Anthrax Toxins
A topical collection in Toxins (ISSN 2072-6651). This collection belongs to the section "Bacterial Toxins".
Viewed by 116008
Share This Topical Collection
Editor
 Dr. Shihui Liu
Dr. Shihui Liu
 Dr. Shihui Liu
Dr. Shihui Liu
E-Mail
Collection Editor
National Institute of Dental and Craniofacial Research, National Institutes of Health, 30 Convent Drive Building 30, Room 3A308, Bethesda, Maryland 20892-4340, USA
Interests: anthrax toxin; toxin receptor; protease; tumor targeting; dipthamide
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Bacillus anthracis is a Gram-positive, rod-shaped, spore-forming bacterium, and is the causative agent of anthrax disease that affects both animals and humans. B. anthracis causes anthrax through a combination of bacterial infection and toxemia. The exotoxins secreted by B. anthracis consist of three individually non-toxic proteins: the cellular binding component, protective antigen (PA), and two enzymatic moieties, lethal factor (LF) and edema factor (EF). PA binds to two host cell-surface integrin-like proteins ̶ tumor endothelium marker-8 (TEM8, also termed anthrax toxin receptor 1) and capillary morphogenesis protein-2 (ANTXR2), and is then processed by ubiquitous cell-associated furin protease to form LF- and EF-binding competent PA oligomers, which translocate LF and/or EF into the cytosol of cells to exert their cytotoxic actions. Thus, LF plus PA form lethal toxin and EF plus PA form edema toxin, both toxins playing essential roles in anthrax pathogenesis. At the initial stages of infection, LT and ET coordinately impair the host innate immune response, enabling the pathogen to establish a successful infection. At late stages, when high concentrations are reached, LT and ET can directly cause host death through targeting distinct vital systems. Although the enzymatic activities and the molecular targets of LF and EF have been known for some time, the detailed mechanisms underlying the toxin-induced tissue damage are still poorly understood.
This Special Issue on “Anthrax Toxins” will cover original studies, reviews, and method papers related to all aspects of anthrax toxins, the toxin receptors, and toxin-host interactions, from basic research to application therapeutics.
Dr. Shihui Liu
Collection Editor
Submission
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are refereed through a peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Toxins is an international peer-reviewed Open Access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1400 CHF (Swiss Francs).
Keywords
- anthrax toxin
- protective antigen
- lethal factor
- edema factor
- anthrax toxin receptor
Published Papers (20 papers)
Open AccessArticle
Whole-Transcriptome Analysis Highlights Adenylyl Cyclase Toxins-Derived Modulation of NF-κB and ERK1/2 Pathways in Macrophages
by
Taoran Zhao, Ruihua Li, Mengyin Qian, Meirong Wang, Xiaozheng Zhang, Yuhan Wang, Xinghui Zhao and Jun Xie
Viewed by 1353
Abstract
Edema toxin (ET), one of the main toxic factors of
Bacillus anthracis (
B. anthracis), is a kind of potent adenylate cyclase (AC).
B. anthracis has adapted to resist macrophage microbicidal mechanisms in part by secreting ET. To date, there is limited
[...] Read more.
Edema toxin (ET), one of the main toxic factors of
Bacillus anthracis (
B. anthracis), is a kind of potent adenylate cyclase (AC).
B. anthracis has adapted to resist macrophage microbicidal mechanisms in part by secreting ET. To date, there is limited information on the pathogenic mechanisms used by ET to manipulate macrophage function, especially at the transcriptome level. We used RNA sequencing to study transcriptional changes in RAW264.7 cells treated with ET. We aimed to identify molecular events associated with the establishment of infection and followed changes in cellular proteins. Our results indicate that ET inhibited TNF-α expression in the RAW264.7 mouse macrophage cell line by activating the cAMP/PKA pathway. ET challenge of macrophages induced a differential expression of genes that participate in multiple macrophage effector functions such as cytokine production, cell adhesion, and the inflammatory response. Furthermore, ET influenced the expression of components of the ERK1/2, as well as the NF-αB signaling pathways. We also showed that ET treatments inhibit the phosphorylation of the ERK1/2 protein. ET also attenuated NF-αB subunit p65 phosphorylation and transcriptional activity of NF-αB via the cAMP/PKA pathway in macrophages. Since the observed modulatory effects were characteristic only of the bacterial exotoxin ET, we propose this may be a mechanism used by B. anthracis to manipulate macrophages and establish systemic infection.
Full article
►▼
Show Figures
Open AccessArticle
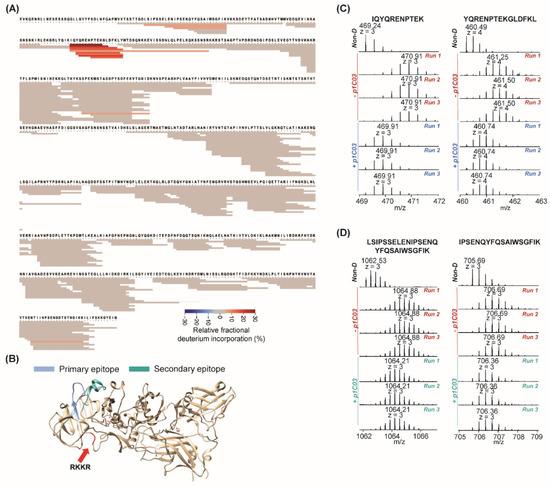
Hydrogen-Deuterium Exchange Mass Spectrometry Reveals a Novel Binding Region of a Neutralizing Fully Human Monoclonal Antibody to Anthrax Protective Antigen
by
Mulin Fang, Zhe Wang, Kathleen Norris, Judith A. James, Si Wu and Kenneth Smith
Cited by 5 | Viewed by 3318
Abstract
Anthrax vaccine adsorbed (AVA) containing protective antigen (PA) is the only FDA-approved anthrax vaccine in the United States. Characterization of the binding of AVA-induced anti-PA human antibodies against the PA antigen after vaccination is crucial to understanding mechanisms of the AVA-elicited humoral immune
[...] Read more.
Anthrax vaccine adsorbed (AVA) containing protective antigen (PA) is the only FDA-approved anthrax vaccine in the United States. Characterization of the binding of AVA-induced anti-PA human antibodies against the PA antigen after vaccination is crucial to understanding mechanisms of the AVA-elicited humoral immune response. Hydrogen deuterium exchange mass spectrometry (HDX-MS) is often coupled with a short liquid chromatography gradient (e.g., 5–10 min) for the characterization of protein interactions. We recently developed a long-gradient (e.g., 90 min), sub-zero temperature, ultra-high performance liquid chromatography HDX-MS (UPLC-HDX-MS) platform that has significantly increased separation power and limited back-exchange for the analysis of protein samples with high complexity. In this study, we demonstrated the utility of this platform for mapping antibody–antigen epitopes by examining four fully human monoclonal antibodies to anthrax PA. Antibody p1C03, with limited neutralizing activity in vivo, bound to a region on domain 1A of PA. p6C04 and p1A06, with no neutralizing activities, bound to the same helix on domain 3 to prevent oligomerization of PA. We found p6C01 strongly bound to domain 3 on a different helix region. We also identified a secondary epitope for p6C01, which likely leads to the blocking of furin cleavage of PA after p6C01 binding. This novel binding of p6C01 results in highly neutralizing activity. This is the first report of this distinct binding mechanism for a highly neutralizing fully human antibody to anthrax protective antigen. Studying such epitopes can facilitate the development of novel therapeutics against anthrax.
Full article
►▼
Show Figures
Open AccessArticle
Establishment of a New Zealand White Rabbit Model for Lethal Toxin (LT) Challenge and Efficacy of Monoclonal Antibody 5E11 in the LT-Challenged Rabbit Model
by
Duanyang Zhang, Weicen Liu, Zhonghua Wen, Bing Li, Shuling Liu, Jianmin Li and Wei Chen
Cited by 9 | Viewed by 4292
Abstract
Anthrax caused by
Bacillus anthracis is a lethal infectious disease, especially when inhaled, and the mortality rate approaches 100% without treatment. The anthrax antitoxin monoclonal antibody (MAb) 5E11 is a humanized antibody that targets the anthrax protective antigen (PA). The efficacy of 5E11
[...] Read more.
Anthrax caused by
Bacillus anthracis is a lethal infectious disease, especially when inhaled, and the mortality rate approaches 100% without treatment. The anthrax antitoxin monoclonal antibody (MAb) 5E11 is a humanized antibody that targets the anthrax protective antigen (PA). The efficacy of 5E11 needs proper animal models. However, anthrax spores are extremely dangerous, so experiments must be conducted under Biosafety Level 3 conditions. Considering the critical effects of lethal toxin (LT) on hosts during infection, we report the establishment of a LT-challenged rabbit model, which caused 100% mortality with a dose of 2 mg PA + 1 mg LF, while a 4 mg PA + 2 mg LF challenge could limit death to within three days. Then, we evaluated 5E11 efficacy against LT. A prophylactic study showed that the i.v. administration of 40 mg/kg 5E11 four days before lethal dose LT challenge could lead to 100% survival. In therapeutic studies, the i.v. administration of 40 mg/kg 5E11 10 min after lethal dose LT challenge could provide complete protection. Overall, we developed a new LT-challenged rabbit model, and our results indicate that 5E11 shows potential for the clinical application in anthrax treatment.
Full article
►▼
Show Figures
Open AccessArticle
Phage Display Analysis of Monoclonal Antibody Binding to Anthrax Toxin Lethal Factor
by
Jason M. Goldstein, Joo Lee, Xiaoling Tang, Anne E. Boyer, John R. Barr, Dennis A. Bagarozzi Jr. and Conrad P. Quinn
Cited by 5 | Viewed by 5827
Abstract
AVR1674 and AVR1675 are monoclonal antibodies (mAbs) that bind with high specificity to anthrax toxin lethal factor (LF) and lethal toxin (LTx). These mAbs have been used as pivotal reagents to develop anthrax toxin detection tests using mass spectrometry. The mAbs were demonstrated
[...] Read more.
AVR1674 and AVR1675 are monoclonal antibodies (mAbs) that bind with high specificity to anthrax toxin lethal factor (LF) and lethal toxin (LTx). These mAbs have been used as pivotal reagents to develop anthrax toxin detection tests using mass spectrometry. The mAbs were demonstrated to bind LF with good affinity (K
D 10
−7–10
−9 M) and to enhance LF-mediated cleavage of synthetic peptide substrates in vitro. Sequence analysis indicated that the mAbs shared 100% amino acid identity in their complementarity determining regions (CDR). A phage display library based on a combinatorial library of random heptapeptides fused to the pIII coat protein of M13 phage was enriched and screened to identify peptide sequences with mAb binding properties. Selection and sequence analysis of 18 anti-LF-reactive phage clones identified a 7-residue (P1–P7) AVR1674/1675 consensus target binding sequence of T
P1-X
P2-K/R
P3-D
P4-D/E
P5-Z
P6-X/Z
P7 (X = aromatic, Z = non-polar). The phage peptide sequence with highest affinity binding to AVR1674/1675 was identified as T-F-K-D-E-I-V. Synthetic oligopeptides were designed based on the phage sequences and interacted with mAbs with high affinity (K
D ~ 10
−9 M). Single amino acid substitutions of A, H, or Q in the peptides identified positions P1–P5 as critical residues for mAb-peptide interactions. CLUSTALW alignment of phage sequences with native LF implicated residues 644–650 (sequence T-H-Q-D-E-I-Y) as a putative linear epitope component located within a structural loop (L2) of LF Domain IV. The activation effects of these mAbs contribute to the analytic sensitivity of function-based LF detection assays.
Full article
►▼
Show Figures
Open AccessArticle
HDAC8 Prevents Anthrax Lethal Toxin-induced Cell Cycle Arrest through Silencing PTEN in Human Monocytic THP-1 Cells
by
Soon-Duck Ha, Woohyun Cho and Sung Ouk Kim
Cited by 11 | Viewed by 6152
Abstract
Anthrax lethal toxin (LeTx) is a cytotoxic virulence factor that causes cell cycle arrest and cell death in various cell types. However, susceptibility to the cytotoxic effects varies depending on cell types. In proliferating monocytes, LeTx has only transient cytotoxic effects due to
[...] Read more.
Anthrax lethal toxin (LeTx) is a cytotoxic virulence factor that causes cell cycle arrest and cell death in various cell types. However, susceptibility to the cytotoxic effects varies depending on cell types. In proliferating monocytes, LeTx has only transient cytotoxic effects due to activation of the phosphoinositide 3-kinase (PI3K)-AKT-mediated adaptive responses. To date, the mechanism of LeTx in activating PI3K-AKT signaling axis is unknown. This study shows that the histone deacetylase 8 (HDAC8) is involved in activating PI3K-AKT signaling axis through down-regulating the phosphatase and tensin homolog 1 (PTEN) in human monocytic THP-1 cells. The HDAC8-specific activator TM-2-51 and inhibitor PCI-34051 enhanced and prevented, respectively, AKT activation and cell cycle progression in LeTx-treated cells. Furthermore, HDAC8 induced tri-methylation of histone H3 lysine 27 (H3K27me3), which is known to suppress PTEN expression, through at least in part down-regulating the H3K27me3 eraser Jumonji Domain Containing (JMJD) 3. Importantly, the JMJD3-specific inhibitor GSK-J4 induced AKT activation and protected cell cycle arrest in LeTx-treated cells, regardless the presence of HDAC8 activity. Collectively, this study for the first time demonstrated that HDAC8 activity determines susceptibility to cell cycle arrest induced by LeTx, through regulating the PI3K-PTEN-AKT signaling axis.
Full article
►▼
Show Figures
Open AccessArticle
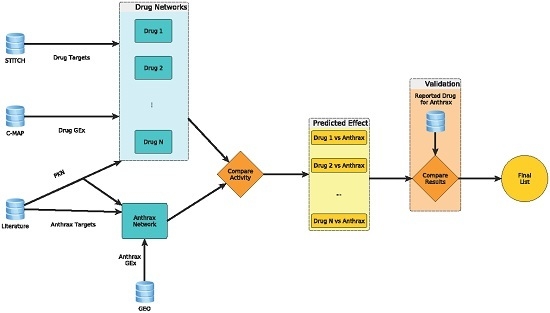
A Biologically-Based Computational Approach to Drug Repurposing for Anthrax Infection
by
Jane P. F. Bai, Theodore Sakellaropoulos and Leonidas G. Alexopoulos
Cited by 3 | Viewed by 6139
Abstract
Developing drugs to treat the toxic effects of lethal toxin (LT) and edema toxin (ET) produced by
B. anthracis is of global interest
. We utilized a computational approach to score 474 drugs/compounds for their ability to reverse the toxic effects of anthrax
[...] Read more.
Developing drugs to treat the toxic effects of lethal toxin (LT) and edema toxin (ET) produced by
B. anthracis is of global interest
. We utilized a computational approach to score 474 drugs/compounds for their ability to reverse the toxic effects of anthrax toxins. For each toxin or drug/compound, we constructed an activity network by using its differentially expressed genes, molecular targets, and protein interactions. Gene expression profiles of drugs were obtained from the Connectivity Map and those of anthrax toxins in human alveolar macrophages were obtained from the Gene Expression Omnibus. Drug rankings were based on the ability of a drug/compound’s mode of action in the form of a signaling network to reverse the effects of anthrax toxins; literature reports were used to verify the top 10 and bottom 10 drugs/compounds identified. Simvastatin and bepridil with reported in vitro potency for protecting cells from LT and ET toxicities were computationally ranked fourth and eighth. The other top 10 drugs were fenofibrate, dihydroergotamine, cotinine, amantadine, mephenytoin, sotalol, ifosfamide, and mefloquine; literature mining revealed their potential protective effects from LT and ET toxicities. These drugs are worthy of investigation for their therapeutic benefits and might be used in combination with antibiotics for treating
B. anthracis infection.
Full article
►▼
Show Figures
Open AccessCommunication
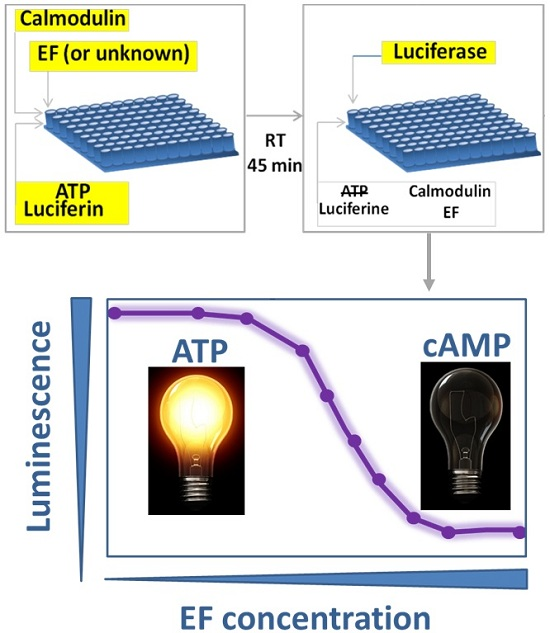
A Simple Luminescent Adenylate-Cyclase Functional Assay for Evaluation of Bacillus anthracis Edema Factor Activity
by
Ma’ayan Israeli, Shahar Rotem, Uri Elia, Erez Bar-Haim, Ofer Cohen and Theodor Chitlaru
Cited by 7 | Viewed by 4687
Abstract
Edema Factor (EF), the toxic sub-unit of the
Bacillus anthracis Edema Toxin (ET) is a calmodulin-dependent adenylate cyclase whose detrimental activity in the infected host results in severe edema. EF is therefore a major virulence factor of
B. anthracis. We describe a
[...] Read more.
Edema Factor (EF), the toxic sub-unit of the
Bacillus anthracis Edema Toxin (ET) is a calmodulin-dependent adenylate cyclase whose detrimental activity in the infected host results in severe edema. EF is therefore a major virulence factor of
B. anthracis. We describe a simple, rapid and reliable functional adenylate-cyclase assay based on inhibition of a luciferase-mediated luminescence reaction. The assay exploits the efficient adenylate cyclase-mediated depletion of adenosine tri-phosphate (ATP), and the strict dependence on ATP of the light-emitting luciferase-catalyzed luciferin-conversion to oxyluciferin, which can be easily visualized. The assay exhibits a robust EF-dose response decrease in luminescence, which may be specifically reverted by anti-EF antibodies. The application of the assay is exemplified in: (a) determining the presence of EF in
B. anthracis cultures, or its absence in cultures of EF-defective strains; (b) evaluating the anti-EF humoral response in experimental animals infected/vaccinated with
B. anthracis; and (c) rapid discrimination between EF producing and non-producing bacterial colonies. Furthermore, the assay may be amenable with high-throughput screening for EF inhibitory molecules.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
The Ins and Outs of Anthrax Toxin
by
Sarah Friebe, F. Gisou Van der Goot and Jérôme Bürgi
Cited by 83 | Viewed by 12493
Abstract
Anthrax is a severe, although rather rare, infectious disease that is caused by the Gram-positive, spore-forming bacterium
Bacillus anthracis. The infectious form is the spore and the major virulence factors of the bacterium are its poly-γ-D-glutamic acid capsule and the tripartite anthrax
[...] Read more.
Anthrax is a severe, although rather rare, infectious disease that is caused by the Gram-positive, spore-forming bacterium
Bacillus anthracis. The infectious form is the spore and the major virulence factors of the bacterium are its poly-γ-D-glutamic acid capsule and the tripartite anthrax toxin. The discovery of the anthrax toxin receptors in the early 2000s has allowed in-depth studies on the mechanisms of anthrax toxin cellular entry and translocation from the endocytic compartment to the cytoplasm. The toxin generally hijacks the endocytic pathway of CMG2 and TEM8, the two anthrax toxin receptors, in order to reach the endosomes. From there, the pore-forming subunit of the toxin inserts into endosomal membranes and enables translocation of the two catalytic subunits. Insertion of the pore-forming unit preferentially occurs in intraluminal vesicles rather than the limiting membrane of the endosome, leading to the translocation of the enzymatic subunits in the lumen of these vesicles. This has important consequences that will be discussed. Ultimately, the toxins reach the cytosol where they act on their respective targets. Target modification has severe consequences on cell behavior, in particular on cells of the immune system, allowing the spread of the bacterium, in severe cases leading to host death. Here we will review the literature on anthrax disease with a focus on the structure of the toxin, how it enters cells and its immunological effects.
Full article
►▼
Show Figures
Open AccessArticle
Quantitative Determination of Lethal Toxin Proteins in Culture Supernatant of Human Live Anthrax Vaccine Bacillus anthracis A16R
by
Xiaodong Zai, Jun Zhang, Ju Liu, Jie Liu, Liangliang Li, Ying Yin, Ling Fu, Junjie Xu and Wei Chen
Cited by 11 | Viewed by 5807
Abstract
Bacillus anthracis (
B. anthracis) is the etiological agent of anthrax affecting both humans and animals. Anthrax toxin (AT) plays a major role in pathogenesis. It includes lethal toxin (LT) and edema toxin (ET), which are formed by the combination of protective
[...] Read more.
Bacillus anthracis (
B. anthracis) is the etiological agent of anthrax affecting both humans and animals. Anthrax toxin (AT) plays a major role in pathogenesis. It includes lethal toxin (LT) and edema toxin (ET), which are formed by the combination of protective antigen (PA) and lethal factor (LF) or edema factor (EF), respectively. The currently used human anthrax vaccine in China utilizes live-attenuated
B. anthracis spores (A16R; pXO1+, pXO2−) that produce anthrax toxin but cannot produce the capsule. Anthrax toxins, especially LT, have key effects on both the immunogenicity and toxicity of human anthrax vaccines. Thus, determining quantities and biological activities of LT proteins expressed by the A16R strain is meaningful. Here, we explored LT expression patterns of the A16R strain in culture conditions using another vaccine strain Sterne as a control. We developed a sandwich ELISA and cytotoxicity-based method for quantitative detection of PA and LF. Expression and degradation of LT proteins were observed in culture supernatants over time. Additionally, LT proteins expressed by the A16R and Sterne strains were found to be monomeric and showed cytotoxic activity, which may be the main reason for side effects of live anthrax vaccines. Our work facilitates the characterization of anthrax vaccines components and establishment of a quality control standard for vaccine production which may ultimately help to ensure the efficacy and safety of the human anthrax vaccine A16R.
Full article
►▼
Show Figures
Open AccessArticle
Diminished but Not Abolished Effect of Two His351 Mutants of Anthrax Edema Factor in a Murine Model
by
Taoran Zhao, Xinghui Zhao, Ju Liu, Yingying Meng, Yingying Feng, Ting Fang, Jinlong Zhang, Xiuxu Yang, Jianmin Li, Junjie Xu and Wei Chen
Cited by 3 | Viewed by 4563
Abstract
Edema toxin (ET), which is composed of a potent adenylate cyclase (AC), edema factor (EF), and protective antigen (PA), is one of the major toxicity factors of
Bacillus anthracis. In this study, we introduced mutations in full-length EF to generate alanine EF(H351A)
[...] Read more.
Edema toxin (ET), which is composed of a potent adenylate cyclase (AC), edema factor (EF), and protective antigen (PA), is one of the major toxicity factors of
Bacillus anthracis. In this study, we introduced mutations in full-length EF to generate alanine EF(H351A) and arginine EF(H351R) variants. In vitro activity analysis displayed that the adenylyl cyclase activity of both the mutants was significantly diminished compared with the wild-type EF. When the native and mutant toxins were administered subcutaneously in a mouse footpad edema model, severe acute swelling was evoked by wild-type ET, while the symptoms induced by mutant toxins were very minor. Systemic administration of these EF variants caused non-lethal hepatotoxicity. In addition, EF(H351R) exhibited slightly higher activity in causing more severe edema than EF(H351A). Our findings demonstrate that the toxicity of ET is not abolished by substitution of EF residue His351 by alanine or arginine. These results also indicate the potential of the mouse footpad edema model as a sensitive method for evaluating both ET toxicity and the efficacy of candidate therapeutic agents.
Full article
►▼
Show Figures
Open AccessReview
Roles of Anthrax Toxin Receptor 2 in Anthrax Toxin Membrane Insertion and Pore Formation
by
Jianjun Sun and Pedro Jacquez
Cited by 15 | Viewed by 8037
Abstract
Interaction between bacterial toxins and cellular surface receptors is an important component of the host-pathogen interaction. Anthrax toxin protective antigen (PA) binds to the cell surface receptor, enters the cell through receptor-mediated endocytosis, and forms a pore on the endosomal membrane that translocates
[...] Read more.
Interaction between bacterial toxins and cellular surface receptors is an important component of the host-pathogen interaction. Anthrax toxin protective antigen (PA) binds to the cell surface receptor, enters the cell through receptor-mediated endocytosis, and forms a pore on the endosomal membrane that translocates toxin enzymes into the cytosol of the host cell. As the major receptor for anthrax toxin
in vivo, anthrax toxin receptor 2 (ANTXR2) plays an essential role in anthrax toxin action by providing the toxin with a high-affinity binding anchor on the cell membrane and a path of entry into the host cell. ANTXR2 also acts as a molecular clamp by shifting the pH threshold of PA pore formation to a more acidic pH range, which prevents premature pore formation at neutral pH before the toxin reaches the designated intracellular location. Most recent studies have suggested that the disulfide bond in the immunoglobulin (Ig)-like domain of ANTXR2 plays an essential role in anthrax toxin action. Here we will review the roles of ANTXR2 in anthrax toxin action, with an emphasis on newly updated knowledge.
Full article
►▼
Show Figures
Open AccessArticle
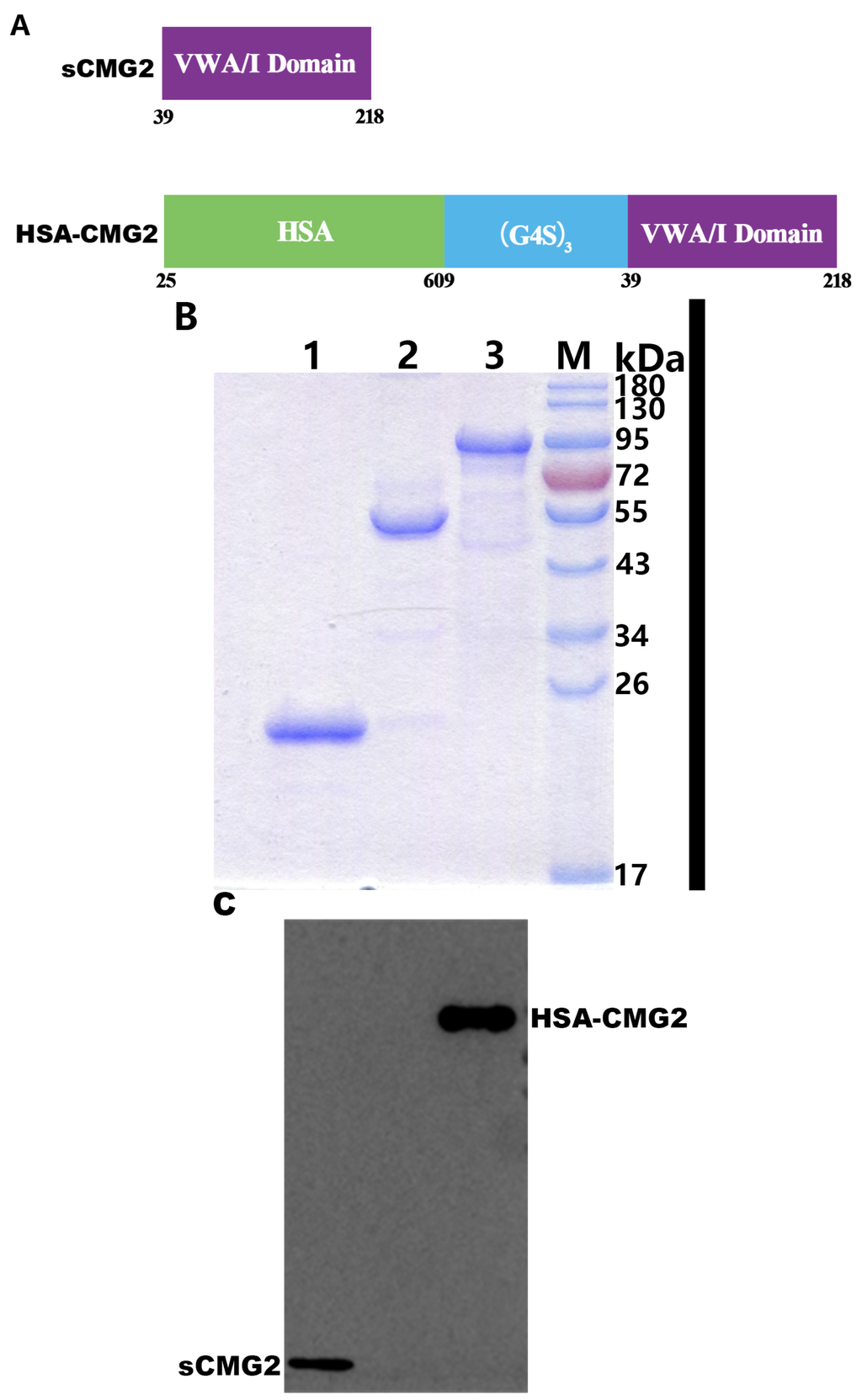
Recombinant HSA-CMG2 Is a Promising Anthrax Toxin Inhibitor
by
Liangliang Li, Qiang Guo, Ju Liu, Jun Zhang, Ying Yin, Dayong Dong, Ling Fu, Junjie Xu and Wei Chen
Cited by 5 | Viewed by 4866
Abstract
Anthrax toxin is the major virulence factor produced by
Bacillus anthracis. Protective antigen (PA) is the key component of the toxin and has been confirmed as the main target for the development of toxin inhibitors. The inhibition of the binding of PA
[...] Read more.
Anthrax toxin is the major virulence factor produced by
Bacillus anthracis. Protective antigen (PA) is the key component of the toxin and has been confirmed as the main target for the development of toxin inhibitors. The inhibition of the binding of PA to its receptor, capillary morphogenesis protein-2 (CMG2), can effectively block anthrax intoxication. The recombinant, soluble von Willebrand factor type A (vWA) domain of CMG2 (sCMG2) has demonstrated potency against anthrax toxin. However, the short half-life of sCMG2
in vivo is a disadvantage for its development as a new anthrax drug. In the present study, we report that HSA-CMG2, a protein combining human serum albumin (HSA) and sCMG2, produced in the
Pichia pastoris expression system prolonged the half-life of sCMG2 while maintaining PA binding ability. The IC
50 of HSA-CMG2 is similar to those of sCMG2 and CMG2-Fc in
in vitro toxin neutralization assays, and HSA-CMG2 completely protects rats from lethal doses of anthrax toxin challenge; these same challenge doses exceed sCMG2 at a sub-equivalent dose ratio and overwhelm CMG2-Fc. Our results suggest that HSA-CMG2 is a promising inhibitor of anthrax toxin and may contribute to the development of novel anthrax drugs.
Full article
►▼
Show Figures
Open AccessCommunication
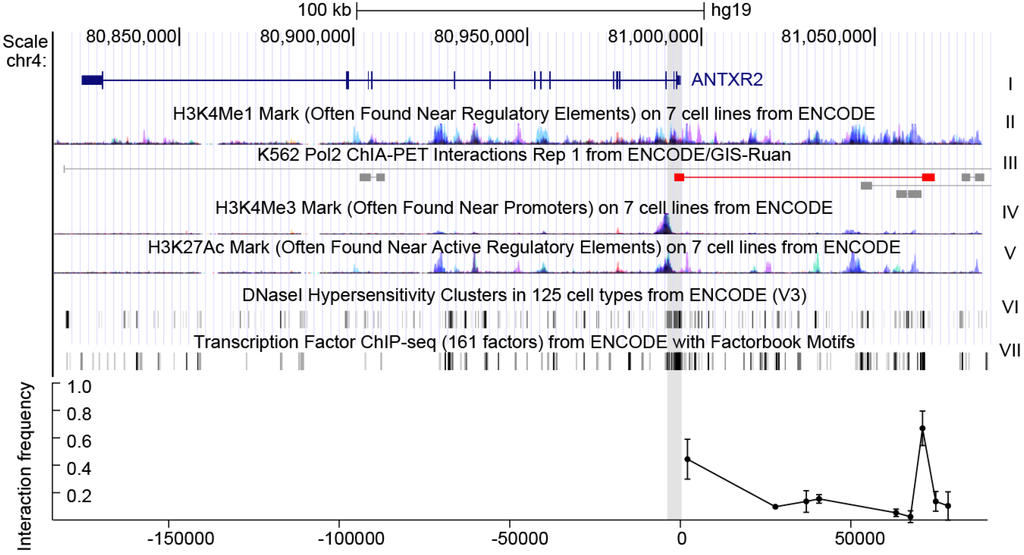
Anthrax Susceptibility: Human Genetic Polymorphisms Modulating ANTXR2 Expression
by
Zhang Zhang, Yan Zhang, Minglei Shi, Bingyu Ye, Wenlong Shen, Ping Li, Lingyue Xing, Xiaopeng Zhang, Lihua Hou, Junjie Xu, Zhihu Zhao and Wei Chen
Cited by 11 | Viewed by 4745
Abstract
Anthrax toxin causes anthrax pathogenesis and expression levels of
ANTXR2 (anthrax toxin receptor 2) are strongly correlated with anthrax toxin susceptibility. Previous studies found that
ANTXR2 transcript abundance varies considerably in individuals of different ethnic/geographical groups, but no eQTLs (expression quantitative trait loci)
[...] Read more.
Anthrax toxin causes anthrax pathogenesis and expression levels of
ANTXR2 (anthrax toxin receptor 2) are strongly correlated with anthrax toxin susceptibility. Previous studies found that
ANTXR2 transcript abundance varies considerably in individuals of different ethnic/geographical groups, but no eQTLs (expression quantitative trait loci) have been identified. By using 3C (chromatin conformation capture), CRISPR-mediated genomic deletion and dual-luciferase reporter assay, gene loci containing cis-regulatory elements of
ANTXR2 were localized. Two SNPs (single nucleotide polymorphism) at the conserved CREB-binding motif, rs13140055 and rs80314910 in the promoter region of the gene, modulating
ANTXR2 promoter activity were identified. Combining these two regulatory SNPs with a previously reported SNP, rs12647691, for the first time, a statistically significant correlation between human genetic variations and anthrax toxin sensitivity was observed. These findings further our understanding of human variability in
ANTXR2 expression and anthrax toxin susceptibility.
Full article
►▼
Show Figures
Open AccessReview
Does Bacillus anthracis Lethal Toxin Directly Depress Myocardial Function? A Review of Clinical Cases and Preclinical Studies
by
Dante A. Suffredini, Hanish Sampath-Kumar, Yan Li, Lernik Ohanjanian, Kenneth E. Remy, Xizhong Cui and Peter Q. Eichacker
Cited by 10 | Viewed by 5424
Abstract
The US outbreak of
B.anthracis infection in 2001 and subsequent cases in the US and Europe demonstrate that anthrax is a continuing risk for the developed world. While several bacterial components contribute to the pathogenesis of
B. anthracis, production of lethal toxin
[...] Read more.
The US outbreak of
B.anthracis infection in 2001 and subsequent cases in the US and Europe demonstrate that anthrax is a continuing risk for the developed world. While several bacterial components contribute to the pathogenesis of
B. anthracis, production of lethal toxin (LT) is strongly associated with the development of hypotension and lethality. However, the mechanisms underlying the cardiovascular instability LT produces are unclear. Some evidence suggests that LT causes shock by impairing the peripheral vasculature, effects consistent with the substantial extravasation of fluid in patients dying with
B. anthracis. Other data suggests that LT directly depresses myocardial function. However a clinical correlate for this latter possibility is less evident since functional studies and post-mortem examination in patients demonstrate absent or minimal cardiac changes. The purposes of this review were to first present clinical studies of cardiac functional and histologic pathology with
B. anthracis infection and to then examine
in vivo,
in vitro, and
ex vivo preclinical studies of LT’s myocardial effects. Together, these data suggest that it is unclear whether that LT directly depresses cardiac function. This question is important for the clinical management and development of new therapies for anthrax and efforts should continue to be made to answer it.
Full article
►▼
Show Figures
Open AccessArticle
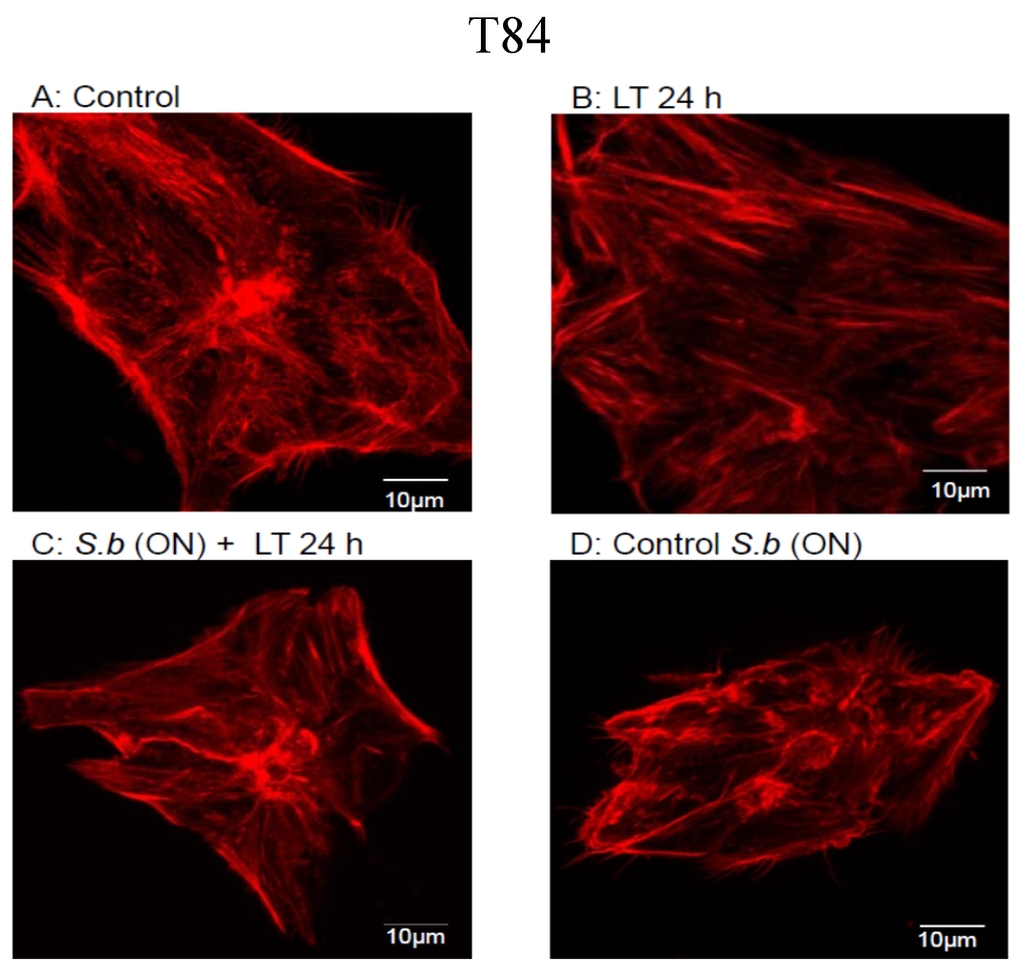
The Saccharomyces boulardii CNCM I-745 Strain Shows Protective Effects against the B. anthracis LT Toxin
by
Rodolphe Pontier-Bres, Patrick Rampal, Jean-François Peyron, Patrick Munro, Emmanuel Lemichez and Dorota Czerucka
Cited by 8 | Viewed by 7586
Abstract
The probiotic yeast
Saccharomyces boulardii (
S. boulardii) has been prescribed for the prophylaxis and treatment of several infectious diarrheal diseases. Gastrointestinal anthrax causes fatal systemic disease. In the present study, we investigated the protective effects conferred by
Saccharomyces boulardii CNCM I-745
[...] Read more.
The probiotic yeast
Saccharomyces boulardii (
S. boulardii) has been prescribed for the prophylaxis and treatment of several infectious diarrheal diseases. Gastrointestinal anthrax causes fatal systemic disease. In the present study, we investigated the protective effects conferred by
Saccharomyces boulardii CNCM I-745 strain on polarized T84 columnar epithelial cells intoxicated by the lethal toxin (LT) of
Bacillus anthracis. Exposure of polarized T84 cells to LT affected cell monolayer integrity, modified the morphology of tight junctions and induced the formation of actin stress fibers. Overnight treatment of cells with
S. boulardii before incubation with LT maintained the integrity of the monolayers, prevented morphological modification of tight junctions, restricted the effects of LT on actin remodeling and delayed LT-induced MEK-2 cleavage. Mechanistically, we demonstrated that in the presence of
S. boulardii, the medium is depleted of both LF and PA sub-units of LT and the appearance of a cleaved form of PA. Our study highlights the potential of the
S. boulardii CNCM I-745 strain as a prophylactic agent against the gastrointestinal form of anthrax.
Full article
►▼
Show Figures
Open AccessArticle
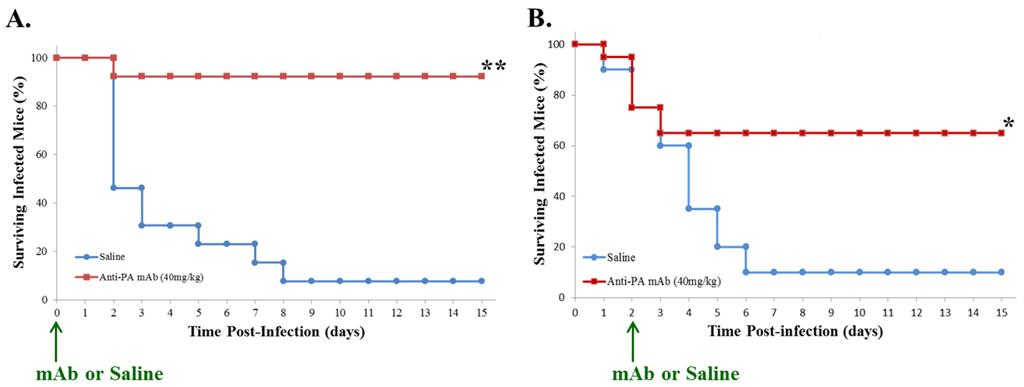
Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax
by
Bruce Huang, Tao Xie, David Rotstein, Hui Fang and David M. Frucht
Cited by 6 | Viewed by 5701
Abstract
The principal portal for anthrax infection in natural animal outbreaks is
the digestive tract. Enteric exposure to anthrax, which is difficult to detect or prevent in a timely manner, could be exploited as an act of terror through contamination of human or animal
[...] Read more.
The principal portal for anthrax infection in natural animal outbreaks is
the digestive tract. Enteric exposure to anthrax, which is difficult to detect or prevent in a timely manner, could be exploited as an act of terror through contamination of human or animal food. Our group has developed a novel animal model of gastrointestinal (GI) anthrax for evaluation of disease pathogenesis and experimental therapeutics, utilizing vegetative
Bacillus anthracis (Sterne strain) administered to A/J mice (a complement-deficient strain) by oral gavage. We hypothesized that a humanized recombinant monoclonal antibody (mAb) * that neutralizes the protective antigen (PA) component of
B. anthracis lethal toxin (LT) and edema toxin (ET) could be an effective treatment. Although the efficacy of this anti-anthrax PA mAb has been shown in animal models of inhalational anthrax, its activity in GI infection had not yet been ascertained. We hereby demonstrate that passive immunotherapy with anti-anthrax PA mAb, administered at the same time as gastrointestinal exposure to
B. anthracis, prevents lethal sepsis in nearly all cases (>90%), while a delay of up to forty-eight hours in treatment still greatly reduces mortality following exposure (65%). Moreover, passive immunotherapy protects against enteric invasion, associated mucosal injury and subsequent dissemination by gastrointestinal
B. anthracis, indicating that it acts to prevent the initial stages of infection. * Expired raxibacumab being cycled off the Strategic National Stockpile; biological activity confirmed by
in vitro assay.
Full article
►▼
Show Figures
Open AccessArticle
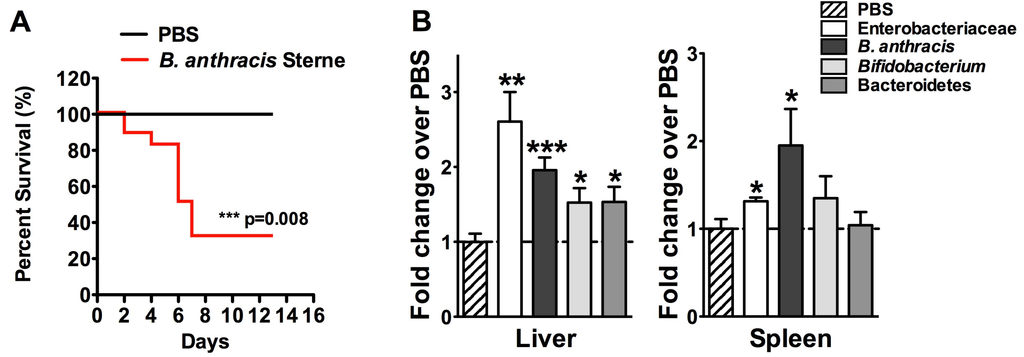
Impact of Gastrointestinal Bacillus anthracis Infection on Hepatic B Cells
by
Natacha Colliou, Bikash Sahay, Mojgan Zadeh, Jennifer L. Owen and Mansour Mohamadzadeh
Cited by 1 | Viewed by 4786
Abstract
Ingestion of
Bacillus anthracis results in rapid gastrointestinal (GI) infection, known as GI anthrax. We previously showed that during GI anthrax, there is swift deterioration of intestinal barrier function leading to translocation of gut-associated bacteria into systemic circulation. Additionally, we described dysfunction in
[...] Read more.
Ingestion of
Bacillus anthracis results in rapid gastrointestinal (GI) infection, known as GI anthrax. We previously showed that during GI anthrax, there is swift deterioration of intestinal barrier function leading to translocation of gut-associated bacteria into systemic circulation. Additionally, we described dysfunction in colonic B cells. In concordance with our previous studies, here, we report early migration of the Sterne strain of
B. anthracis along with other gut-resident bacteria into the infected murine liver. Additionally, despite a global decrease in the B cell population, we observed an increase in both B-1a and marginal zone (MZ)-like B cells. Both of these cell types are capable of producing immunoglobulins against common pathogens and commensals, which act as a general antibody barrier before an antigen-specific antibody response. Accumulation of these cells in the liver was associated with an increase in chemokine expression. These data suggest that the presence of Sterne and other commensals in the liver trigger migration of MZ-like B cells from the spleen to the liver to neutralize systemic spread. Further research is required to evaluate the possible cause of their failure to clear the infection within the liver, including the potential role of dysfunctional mitogen-activated protein kinase (MAPK) signaling.
Full article
►▼
Show Figures
Open AccessReview
Anthrax Toxins in Context of Bacillus anthracis Spores and Spore Germination
by
Christopher K. Cote and Susan L. Welkos
Cited by 18 | Viewed by 7182
Abstract
The interaction of anthrax toxin or toxin components with
B. anthracis spores has been demonstrated. Germinating spores can produce significant amounts of toxin components very soon after the initiation of germination. In this review, we will summarize the work performed that has led
[...] Read more.
The interaction of anthrax toxin or toxin components with
B. anthracis spores has been demonstrated. Germinating spores can produce significant amounts of toxin components very soon after the initiation of germination. In this review, we will summarize the work performed that has led to our understanding of toxin and spore interactions and discuss the complexities associated with these interactions
Full article
►▼
Show Figures
Open AccessArticle
Different Roles of N-Terminal and C-Terminal Domains in Calmodulin for Activation of Bacillus anthracis Edema Factor
by
Carolin Lübker, Stefan Dove, Wei-Jen Tang, Ramona J. Bieber Urbauer, Jackob Moskovitz, Jeffrey L. Urbauer and Roland Seifert
Cited by 3 | Viewed by 5740
Abstract
Bacillus anthracis adenylyl cyclase toxin edema factor (EF) is one component of the anthrax toxin and is essential for establishing anthrax disease. EF activation by the eukaryotic Ca
2+-sensor calmodulin (CaM) leads to massive cAMP production resulting in edema. cAMP also inhibits
[...] Read more.
Bacillus anthracis adenylyl cyclase toxin edema factor (EF) is one component of the anthrax toxin and is essential for establishing anthrax disease. EF activation by the eukaryotic Ca
2+-sensor calmodulin (CaM) leads to massive cAMP production resulting in edema. cAMP also inhibits the nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, thus reducing production of reactive oxygen species (ROS) used for host defense in activated neutrophils and thereby facilitating bacterial growth. Methionine (Met) residues in CaM, important for interactions between CaM and its binding partners, can be oxidized by ROS. We investigated the impact of site-specific oxidation of Met in CaM on EF activation using thirteen CaM-mutants (CaM-mut) with Met to leucine (Leu) substitutions. EF activation shows high resistance to oxidative modifications in CaM. An intact structure in the C-terminal region of oxidized CaM is sufficient for major EF activation despite altered secondary structure in the N-terminal region associated with Met oxidation. The secondary structures of CaM-mut were determined and described in previous studies from our group. Thus, excess cAMP production and the associated impairment of host defence may be afforded even under oxidative conditions in activated neutrophils.
Full article
►▼
Show Figures
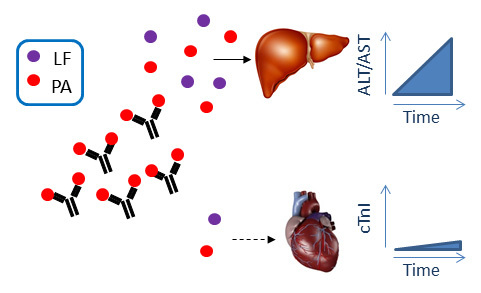
Open AccessArticle
Immunization of Mice with Anthrax Protective Antigen Limits Cardiotoxicity but Not Hepatotoxicity Following Lethal Toxin Challenge
by
T. Scott Devera, Dawn K. Prusator, Sunil K. Joshi, Jimmy D. Ballard and Mark L. Lang
Cited by 3 | Viewed by 5241
Abstract
Protective immunity against anthrax is inferred from measurement of vaccine antigen-specific neutralizing antibody titers in serum samples. In animal models,
in vivo challenges with toxin and/or spores can also be performed. However, neither of these approaches considers toxin-induced damage to specific organ systems.
[...] Read more.
Protective immunity against anthrax is inferred from measurement of vaccine antigen-specific neutralizing antibody titers in serum samples. In animal models,
in vivo challenges with toxin and/or spores can also be performed. However, neither of these approaches considers toxin-induced damage to specific organ systems. It is therefore important to determine to what extent anthrax vaccines and existing or candidate adjuvants can provide organ-specific protection against intoxication. We therefore compared the ability of Alum, CpG DNA and the CD1d ligand α-galactosylceramide (αGC) to enhance protective antigen-specific antibody titers, to protect mice against challenge with lethal toxin, and to block cardiotoxicity and hepatotoxicity. By measurement of serum cardiac Troponin I (cTnI), and hepatic alanine aminotransferase (ALT), and aspartate aminotransferase (AST), it was apparent that neither vaccine modality prevented hepatic intoxication, despite high Ab titers and ultimate survival of the subject. In contrast, cardiotoxicity was greatly diminished by prior immunization. This shows that a vaccine that confers survival following toxin exposure may still have an associated morbidity. We propose that organ-specific intoxication should be monitored routinely during research into new vaccine modalities.
Full article
►▼
Show Figures