Surface Functionalization of TiO2 Nanotubes Modified with a Thin Film of BiFeO3
Abstract
:1. Introduction
2. Materials and Methods
3. Results
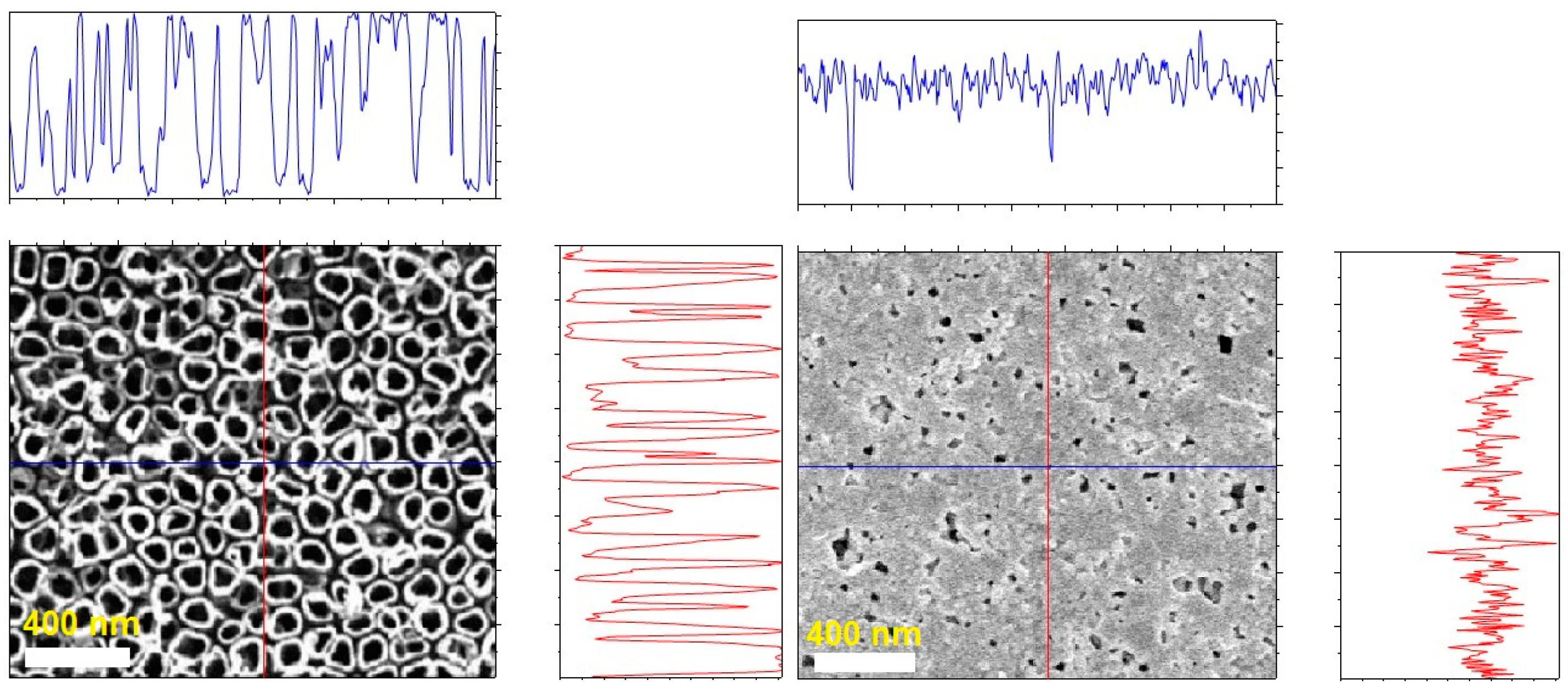
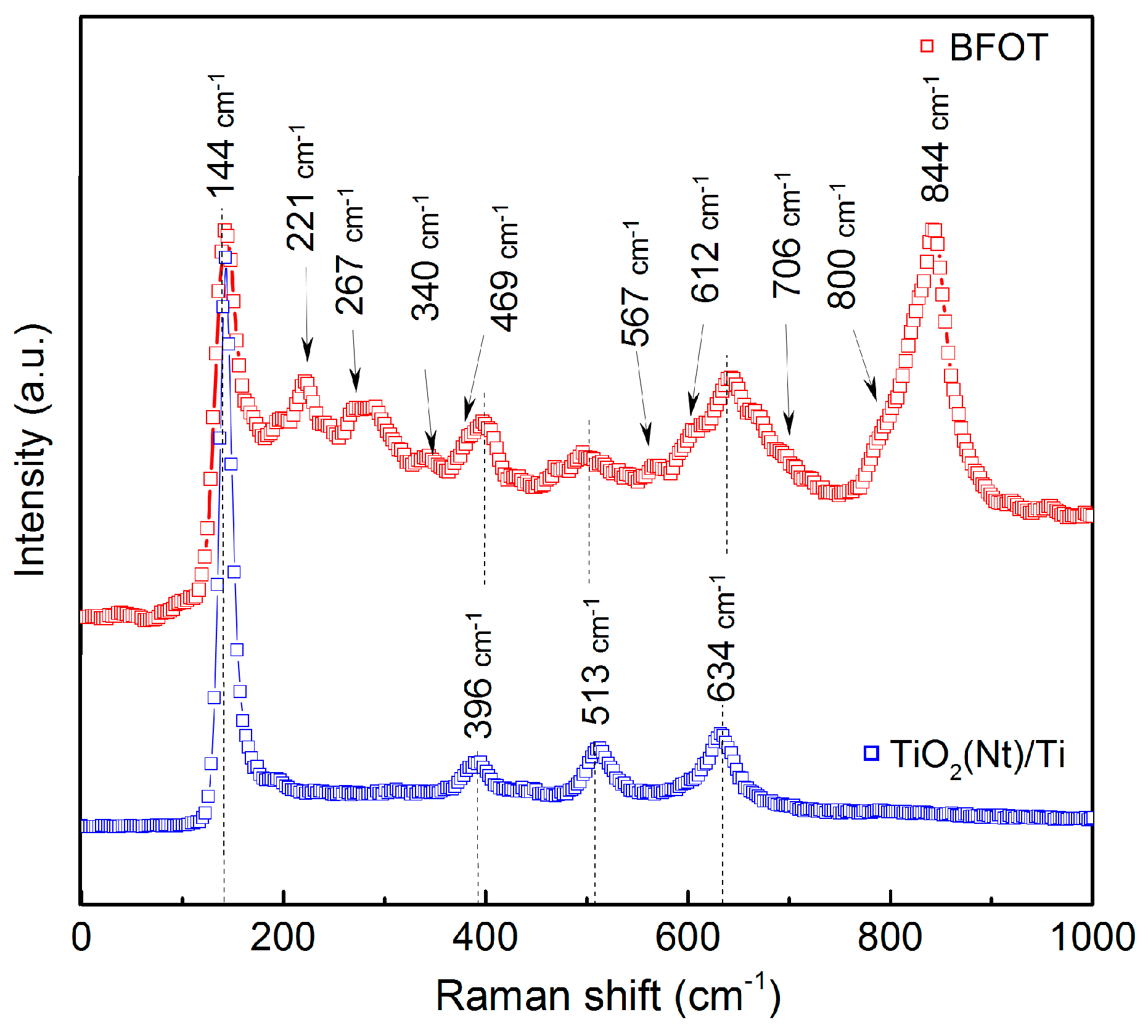
3.1. Structural Characteristics
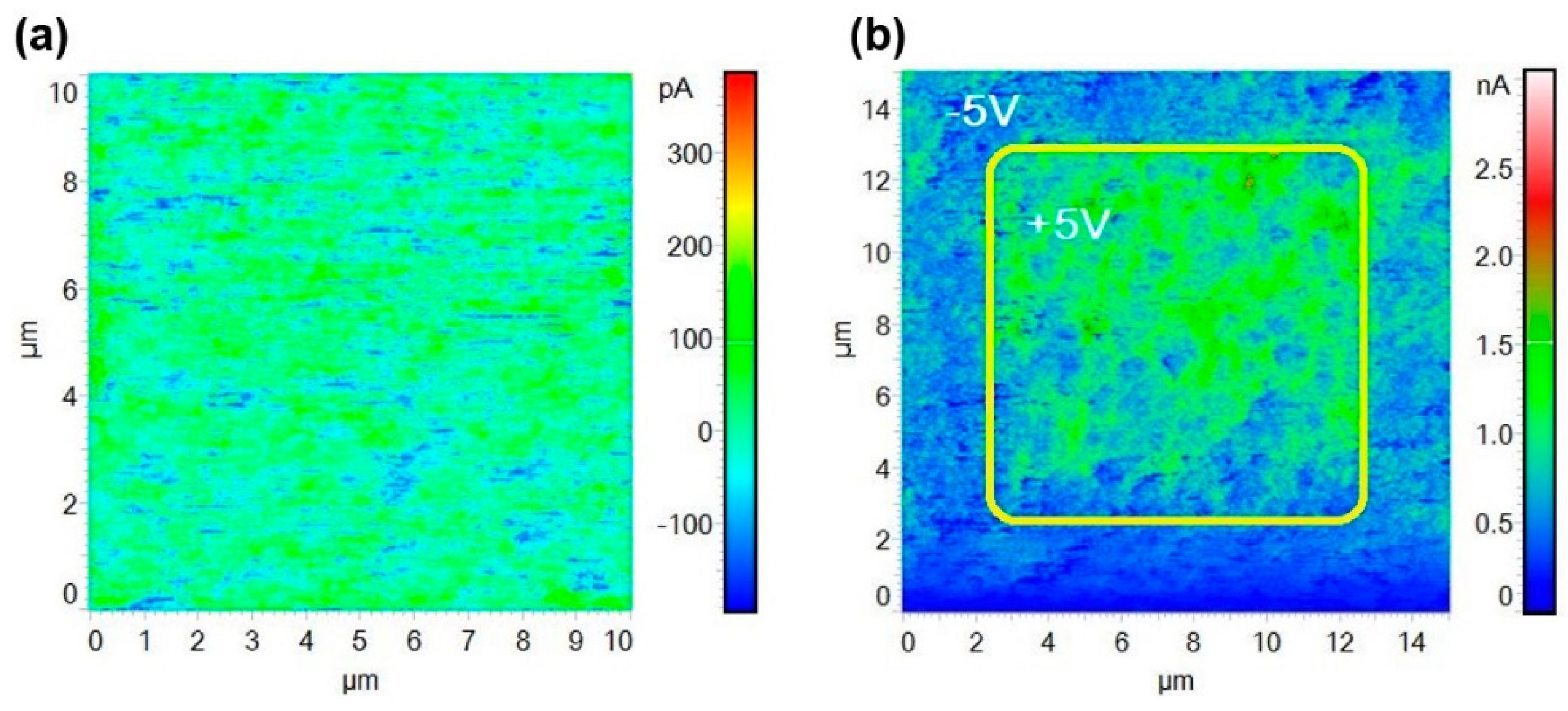
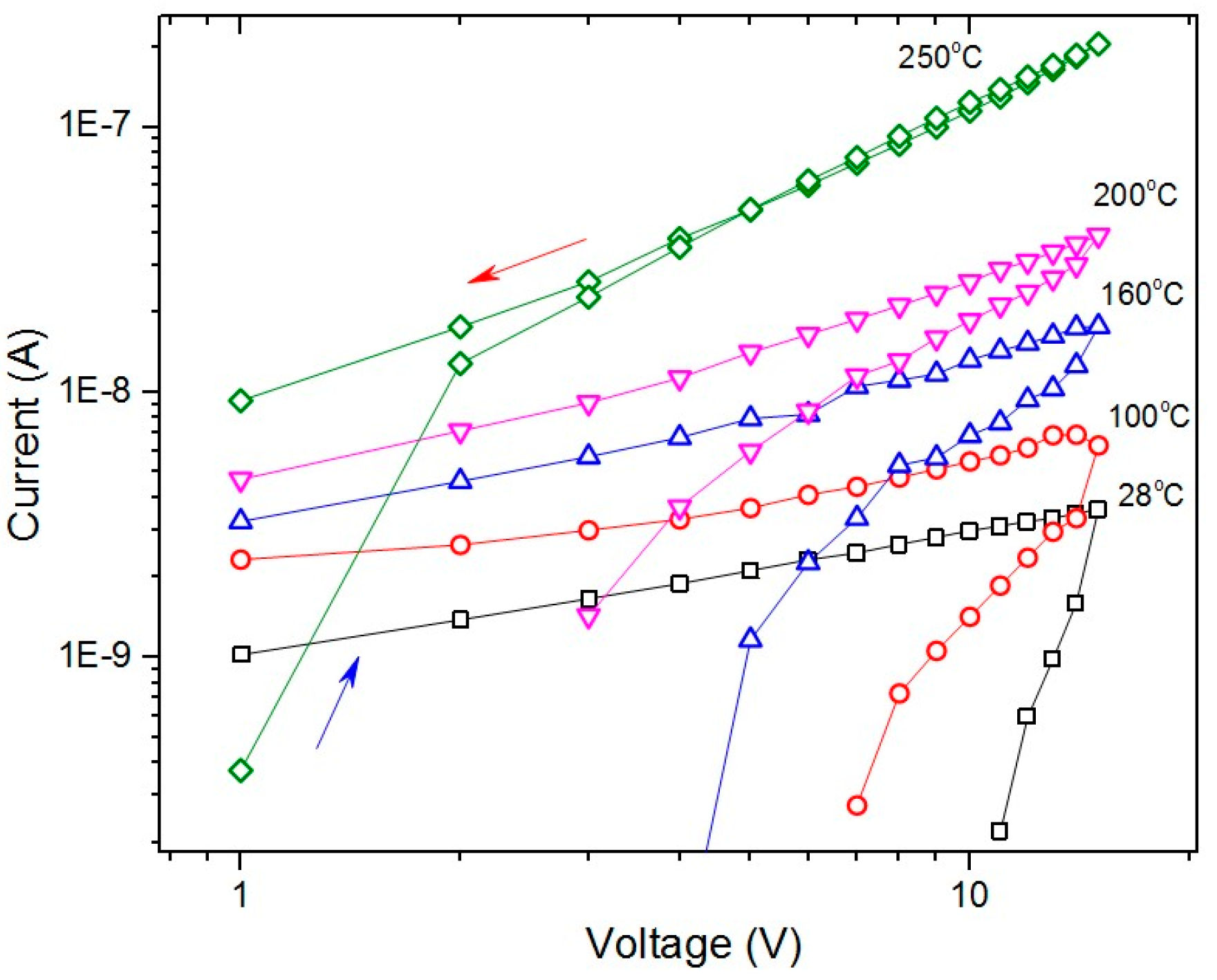
3.2. Electrical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Y.; Zhang, J.; Yan, M.; Jiang, Y.; Jawad, H.; Tian, B.; Wang, W.; Zhan, Y.; Qin, Y.; Xiong, S.; et al. Molecular ferroelectric/semiconductor interfacial memristors for artificial synapses. NPJ Flex. Electron. 2022, 6, 16. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, X. Ferroelectric Memristor Based on Hf0.5Zr0.5O2 Thin Film Combining Memristive and Neuromorphic Functionalities. Phys. Status Solidi–Rapid Res. Lett. 2020, 14, 2000224. [Google Scholar] [CrossRef]
- Luk’yanchuk, I.; Razumnaya, A.; Sené, A.; Tikhonov, Y.; Vinokur, V.M. The ferroelectric field-effect transistor with negative capacitance. NPJ Comput. Mater. 2022, 8, 52. [Google Scholar] [CrossRef]
- Hoffman, J.; Pan, X.; Reiner, J.W.; Walker, F.J.; Han, J.P.; Ahn, C.H.; Ma, T.P. Ferroelectric Field Effect Transistors for Memory Applications. Adv. Mater. 2010, 22, 2957–2961. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, M.J.; Jang, H.W. Ferroelectric field effect transistors: Progress and perspective. APL Mater. 2021, 9, 021102. [Google Scholar] [CrossRef]
- Panda, D.; Patnaik, A. Novel TiO2-based memristors FET with programmable SET/RESET for neuromorphic computing. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Chen, A. A review of emerging non-volatile memory (NVM) technologies and applications. Solid. State. Electron. 2016, 125, 25–38. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, J.; Poremba, M.; Xu, C.; Xie, Y. Memory that never forgets: Emerging nonvolatile memory and the implication for architecture design. Natl. Sci. Rev. 2018, 5, 577–592. [Google Scholar] [CrossRef]
- Cardona Rodríguez, A.; Reiber, A.; Schuller, I.K.; Muraca, D.; Gabriel Ramírez, J. Evidence of a glassy magnetic transition driven by structural disorder in BiFeO3 nanoparticles. J. Magn. Magn. Mater. 2022, 563, 169917. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef]
- Schmidt, R.; Eerenstein, W.; Winiecki, T.; Morrison, F.D.; Midgley, P.A. Impedance spectroscopy of epitaxial multiferroic thin films. Phys. Rev. B-Condens. Matter Mater. Phys. 2007, 75, 245111. [Google Scholar] [CrossRef]
- Tomczyk, M.; Mahajan, A.; Tkach, A.; Vilarinho, P.M. Interface-based reduced coercivity and leakage currents of BiFeO3 thin films: A comparative study. Mater. Des. 2018, 160, 1322–1334. [Google Scholar] [CrossRef]
- Wani, W.A.; Naaz, N.; Venkataraman, B.H.; Kundu, S.; Ramaswamy, K. Significantly reduced leakage current density in Mn-doped BiFeO3 thin films deposited using spin coating technique. J. Phys. Conf. Ser. 2021, 2070, 012088. [Google Scholar] [CrossRef]
- Tuluk, A.; Joshi, S.; Mahon, T.; Van Der Zwaag, S. Tuning piezoproperties of BiFeO3ceramic by cobalt and titanium dual doping. J. Appl. Phys. 2022, 131, 214104. [Google Scholar] [CrossRef]
- Zhao, Z.; Abdelsamie, A.; Guo, R.; Shi, S.; Zhao, J.; Lin, W.; Sun, K.; Wang, J.; Wang, J.; Yan, X.; et al. Flexible artificial synapse based on single-crystalline BiFeO3 thin film. Nano Res. 2022, 15, 2682–2688. [Google Scholar] [CrossRef]
- Illarionov, G.A.; Morozova, S.M.; Chrishtop, V.V.; Einarsrud, M.A.; Morozov, M.I. Memristive TiO2: Synthesis, Technologies, and Applications. Front. Chem. 2020, 8, 556815. [Google Scholar] [CrossRef]
- Adamaki, V.; Clemens, F.; Ragulis, P.; Pennock, S.R.; Taylor, J.; Bowen, C.R. Manufacturing and characterization of Magnéli phase conductive fibres. J. Mater. Chem. A 2014, 2, 8328–8333. [Google Scholar] [CrossRef]
- Ramazanov, S.; Sobola, D.; Ţălu, Ş.; Orudzev, F.; Arman, A.; Kaspar, P.; Dallaev, R.; Ramazanov, G. Multiferroic behavior of the functionalized surface of a flexible substrate by deposition of Bi2O3 and Fe2O3. Microsc. Res. Tech. 2022, 85, 1300–1310. [Google Scholar] [CrossRef]
- Ramazanov, S.; Ţălu, Ş.; Sobola, D.; Orudzev, F.; Ramazanov, G.; Selimov, D.; Kaspar, P.; Macků, R.; Nazarov, A. Crack resistance of bismuth ferrite films obtained on a flexible substrate. E3S Web Conf. 2021, 295, 04008. [Google Scholar] [CrossRef]
- Cai, S.; Lun, Y.; Ji, D.; Lv, P.; Han, L.; Guo, C.; Zang, Y.; Gao, S.; Wei, Y.; Gu, M.; et al. Enhanced polarization and abnormal flexural deformation in bent freestanding perovskite oxides. Nat. Commun. 2022, 13, 5116. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; You, L.; Lin, W.; Abdelsamie, A.; Shu, X.; Zhou, G.; Chen, S.; Liu, L.; Yan, X.; Wang, J.; et al. Continuously controllable photoconductance in freestanding BiFeO3 by the macroscopic flexoelectric effect. Nat. Commun. 2020, 11, 2571. [Google Scholar] [CrossRef]
- Ramazanov, S.; Orudzhev, F.; Gajiev, G.; Holcman, V.; Matos, R.S.; da Fonseca Filho, H.D.; Ţălu, Ş.; Selimov, D. Local electrical characteristic of memristor structure in a high-resistance state obtained using electrostatic force microscopy: Fractal and multifractal dynamics of surface. Appl. Surf. Sci. 2023, 647, 158863. [Google Scholar] [CrossRef]
- Min, Y.-S.; Cho, Y.J.; Ko, J.-H.; Bae, E.J.; Park, W.; Hwang, C.S. Atomic Layer Deposition of Bi1−x−yTixSiyOz Thin Films from Alkoxide Precursors and Water. J. Electrochem. Soc. 2005, 152, F124. [Google Scholar] [CrossRef]
- Martinson, A.B.F.; DeVries, M.J.; Libera, J.A.; Christensen, S.T.; Hupp, J.T.; Pellin, M.J.; Elam, J.W. Atomic Layer Deposition of Fe2O3 Using Ferrocene and Ozone. J. Phys. Chem. C 2011, 115, 4333–4339. [Google Scholar] [CrossRef]
- Lie, M.; Fjellvåg, H.; Kjekshus, A. Growth of Fe2O3 thin films by atomic layer deposition. Thin Solid Films 2005, 488, 74–81. [Google Scholar] [CrossRef]
- Akbashev, A.R.; Chen, G.; Spanier, J.E. A Facile Route for Producing Single-Crystalline Epitaxial Perovskite Oxide Thin Films. Nano Lett. 2014, 14, 44–49. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Sarkar, T.; Fortalnova, E.A.; Politova, E.D.; Stefanovich, S.Y.; Safronenko, M.G.; Nordblad, P.; Mathieu, R. Composition dependence of the multifunctional properties of Nd-doped Bi4Ti3O12 ceramics. J. Mater. Sci. Mater. Electron. 2017, 28, 7692–7707. [Google Scholar] [CrossRef]
- Zhang, S.T.; Lu, M.H.; Wu, D.; Chen, Y.F.; Ming, N.B. Larger polarization and weak ferromagnetism in quenched BiFeO3 ceramics with a distorted rhombohedral crystal structure. Appl. Phys. Lett. 2005, 87, 262907. [Google Scholar] [CrossRef]
- Lisińska-Czekaj, A.; Lubina, M.; Czekaj, D.; Rerak, M.; Garbarz-Glos, B.; Bąk, W. Influence of Processing Conditions on Crystal Structure of Bi6Fe2Ti3O18 Ceramics. Arch. Metall. Mater. 2016, 61, 881–886. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman spectrum of brookite, TiO2 (Pbca, Z = 8). J. Raman Spectrosc. 1995, 26, 57–62. [Google Scholar] [CrossRef]
- Kooriyattil, S.; Pavunny, S.P.; Barrionuevo, D.; Katiyar, R.S. Optical, ferroelectric, and piezoresponse force microscopy studies of pulsed laser deposited Aurivillius Bi5FeTi3O15 thin films. J. Appl. Phys. 2014, 116, 144101. [Google Scholar] [CrossRef]
- Lomanova, N.A. Aurivillius Phases Bim + 1Fem – 3Ti3O3m + 3: Synthesis, Structure, and Properties (a Review). Russ. J. Inorg. Chem. 2022, 67, 741–753. [Google Scholar] [CrossRef]
- Lu, C.D.; Chang, L.S.; Lu, Y.F.; Lu, F.H. The growth of interfacial compounds between titanium dioxide and bismuth oxide. Ceram. Int. 2009, 35, 2699–2704. [Google Scholar] [CrossRef]
- Orudzhev, F.F.; Ramazanov, S.M.; Isaev, A.B.; Alikhanov, N.M.R.; Sobola, D.; Presniakov, M.Y.; Kaviyarasu, K. Self-organization of layered perovskites on TiO2 nanotubes surface by atomic layer deposition. Mater. Today Proc. 2021, 36, 364–367. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Z.; Wang, G.; Geng, X.; Xiao, Z.; Sun, Z.; Sun, Z.; Peng, R.; Lu, Y. Nanoscale Structural Modulation and Low-temperature Magnetic Response in Mixed-layer Aurivillius-type Oxides. Sci. Rep. 2018, 8, 871. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, G.; Huang, Y.; Wang, J.; Peng, R.; Lu, Y. Structural transformation and multiferroic properties in Gd-doped Bi7Fe3Ti3O21 ceramics. RSC Adv. 2014, 4, 30440–30446. [Google Scholar] [CrossRef]
- Sun, S.; Yan, H.; Wang, G.; Wang, J.; Peng, R.; Fu, Z.; Zhai, X.; Mao, X.; Chen, X.; Lu, Y. Room-temperature multiferroic responses arising from 1D phase modulation in correlated Aurivillius-type layer structures. J. Phys. D Appl. Phys. 2016, 49, 125005. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Newnham, R.E. Bismuth titanate solid solutions. Mater. Res. Bull. 1972, 7, 1025–1034. [Google Scholar] [CrossRef]
- Kikuchi, T. Stability of layered bismuth compounds in relation to the structural mismatch. Mater. Res. Bull. 1979, 14, 1561–1569. [Google Scholar] [CrossRef]
- Yan, H.; Inam, F.; Viola, G.; Ning, H.; Zhang, H.; Jiang, Q.; Zeng, T.A.O.; Gao, Z.; Reece, M.J. The Contribution of Electrical Conductivity, Dielectric Permittivity and Domain Switching in Ferroelectric Hysteresis Loops. J. Adv. Dielectr. 2011, 01, 107–118. [Google Scholar] [CrossRef]
- Orudzhev, F.; Ramazanov, S.; Sobola, D.; Isaev, A.; Wang, C.; Magomedova, A.; Kadiev, M.; Kaviyarasu, K. Atomic layer deposition of mixed-layered aurivillius phase on TIO2 nanotubes: Synthesis, characterization and photoelectrocatalytic properties. Nanomaterials 2020, 10, 2183. [Google Scholar] [CrossRef] [PubMed]
- Orudzhev, F.; Ramazanov, S.; Sobola, D.; Alikhanov, N.; Holcman, V.; Škvarenina, L.; Kaspar, P.; Gadjilov, G. Piezoelectric Current Generator Based on Bismuth Ferrite Nanoparticles. Sensors 2020, 20, 6736. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, L.; Wang, Z.; Alshareef, H.; Zhang, X.X. Electrical and piezoelectric properties of BiFeO 3 thin films grown on SrxCa1−xRuO3-buffered SrTiO3 substrates. J. Appl. Phys. 2012, 111, 114102. [Google Scholar] [CrossRef]
- Heo, Y.; Zhang, H.; Alexe, M. Dynamic Control of Piezoelectricity Enhancement via Modulation of the Bulk Photovoltaic Effect in a BiFeO3 Thin Film. Adv. Electron. Mater. 2022, 8, 2200785. [Google Scholar] [CrossRef]
- Ramazanov, S.; Sobola, D.; Gajiev, G.; Orudzhev, F.; Kaspar, P.; Gummetov, A. Multiferroic/Polymer Flexible Structures Obtained by Atomic Layer Deposition. Nanomaterials 2022, 13, 139. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Hu, N.; Bell, J.; Yan, C. Atomistic investigation into the mechanical behaviour of crystalline and amorphous TiO2 nanotubes. RSC Adv. 2016, 6, 28121–28129. [Google Scholar] [CrossRef]
- Fischer, K.; Mayr, S.G. In-Plane Mechanical Response of TiO2 Nanotube Arrays—Intrinsic Properties and Impact of Adsorbates for Sensor Applications. Adv. Mater. 2011, 23, 3838–3841. [Google Scholar] [CrossRef]
- Quddus, M.T.; Mudholkar, M.; Salih, A. Carrier separation technique to optimize conductivity modulation in high voltage rectifiers. In Proceedings of the 2015 IEEE International Conference on Electron Devices and Solid-State Circuits (EDSSC), Singapore, 1–4 June 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 507–510. [Google Scholar]
- Paul, F.; Nama Manjunatha, K.; Paul, S. Non-Zero and Open-Loop Current–Voltage Characteristics in Electronic Memory Devices. Adv. Electron. Mater. 2023, 9, 2300324. [Google Scholar] [CrossRef]
- Pompl, T.; Engel, C.; Wurzer, H.; Kerber, M. Soft breakdown and hard breakdown in ultra-thin oxides. Microelectron. Reliab. 2001, 41, 543–551. [Google Scholar] [CrossRef]
- Funck, C.; Menzel, S. Comprehensive Model of Electron Conduction in Oxide-Based Memristive Devices. ACS Appl. Electron. Mater. 2021, 3, 3674–3692. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramazanov, S.; Orudzhev, F.; Gajiev, G. Surface Functionalization of TiO2 Nanotubes Modified with a Thin Film of BiFeO3. Surfaces 2024, 7, 1-11. https://doi.org/10.3390/surfaces7010001
Ramazanov S, Orudzhev F, Gajiev G. Surface Functionalization of TiO2 Nanotubes Modified with a Thin Film of BiFeO3. Surfaces. 2024; 7(1):1-11. https://doi.org/10.3390/surfaces7010001
Chicago/Turabian StyleRamazanov, Shikhgasan, Farid Orudzhev, and Gaji Gajiev. 2024. "Surface Functionalization of TiO2 Nanotubes Modified with a Thin Film of BiFeO3" Surfaces 7, no. 1: 1-11. https://doi.org/10.3390/surfaces7010001






