossRef]
- Penman, T.D.; Binns, D.; Shiels, R.; Allen, R.; Penman, S. Hidden effects of forest management practices: Responses of a soil stored seed bank to logging and repeated prescribed fire. Austral Ecol. 2011, 36, 571–580. [Google Scholar] [CrossRef]
- Chick, M.P.; Nitschke, C.R.; Cohn, J.S.; Penman, T.D.; York, A. Factors influencing above-ground and soil seed bank vegetation diversity at different scales in a quasi-Mediterranean ecosystem. J. Veg. Sci. 2018, 29, 684–694. [Google Scholar] [CrossRef]
- Chick, M.P.; York, A.; Sitters, H.; Di Stefano, J.; Nitschke, C.R. Combining optimization and simulation modelling to measure the cumulative impacts of prescribed fire and wildfire on vegetation species diversity. J. Appl. Ecol. 2019, 56, 722–732. [Google Scholar] [CrossRef]
- Van der Veken, S.; Bellemare, J.; Verheyen, K.; Hermy, M. Life-history traits are correlated with geographical distribution patterns of western European forest herb species. J. Biogeogr. 2007, 34, 1723–1735. [Google Scholar] [CrossRef]
- Vandvik, V.; Klanderud, K.; Meineri, E.; Måren, I.E.; Töpper, J. Seed banks are biodiversity reservoirs: Species–area relationships above versus below ground. Oikos 2016, 125, 218–228. [Google Scholar] [CrossRef]
- Palmer, H.D.; Denham, A.J.; Ooi, M.K.J. Fire severity drives variation in post-fire recruitment and residual seed bank size of Acacia species. Plant Ecol. 2018, 219, 527–537. [Google Scholar] [CrossRef]
- Syphard, A.D.; Franklin, J.; Keeley, J.E. Simulating the effects of frequent fire on southern California coastal shrublands. Ecol. Appl. 2006, 16, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, E.; Wagner, B.; Nitschke, C.R.; Kasel, S. Short-interval, high-severity wildfires cause declines in soil seed bank diversity in montane forests of south-eastern Australia. For. Ecol. Manag. 2024, 553, 121627. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Plant Functional Types and Ecosystem Function in Relation to Global Change. J. Veg. Sci. 1997, 8, 463–474. [Google Scholar] [CrossRef]
- Lipoma, M.L.; Funes, G.; Diaz, S. Fire effects on the soil seed bank and post-fire resilience of a semi-arid shrubland in central Argentina. Austral Ecol. 2018, 43, 46–55. [Google Scholar] [CrossRef]
- Fairman, T.A.; Bennett, L.T.; Nitschke, C.R. Short-interval wildfires increase likelihood of resprouting failure in fire-tolerant trees. J. Environ. Manag. 2019, 231, 59–65. [Google Scholar] [CrossRef]
- Stewart, S.B.; Nitschke, C.R. Improving temperature interpolation using MODIS LST and local topography: A comparison of methods in south east Australia. Int. J. Climatol. 2017, 37, 3098–3110. [Google Scholar] [CrossRef]
- DEECA. Victorian Ecological Vegetation Communities. Available online: https://www.environment.vic.gov.au/biodiversity/bioregions-and-evc-benchmarks (accessed on 31 May 2023).
- Murphy, B.P.; Bradstock, R.A.; Boer, M.M.; Carter, J.; Cary, G.J.; Cochrane, M.A.; Fensham, R.J.; Russell-Smith, J.; Williamson, G.J.; Bowman, D. Fire regimes of Australia: A pyrogeographic model system. J. Biogeogr. 2013, 40, 1048–1058. [Google Scholar] [CrossRef]
- Cheal, D. Growth Stages and Tolerable Fire Intervals for Victoria’s Native Vegetation Data Sets. Fire and Adaptive Management Report No. 84; Department of Sustainability and Environment: East Melbourne, VIC, Australia, 2010. [Google Scholar]
- Volkova, L.; Weiss Aparicio, A.G.; Weston, C.J. Fire intensity effects on post-fire fuel recovery in Eucalyptus open forests of south-eastern Australia. Sci. Total Environ. 2019, 670, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Costermans, L. Native Trees and Shrubs of South-Eastern Australia; New Holland: Melbourne, VIC, Australia, 2009. [Google Scholar]
- Ferrar, P.J.; Cochrane, P.M.; Slatyer, R.O. Factors influencing germination and establishment of Eucalyptus pauciflora near the alpine tree line. Tree Physiol. 1988, 4, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Green, K. Causes of stability in the alpine treeline in the Snowy Mountains of Australia a natural experiment. Aust. J. Bot. 2009, 57, 171–179. [Google Scholar] [CrossRef]
- Slatyer, R.O.; Noble, I.R. Dynamics of montane treelines. In Landscape Boundaries; Hansen, A.J., Di Castri, F., Eds.; Springer: New York, NY, USA, 1992. [Google Scholar]
- Williams, R.J.; Wahren, C.-H.; Tolsma, A.D.; Sanecki, G.M.; Papst, W.A.; Myers, B.A.; McDougall, K.L.; Heinze, D.A.; Green, K. Large fires in Australian alpine landscapes: Their part in the historical fire regime and their impacts on alpine biodiversity. Int. J. Wildland Fire 2009, 17, 793–808. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Cavieres, L.A.; Carmen, C.; Humaña, A.M. Persistent Soil Seed Bank and Standing Vegetation at a High Alpine Site in the Central Chilean Andes. Oecologia 1999, 119, 126–132. [Google Scholar]
- Venn, S.E.; Morgan, J.W. Soil seedbank composition and dynamics across alpine summits in south-eastern Australia. Aust. J. Bot. 2010, 58, 349–362. [Google Scholar] [CrossRef]
- Lipoma, M.L.; Fortunato, V.; Enrico, L.; Díaz, S. Where does the forest come back from? Soil and litter seed banks and the juvenile bank as sources of vegetation resilience in a semiarid Neotropical forest. J. Veg. Sci. 2020, 31, 1017–1027. [Google Scholar] [CrossRef]
- Younis, S.; Kasel, S. Do Fire Cues Enhance Germination of Soil Seed Stores across an Ecotone of Wet Eucalypt Forest to Cool Temperate Rainforest in the Central Highlands of South-Eastern Australia? Fire 2023, 6, 138. [Google Scholar] [CrossRef]
- Beadle, N.C.W. Soil Temperatures during Forest Fires and Their Effect on the Survival of Vegetation. J. Ecol. 1940, 28, 180–192. [Google Scholar] [CrossRef]
- Warcup, J. Effect of heat treatment of forest soil on germination of buried seed. Aust. J. Bot. 1980, 28, 567–571. [Google Scholar] [CrossRef]
- Meers, T.L.; Enright, N.J.; Bell, T.L.; Kasel, S. Deforestation strongly affects soil seed banks in eucalypt forests: Generalisations in functional traits and implications for restoration. For. Ecol. Manag. 2012, 266, 94–107. [Google Scholar] [CrossRef]
- Hoyle, G.L.; Venn, S.E.; Steadman, K.J.; Good, R.B.; McAuliffe, E.J.; Williams, E.R.; Nicotra, A.B. Soil warming increases plant species richness but decreases germination from the alpine soil seed bank. Glob. Chang. Biol. 2013, 19, 1549–1561. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Brown, D. Estimating the composition of a forest seed bank: A comparison of the seed extraction and seedling emergence methods. Can. J. Bot. 1992, 70, 1603–1612. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: Oxford, UK, 1934. [Google Scholar]
- McIntyre, S.; Lavorel, S.; Tremont, R.M. Plant life-history attributes: Their relationships to disturbance response in herbaceous vegetation. J. Ecol. 1995, 83, 31–44. [Google Scholar] [CrossRef]
- Leishman, M.R.; Westoby, M.; Jurado, E. Correlates of Seed Size Variation: A Comparison among Five Temperate Floras. J. Ecol. 1995, 83, 517–529. [Google Scholar] [CrossRef]
- NPWS. NSW Flora Fires Response Database, Version 1.3a; NSW National Parks and Wildlife Service: Hurstville, NSW, Australia, 2002. [Google Scholar]
- Vivian, L.M.; Doherty, M.D.; Cary, G.J. Classifying the fire-response traits of plants: How reliable are species-level classifications? Austral Ecol. 2010, 35, 264–273. [Google Scholar] [CrossRef]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Paula, S.; Kaneakua-Pia, I.N.; Davis, S.D. Towards understanding resprouting at the global scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef]
- Vesk, P.A.; Westoby, M. Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 2004, 92, 310–320. [Google Scholar] [CrossRef]
- Penman, T.D.; Binns, D.; Allen, R.; Shiels, R.; Plummer, S. Germination responses of a dry sclerophyll forest soil-stored seedbank to fire related cues. Cunninghamia 2008, 10, 547–555. [Google Scholar]
- Trezise, J.E.; Facelli, J.M.; Paton, D.C.; Davies, R.J.-P. The effect of heat and smoke on the soil seed banks of heathlands on permanent freshwater swamps. Austral Ecol. 2021, 46, 39–51. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, MA, USA, 2008. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Anderson, M.J.; Connell, S.D.; Gillanders, B.M.; Diebel, C.E.; Blom, W.M.; Saunders, J.E.; Landers, T.J. Relationships between taxonomic resolution and spatial scales of multivariate variation. J. Anim. Ecol. 2005, 74, 636–646. [Google Scholar] [CrossRef]
- Burrows, N.; Ward, B.; Wills, A.; Williams, M.; Cranfield, R. Fine-scale temporal turnover of jarrah forest understory vegetation assemblages is independent of fire regime. Fire Ecol. 2019, 15, 18. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Primer-E Ltd.: Plymouth Marine Laboratory, UK, 2001. [Google Scholar]
- Keith, D.A. Functional traits: Thier roles in undertanding and predicting biotic responses to fire regimes from individuals to landscapes. In Flammable Australia: Fire Regimes, Biodiversity and Ecosystems in a Changing World, 2nd ed.; Bradstock, R.A., Gill, A.M., Williams, R.J., Eds.; CSIRO: Melbourne, VIC, Australia, 2012; pp. 97–125. [Google Scholar]
- Keith, D.A.; Holman, L.; Rodoreda, S.; Lemmon, J.; Bedward, M. Plant functional types can predict decade-scale changes in fire-prone vegetation. J. Ecol. 2007, 95, 1324–1337. [Google Scholar] [CrossRef]
- Anderson, B.J.; Chiarucci, A.; Williamson, M. How differences in plant abundance measures produce different species-abundance distributions. Methods Ecol. Evol. 2012, 3, 783–786. [Google Scholar] [CrossRef]
- Clarke, P.J.; Dorji, K. Are trade-offs in plant resprouting manifested in community seed banks. Ecology 2008, 89, 1850–1858. [Google Scholar] [CrossRef]
- Vázquez-Ramírez, J.; Venn, S.E. Snow, fire and drought: How alpine and treeline soil seed banks are affected by simulated climate change. Ann. Bot. 2023, mcad184. [Google Scholar] [CrossRef] [PubMed]
- Ashton, D.H. The Big Ash forest, Wallaby Creek, Victoria; changes during one lifetime. Aust. J. Bot. 2000, 48, 1–26. [Google Scholar] [CrossRef]
- Campbell, M.L.; Clarke, P.J. Response of montane wet sclerophyll forest understorey species to fire: Evidence from high and low intensity fires. Proc. Linn. Soc. New South Wales 2006, 127, 63. [Google Scholar]
- Williams, R.J. Gap Dynamics in Subalpine Heathland and Grassland Vegetation in South- Eastern Australia. J. Ecol. 1992, 80, 343–352. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lamont, B.B. Fire-released seed dormancy—A global synthesis. Biol. Rev. 2022, 97, 1612–1639. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-F.; Shi, S.-H.; Jiang, Y.-S.; Liu, J. A global synthesis of fire effects on soil seed banks. Glob. Ecol. Conserv. 2022, 36, e02132. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A Compound from Smoke That Promotes Seed Germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Falster, D.; Gallagher, R.; Wenk, E.H.; Wright, I.J.; Indiarto, D.; Andrew, S.C.; Baxter, C.; Lawson, J.; Allen, S.; Fuchs, A.; et al. AusTraits, a curated plant trait database for the Australian flora. Sci. Data 2021, 8, 254. [Google Scholar] [CrossRef]
- Taranto, M.; Downe, J.; Coates, F.; Oates, A. Recovery of Montane Swamp Complex after Bushfires in North East Victoria 2003. Arthur Rylah Institute for Environmental Research Technical Report Series No. 152; Victorian Government Department of Sustainability and Environment: Melbourne, VIC, Australia, 2004; p. 44. [Google Scholar]
- Emery, N.J.; Offord, C.A. Managing Persoonia (Proteaceae) species in the landscape through a better understanding of their seed biology and ecology. Cunninghamia J. Plant Ecol. East. Aust. 2018, 18, 89–107. [Google Scholar]
- Walsh, N.G.; McDougall, K.L. Progress in the recovery of the flora of treeless subalpine vegetation in Kosciuszko National Park after the 2003 fires. Cunninghamia 2004, 8, 439–452. [Google Scholar]
- Bargmann, T.; Måren, I.E.; Vandvik, V.; Fraser, L. Life after fire: Smoke and ash as germination cues in ericads, herbs and graminoids of northern heathlands. Appl. Veg. Sci. 2014, 17, 670–679. [Google Scholar] [CrossRef]
- Keeley, J.E. Seed-germination patterns in fire-prone Mediterranean-climate regions. In Ecology and Biogeography of Mediterranean Ecosystems in Chile, California, and Australia; Arroyo, M., Zedler, P.H., Fox, M.D., Eds.; Springer: New York, NY, USA, 1995; pp. 239–273. [Google Scholar]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Kasel, S.; Bennett, L.; Aponte, C.; Fedrigo, M.; Nitschke, C. Environmental heterogeneity promotes floristic turnover in temperate forests of south-eastern Australia more than dispersal limitation and disturbance. Landsc. Ecol. 2017, 32, 1613–1629. [Google Scholar] [CrossRef]
- Kiel, N.G.; Braziunas, K.H.; Turner, M.G. Peeking under the canopy: Anomalously short fire-return intervals alter subalpine forest understory plant communities. New Phytol 2023, 239, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.W.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Halpern, C.B.; Antos, J.A. Burn severity and pre-fire seral state interact to shape vegetation responses to fire in a young, western Cascade Range forest. For. Ecol. Manag. 2022, 507, 18. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Wright, B.R.; Clarke, P.J. Fire, aridity and seed banks. What does seed bank composition reveal about community processes in fire-prone desert? J. Veg. Sci. 2009, 20, 663–674. [Google Scholar] [CrossRef]
- Bowd, E.J.; Blair, D.P.; Lindenmayer, D.B. Prior disturbance legacy effects on plant recovery post-high-severity wildfire. Ecosphere 2021, 12, e03480. [Google Scholar] [CrossRef]
- Blair, D.P.; McBurney, L.M.; Blanchard, W.; Banks, S.C.; Lindenmayer, D.B. Disturbance gradient shows logging affects plant functional groups more than fire. Ecol. Appl. 2016, 26, 2280–2301. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cai, W.; Prentice, I.C.; Harrison, S.P. Community Abundance of Resprouting in Woody Plants Reflects Fire Return Time, Intensity, and Type. Forests 2023, 14, 878. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef] [PubMed]
- McDougall, K.L.; Walsh, N.G.; Wright, G.T. Recovery of treeless subalpine vegetation in Kosciuszko National Park after the landscape-scale fire of 2003. Aust. J. Bot. 2015, 63, 597–607. [Google Scholar] [CrossRef]
- Nolan, R.H.; Collins, L.; Leigh, A.; Ooi, M.K.J.; Curran, T.J.; Fairman, T.A.; de Dios, V.R.; Bradstock, R. Limits to post-fire vegetation recovery under climate change. Plant Cell Environ. 2021, 44, 3471–3489. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Baker, P.J.; Kasel, S.; Trouvé, R.; Stewart, S.B.; Nitschke, C.R. The role of climatic variability on Eucalyptus regeneration in southeastern Australia. Glob. Ecol. Conserv. 2021, 32, e01929. [Google Scholar] [CrossRef]
- Singh, A.; Kasel, S.; Hui, F.K.C.; Trouvé, R.; Baker, P.J.; Nitschke, C.R. Acacia Density, Edaphic, and Climatic Factors Shape Plant Assemblages in Regrowth Montane Forests in Southeastern Australia. Forests 2023, 14, 1166. [Google Scholar] [CrossRef]
- Barker, J.W.; Price, O.F.; Jenkins, M.E. High severity fire promotes a more flammable eucalypt forest structure. Austral Ecol. 2022, 47, 519–529. [Google Scholar] [CrossRef]
- Zylstra, P.J. Flammability dynamics in the Australian Alps. Austral Ecol. 2018, 43, 578–591. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Taylor, C. New spatial analyses of Australian wildfires highlight the need for new fire, resource, and conservation policies. Proc. Natl. Acad. Sci. USA 2020, 117, 12481–12485. [Google Scholar] [CrossRef] [PubMed]

| Density | Richness | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait and Attribute | Soil Seedbank Treatment A | Fire Frequency B | Soil Seedbank Treatment | Fire Frequency | ||||||||||||
| C | S | LHS | HHS | UB | S07 | S13 | D | C | S | LHS | HHS | UB | S07 | S13 | D | |
| Life Form C | F(3, 64) = 1.72, p = 0.0379 | F(3,64) = 4.09, p = 0.0001 | F(3, 64) = 2.06, p = 0.0062 | F(3, 64) = 3.45, p = 0.0003 | ||||||||||||
| ab | a | ab | b | a | a | a | b | a | a | ab | b | a | a | a | b | |
| HE | 7.6 | 4 | 3.8 | 7.1 | 3.7 | 3.7 | 8.9 | 6.1 | 9.7 | 7 | 5 | 8.5 | 8.8 | 8 | 8 | 5.4 |
| T | 35.9 | 57.3 | 40.1 | 34.2 | 22.8 | 45.6 | 36.8 | 62.4 | 33.7 | 50 | 36.9 | 23.9 | 23.5 | 40 | 33.6 | 47.4 |
| C | 3.5 | 3.2 | 7.6 | 6.4 | 6.5 | 5.1 | 5.4 | 3.7 | 4 | 8.8 | 10.9 | 8.1 | 8.4 | 7.3 | 7.6 | 7.5 |
| HPr | 29.4 | 16.3 | 23.5 | 25.8 | 39.8 | 24 | 29.3 | 2 | 19.9 | 13.2 | 17.4 | 19.2 | 24.5 | 17.9 | 21.5 | 5.9 |
| HF | 3.1 | 1.3 | 1.8 | 1.1 | 4.3 | 1.9 | 0.9 | 0.2 | 6.1 | 3.1 | 1.6 | 1.5 | 2.8 | 5.6 | 2.6 | 1.2 |
| G | 3.1 | 4.9 | 1.5 | 1.3 | 0.8 | 0.4 | 3.8 | 5.8 | 4 | 5.8 | 1.8 | 2.7 | 2.5 | 1.3 | 7 | 3.7 |
| P | 10.4 | 11.6 | 21.1 | 20.9 | 18.7 | 19 | 11.5 | 14.8 | 14.9 | 10.9 | 24.9 | 31.6 | 23.5 | 18.7 | 16.2 | 23.8 |
| E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HP | 2 | 1.4 | 0.5 | 3.1 | 3.3 | 0.3 | 3.5 | 0 | 2.6 | 2.2 | 1.6 | 4.4 | 5.9 | 1.2 | 3.6 | 0 |
| Dispersal Mode D | F(3, 64) = 2.94, p = 0.0010 | F(3, 64) = 5.51, p = 0.0001 | F(3, 64) = 3.01, p = 0.0002 | F(3, 64) = 5.29, p = 0.0001 | ||||||||||||
| a | a | b | b | a | b | a | b | a | a | b | b | a | b | c | d | |
| bar | 11.8 | 9.7 | 22.7 | 23.4 | 27.5 | 14.4 | 16.2 | 9.5 | 12.4 | 18.8 | 20.1 | 23.4 | 25.1 | 17.3 | 17.9 | 14.3 |
| ane | 34.8 | 56.1 | 38.9 | 38.3 | 20.2 | 51.3 | 37.5 | 59 | 38.3 | 43.7 | 39.6 | 26.5 | 23.9 | 43.8 | 34.1 | 46.4 |
| myr | 4.5 | 1.9 | 16 | 14.7 | 8.9 | 9.9 | 5.6 | 12.6 | 7.7 | 4.7 | 18 | 22 | 13.5 | 12.2 | 7.1 | 19.7 |
| end | 1.8 | 1.8 | 2.2 | 1 | 2 | 2.6 | 1.5 | 0.6 | 2.9 | 1.8 | 3.9 | 3.3 | 3.6 | 4.7 | 1.6 | 2.1 |
| mob | 38.7 | 30.2 | 19.7 | 21.8 | 40.1 | 21.1 | 37.8 | 11.4 | 28.4 | 30.1 | 17.3 | 21.9 | 31.5 | 20.1 | 36.3 | 10 |
| epi | 3.4 | 0.3 | 0.5 | 0.9 | 1.3 | 0.6 | 1.3 | 1.8 | 5.2 | 1 | 1 | 2.8 | 2.5 | 2 | 3.1 | 2.5 |
| Fire Response E | F(3, 64) = 1.35, p = 0.1861 | F(3, 64) = 3.71, p = 0.0002 | F(3, 64) = 2.02, p = 0.0198 | F(3, 64) = 3.78, p = 0.0001 | ||||||||||||
| a | a | a | b | a | a | a | b | a | a | a | b | |||||
| R | 14.5 | 12.1 | 22 | 27.9 | 21.8 | 14.6 | 18.3 | 21.8 | 20.9 | 17.3 | 20.2 | 34.1 | 27.2 | 18.3 | 20.7 | 26.3 |
| S | 50.7 | 67.4 | 50.3 | 46.4 | 40.5 | 57.4 | 49.7 | 67.2 | 49.8 | 62.4 | 53.3 | 37.9 | 44.5 | 51.1 | 50.3 | 57.4 |
| Rs | 8.2 | 4.4 | 5.5 | 8.5 | 6.4 | 8.8 | 10.8 | 0.6 | 9 | 8.8 | 6.4 | 6.9 | 7.7 | 7.7 | 13.8 | 2 |
| SR | 2.4 | 2.5 | 4.3 | 3.8 | 1.9 | 5.3 | 3.7 | 2.2 | 3.2 | 3.6 | 4.7 | 9.1 | 3.8 | 7.6 | 5.6 | 3.5 |
| Sr | 19.2 | 13.6 | 17.8 | 13.3 | 29.4 | 13.8 | 17.5 | 3.2 | 12 | 7.9 | 15.4 | 12 | 16.8 | 15.3 | 9.5 | 5.7 |
| Density | Richness | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait and Attribute C | Soil Seedbank Treatment A | Fire Frequency B | Soil Seedbank Treatment | Fire Frequency | ||||||||||||
| C | S | LHS | HHS | UB | S | D | T | C | S | LHS | HHS | UB | S | D | T | |
| Life Form | F(3, 48) = 0.39, p = 0.9883 | F(3, 48) = 4.06, p = 0.0001 | F(3, 48) = 0.67, p = 0.8468 | F(3, 48) = 2.89, p = 0.0008 | ||||||||||||
| a | a | b | a | a | a | b | ab | |||||||||
| HE | 61.9 | 59.7 | 60.3 | 58 | 66 | 49.9 | 86 | 37.9 | 44.7 | 41.6 | 38.2 | 40.2 | 36 | 35.5 | 56.5 | 36.7 |
| T | 3 | 4.6 | 2 | 3.4 | 2.9 | 3.3 | 1.8 | 4.9 | 8.5 | 11.9 | 7.1 | 9.9 | 9 | 9.5 | 7.4 | 11.5 |
| C | 0.9 | 0.6 | 0.8 | 0.4 | 1.3 | 0.3 | 0.7 | 0.4 | 3.4 | 1.4 | 1.1 | 1.2 | 3.4 | 0.8 | 1.4 | 1.6 |
| HPr | 14.2 | 13.8 | 10.5 | 11 | 10 | 17.4 | 7.4 | 14.7 | 17.7 | 20.6 | 18.8 | 20.6 | 21.3 | 21.7 | 19.4 | 15.4 |
| HF | 6.8 | 6.3 | 6.7 | 7.5 | 10.5 | 9.9 | 1.6 | 5.2 | 8.5 | 8.8 | 9.6 | 9.5 | 14.2 | 10.6 | 4.5 | 7.2 |
| G | 4.6 | 5.7 | 4.6 | 4.7 | 0.1 | 0.7 | 0.3 | 18.5 | 2.9 | 2.6 | 2.7 | 2.1 | 0.6 | 1.2 | 1.6 | 7 |
| P | 0.5 | 1.6 | 3.4 | 2.1 | 0.8 | 5.3 | 1.1 | 0.3 | 1.5 | 3.1 | 8.9 | 5.3 | 4.6 | 9.4 | 4.2 | 0.6 |
| E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HP | 8.1 | 7.8 | 11.7 | 12.9 | 8.4 | 13.1 | 1 | 18 | 12.8 | 9.9 | 13.5 | 11.1 | 10.8 | 11.4 | 5.1 | 20.1 |
| Dispersal Mode | F(3, 48) = 0.52, p = 0.8875 | F(3, 48) = 4.03, p = 0.0001 | F(3, 48) = 0.31, p = 0.8998 | F(3, 48) = 4.19, p = 0.0008 | ||||||||||||
| a | ac | b | c | a | a | b | ab | |||||||||
| Bar | 76.3 | 73.6 | 76.4 | 76.6 | 74.4 | 73.3 | 90.5 | 64.6 | 63.8 | 59.3 | 62.1 | 65.6 | 60 | 54.7 | 74.6 | 61.5 |
| Ane | 11 | 11.8 | 7.4 | 8.2 | 6 | 16.5 | 3.5 | 12.4 | 20.9 | 23 | 17.1 | 15.9 | 15.6 | 25.3 | 13.3 | 22.6 |
| Myr | 4.6 | 5.9 | 8.2 | 8.1 | 12.8 | 6.8 | 5.2 | 2 | 6.3 | 8.6 | 10.3 | 10.2 | 12 | 10.6 | 8.9 | 3.9 |
| End | 0 | 0.1 | 0.5 | 0.3 | 0.6 | 0 | 0 | 0.4 | 0 | 0.5 | 1.4 | 0.3 | 1.5 | 0 | 0 | 0.7 |
| Mob | 7.8 | 8 | 7.2 | 6.3 | 5.3 | 3.2 | 0.7 | 20.1 | 7.9 | 7.2 | 7.7 | 6.1 | 7.1 | 9 | 2.6 | 10.1 |
| Epi | 0.3 | 0.6 | 0.3 | 0.5 | 0.9 | 0.2 | 0.1 | 0.5 | 1.1 | 1.5 | 1.5 | 1.9 | 3.8 | 0.4 | 0.6 | 1.3 |
| Fire Response | F(3, 48) = 0.56, p = 0.8481 | F(3, 48) = 4.76, p = 0.0002 | F(3, 48) = 0.40, p = 0.9636 | F(3, 48) = 3.53, p = 0.0009 | ||||||||||||
| a | b | b | a | a | abc | b | c | |||||||||
| R | 66.5 | 65.3 | 65.2 | 60.7 | 62 | 52.2 | 75.2 | 68.2 | 54 | 50.6 | 47.3 | 48.8 | 40.3 | 43.4 | 64.9 | 52.1 |
| S | 16.4 | 17.1 | 14.1 | 13.1 | 8.9 | 22.9 | 20.6 | 8.4 | 20.7 | 22.5 | 26.1 | 21.4 | 23.3 | 28.1 | 20.5 | 18.7 |
| Rs | 7.2 | 6.2 | 4.1 | 4.9 | 6.8 | 8.8 | 1.4 | 5.3 | 7.9 | 11.4 | 7.8 | 7.4 | 10.5 | 11.1 | 4.8 | 8.2 |
| SR | 6.9 | 7.8 | 11.7 | 16.5 | 11.8 | 14.1 | 1.3 | 15.6 | 13.1 | 11.3 | 14.2 | 15 | 18.5 | 11.6 | 6.3 | 17.2 |
| Sr | 3.1 | 3.6 | 4.9 | 4.8 | 10.5 | 2 | 1.5 | 2.5 | 4.2 | 4.2 | 4.6 | 7.4 | 7.4 | 5.7 | 3.5 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasel, S.; Fairman, T.A.; Nitschke, C.R. Short-Interval, High-Severity Wildfire Depletes Diversity of Both Extant Vegetation and Soil Seed Banks in Fire-Tolerant Eucalypt Forests. Fire 2024, 7, 148. https://doi.org/10.3390/fire7040148
Kasel S, Fairman TA, Nitschke CR. Short-Interval, High-Severity Wildfire Depletes Diversity of Both Extant Vegetation and Soil Seed Banks in Fire-Tolerant Eucalypt Forests. Fire. 2024; 7(4):148. https://doi.org/10.3390/fire7040148
Chicago/Turabian StyleKasel, Sabine, Thomas A. Fairman, and Craig R. Nitschke. 2024. "Short-Interval, High-Severity Wildfire Depletes Diversity of Both Extant Vegetation and Soil Seed Banks in Fire-Tolerant Eucalypt Forests" Fire 7, no. 4: 148. https://doi.org/10.3390/fire7040148





