Tau Protein in Neurodegenerative Diseases
(Closed)
Share This Topical Collection
Editors
 Prof. Claude M. Wischik
Prof. Claude M. Wischik
 Prof. Claude M. Wischik
Prof. Claude M. Wischik
E-Mail
Website
Collection Editor
TauRx Therapeutics Ltd., 395 King Street, Aberdeen AB24 5RP, UKSchool of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Foresterhill Road, Aberdeen AB25 2ZP, UK
Interests: Alzheimer’s disease; frontotemporal dementia; clinical trials; tau protein
Special Issues, Collections and Topics in MDPI journals
 Dr. Renato Santos
Dr. Renato Santos
 Dr. Renato Santos
Dr. Renato Santos
E-Mail
Website
Collection Editor
School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Foresterhill Road, Aberdeen AB25 2ZP, UK
Interests: Alzheimer’s disease; Tau protein; mitochondria
 Dr. Charles R. Harrington
Dr. Charles R. Harrington
 Dr. Charles R. Harrington
Dr. Charles R. Harrington
E-Mail
Website
Collection Editor
TauRx Therapeutics Ltd., 395 King Street, Aberdeen AB24 5RP, UKSchool of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Foresterhill Road, Aberdeen AB25 2ZP, UK
Interests: Alzheimer’s disease; neurodegeneration; tau protein; tau-based therapeutics; clinical trials
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Tau is a family of proteins providing numerous functions in the brain. The clearest evidence of the importance of tau proteins can be seen from the pathological changes in tau that arise in Alzheimer’s disease and other neurodegenerative disorders. These changes are closely associated with cognitive disfunction.
This Topical Collection aims to provide new concepts in the pathogenesis and molecular mechanism of Alzheimer's disease and related tauopathies. Original articles and comprehensive reviews are welcome.
This Topical Collection of Cells aims to summarize our understanding of the molecular and cellular basis of the aetiopathogenesis of Alzheimer’s disease and related disorders, with a special focus on tau-targeted therapeutic approaches both in the clinic and in disease models.
Prof. Claude M. Wischik
Dr. Renato Santos
Dr. Charles R. Harrington
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (5 papers)
Open AccessReview
Populations of Tau Conformers Drive Prion-like Strain Effects in Alzheimer’s Disease and Related Dementias
by
Lenka Hromadkova, Mohammad Khursheed Siddiqi, He Liu and Jiri G. Safar
Cited by 7 | Viewed by 2795
Abstract
Recent findings of diverse populations of prion-like conformers of misfolded tau protein expand the prion concept to Alzheimer’s disease (AD) and monogenic frontotemporal lobar degeneration (FTLD)-MAPT P301L, and suggest that distinct strains of misfolded proteins drive the phenotypes and progression rates in many
[...] Read more.
Recent findings of diverse populations of prion-like conformers of misfolded tau protein expand the prion concept to Alzheimer’s disease (AD) and monogenic frontotemporal lobar degeneration (FTLD)-MAPT P301L, and suggest that distinct strains of misfolded proteins drive the phenotypes and progression rates in many neurodegenerative diseases. Notable progress in the previous decades has generated many lines of proof arguing that yeast, fungal, and mammalian prions determine heritable as well as infectious traits. The extraordinary phenotypic diversity of human prion diseases arises from structurally distinct prion strains that target, at different progression speeds, variable brain structures and cells. Although human prion research presents beneficial lessons and methods to study the mechanism of strain diversity of protein-only pathogens, the fundamental molecular mechanism by which tau conformers are formed and replicate in diverse tauopathies is still poorly understood. In this review, we summarize up to date advances in identification of diverse tau conformers through biophysical and cellular experimental paradigms, and the impact of heterogeneity of pathological tau strains on personalized structure- and strain-specific therapeutic approaches in major tauopathies.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Microtubules as Regulators of Neural Network Shape and Function: Focus on Excitability, Plasticity and Memory
by
Fernando Peña-Ortega, Ángel Abdiel Robles-Gómez and Lorena Xolalpa-Cueva
Cited by 6 | Viewed by 4236
Abstract
Neuronal microtubules (MTs) are complex cytoskeletal protein arrays that undergo activity-dependent changes in their structure and function as a response to physiological demands throughout the lifespan of neurons. Many factors shape the allostatic dynamics of MTs and tubulin dimers in the cytosolic microenvironment,
[...] Read more.
Neuronal microtubules (MTs) are complex cytoskeletal protein arrays that undergo activity-dependent changes in their structure and function as a response to physiological demands throughout the lifespan of neurons. Many factors shape the allostatic dynamics of MTs and tubulin dimers in the cytosolic microenvironment, such as protein–protein interactions and activity-dependent shifts in these interactions that are responsible for their plastic capabilities. Recently, several findings have reinforced the role of MTs in behavioral and cognitive processes in normal and pathological conditions. In this review, we summarize the bidirectional relationships between MTs dynamics, neuronal processes, and brain and behavioral states. The outcomes of manipulating the dynamicity of MTs by genetic or pharmacological approaches on neuronal morphology, intrinsic and synaptic excitability, the state of the network, and behaviors are heterogeneous. We discuss the critical position of MTs as responders and adaptative elements of basic neuronal function whose impact on brain function is not fully understood, and we highlight the dilemma of artificially modulating MT dynamics for therapeutic purposes.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Impairments in Brain Bioenergetics in Aging and Tau Pathology: A Chicken and Egg Situation?
by
Amandine Grimm
Cited by 14 | Viewed by 4044
Abstract
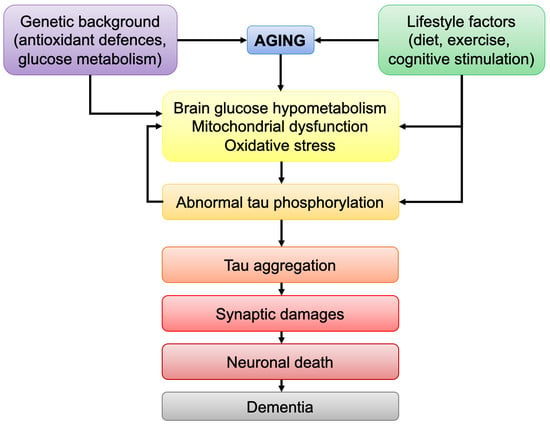
The brain is the most energy-consuming organ of the body and impairments in brain energy metabolism will affect neuronal functionality and viability. Brain aging is marked by defects in energetic metabolism. Abnormal tau protein is a hallmark of tauopathies, including Alzheimer’s disease (AD).
[...] Read more.
The brain is the most energy-consuming organ of the body and impairments in brain energy metabolism will affect neuronal functionality and viability. Brain aging is marked by defects in energetic metabolism. Abnormal tau protein is a hallmark of tauopathies, including Alzheimer’s disease (AD). Pathological tau was shown to induce bioenergetic impairments by affecting mitochondrial function. Although it is now clear that mutations in the tau-coding gene lead to tau pathology, the causes of abnormal tau phosphorylation and aggregation in non-familial tauopathies, such as sporadic AD, remain elusive. Strikingly, both tau pathology and brain hypometabolism correlate with cognitive impairments in AD. The aim of this review is to discuss the link between age-related decrease in brain metabolism and tau pathology. In particular, the following points will be discussed: (i) the common bioenergetic features observed during brain aging and tauopathies; (ii) how age-related bioenergetic defects affect tau pathology; (iii) the influence of lifestyle factors known to modulate brain bioenergetics on tau pathology. The findings compiled here suggest that age-related bioenergetic defects may trigger abnormal tau phosphorylation/aggregation and cognitive impairments after passing a pathological threshold. Understanding the effects of aging on brain metabolism may therefore help to identify disease-modifying strategies against tau-induced neurodegeneration.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
The Many Faces of Post-Ischemic Tau Protein in Brain Neurodegeneration of the Alzheimer’s Disease Type
by
Ryszard Pluta, Stanisław J. Czuczwar, Sławomir Januszewski and Mirosław Jabłoński
Cited by 15 | Viewed by 3459
Abstract
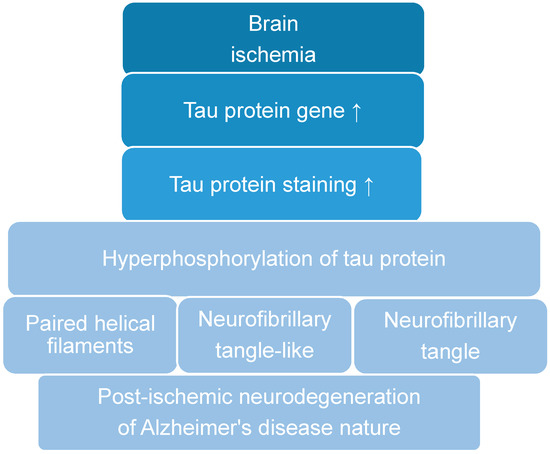
Recent data suggest that post-ischemic brain neurodegeneration in humans and animals is associated with the modified tau protein in a manner typical of Alzheimer’s disease neuropathology. Pathological changes in the tau protein, at the gene and protein level due to cerebral ischemia, can
[...] Read more.
Recent data suggest that post-ischemic brain neurodegeneration in humans and animals is associated with the modified tau protein in a manner typical of Alzheimer’s disease neuropathology. Pathological changes in the tau protein, at the gene and protein level due to cerebral ischemia, can lead to the development of Alzheimer’s disease-type neuropathology and dementia. Some studies have shown increased tau protein staining and gene expression in neurons following ischemia-reperfusion brain injury. Recent studies have found the tau protein to be associated with oxidative stress, apoptosis, autophagy, excitotoxicity, neuroinflammation, blood-brain barrier permeability, mitochondrial dysfunction, and impaired neuronal function. In this review, we discuss the interrelationship of these phenomena with post-ischemic changes in the tau protein in the brain. The tau protein may be at the intersection of many pathological mechanisms due to severe neuropathological changes in the brain following ischemia. The data indicate that an episode of cerebral ischemia activates the damage and death of neurons in the hippocampus in a tau protein-dependent manner, thus determining a novel and important mechanism for the survival and/or death of neuronal cells following ischemia. In this review, we update our understanding of proteomic and genomic changes in the tau protein in post-ischemic brain injury and present the relationship between the modified tau protein and post-ischemic neuropathology and present a positive correlation between the modified tau protein and a post-ischemic neuropathology that has characteristics of Alzheimer’s disease-type neurodegeneration.
Full article
►▼
Show Figures
Open AccessArticle
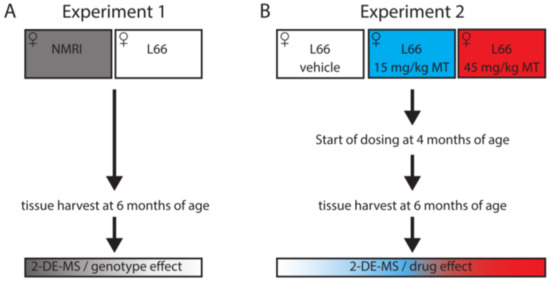
Proteomic Analysis of Hydromethylthionine in the Line 66 Model of Frontotemporal Dementia Demonstrates Actions on Tau-Dependent and Tau-Independent Networks
by
Karima Schwab, Valeria Melis, Charles R. Harrington, Claude M. Wischik, Mandy Magbagbeolu, Franz Theuring and Gernot Riedel
Cited by 3 | Viewed by 4056
Abstract
Abnormal aggregation of tau is the pathological hallmark of tauopathies including frontotemporal dementia (FTD). We have generated tau-transgenic mice that express the aggregation-prone P301S human tau (line 66). These mice present with early-onset, high tau load in brain and FTD-like behavioural deficiencies. Several
[...] Read more.
Abnormal aggregation of tau is the pathological hallmark of tauopathies including frontotemporal dementia (FTD). We have generated tau-transgenic mice that express the aggregation-prone P301S human tau (line 66). These mice present with early-onset, high tau load in brain and FTD-like behavioural deficiencies. Several of these behavioural phenotypes and tau pathology are reversed by treatment with hydromethylthionine but key pathways underlying these corrections remain elusive. In two proteomic experiments, line 66 mice were compared with wild-type mice and then vehicle and hydromethylthionine treatments of line 66 mice were compared. The brain proteome was investigated using two-dimensional electrophoresis and mass spectrometry to identify protein networks and pathways that were altered due to tau overexpression or modified by hydromethylthionine treatment. Overexpression of mutant tau induced metabolic/mitochondrial dysfunction, changes in synaptic transmission and in stress responses, and these functions were recovered by hydromethylthionine. Other pathways, such as NRF2, oxidative phosphorylation and protein ubiquitination were activated by hydromethylthionine, presumably independent of its function as a tau aggregation inhibitor. Our results suggest that hydromethylthionine recovers cellular activity in both a tau-dependent and a tau-independent fashion that could lead to a wide-spread improvement of homeostatic function in the FTD brain.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: To be determined
Authors: Koorosh Shahpasand; et al.
Affiliation: Department of Brain and Cognitive Sciences, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
Title: Cryo-EM structures of tau filaments from human brain
Authors: Michel Goedert
Affiliation: MRC Laboratory of Molecular Biology, Francis Crick Avenue, Cambridge CB1 7PH, UK
Title: Tau-based therapies and the use of biomarkers in clinical development
Authors: Miguel Medina; et al.
Affiliation: Network Center for Biomedical Research on Neurodegenerative Diseases (CIBERNED), 28031 Madrid, Spain
Title: Neuroprotection of cholinergic neurons with tau aggregation inhibitor and rivastigmine in L1 mice with Alzheimer's-like tauopathy
Authors: Maciej Zadrozny; Marta Steczkowska; Malgorzata Wydrych; Patrycja Drapich; Anna Gasiorowska; Wiktor Niewiadomski; Gernot Riedel; Claude Wischik; Grazyna Niewiadomska
Affiliation: Nencki Institute of Experimental Biology, Polish Academy of Sciences, 02-093 Warsaw, Poland