The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases
Abstract
:1. Introduction
2. Roles of Glutamine in the Intestine
2.1. Tissue Integrity
2.2. Inflammatory Pathway
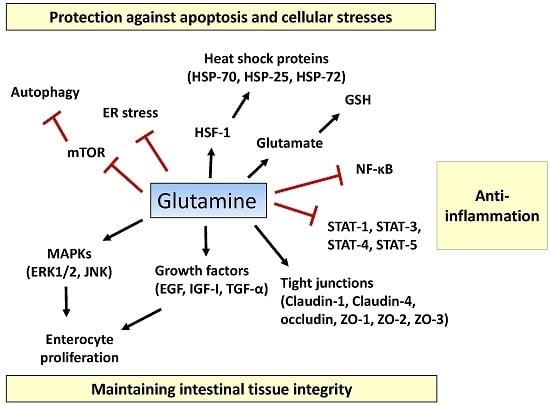
2.3. Apoptosis and Cellular Stresses
3. Clinical Implications for Intestinal Diseases
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calder, P. Glutamine and the immune system. Clin. Nutr. 1994, 13, 2–8. [Google Scholar] [CrossRef]
- Albrecht, J.; Sidoryk-Wegrzynowicz, M.; Zielinska, M.; Aschner, M. Roles of glutamine in neurotransmission. Neuron. Glia Biol. 2010, 6, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Amores-Sanchez, M.I.; Medina, M.A. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Coster, J.; McCauley, R.; Hall, J. Glutamine: Metabolism and application in nutrition support. Asia Pac. J. Clin. Nutr. 2004, 13, 25–31. [Google Scholar] [PubMed]
- Patience, J.F. A review of the role of acid-base balance in amino acid nutrition. J. Anim. Sci. 1990, 68, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Curi, R.; Pithon Curi, T.C.; Murphy, C.J.; Garcia, C.; Pires de Melo, M. Glutamine metabolism by lymphocytes, macrophages, and neutrophils: Its importance in health and disease. J. Nutr. Biochem. 1999, 10, 316–324. [Google Scholar] [CrossRef]
- Newsholme, P. Why is l-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131, 2515S–2522S. [Google Scholar] [PubMed]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, H. Oncogenes and tumor suppressors regulate glutamine metabolism in cancer cells. J. Cancer Prev. 2013, 18, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.M.; Wilmore, D.W. Is glutamine a conditionally essential amino acid? Nutr. Rev. 1990, 48, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E. Glutamine: Mode of action in critical illness. Crit. Care Med. 2007, 35, S541–S544. [Google Scholar] [CrossRef] [PubMed]
- Karinch, A.M.; Pan, M.; Lin, C.M.; Strange, R.; Souba, W.W. Glutamine metabolism in sepsis and infection. J. Nutr. 2001, 131, 2535S–2538S. [Google Scholar] [PubMed]
- Askanazi, J.; Carpentier, Y.A.; Michelsen, C.B.; Elwyn, D.H.; Furst, P.; Kantrowitz, L.R.; Gump, F.E.; Kinney, J.M. Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann. Surg. 1980, 192, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [PubMed]
- Newsholme, E.A.; Carrie, A.L. Quantitative aspects of glucose and glutamine metabolism by intestinal cells. Gut 1994, 35, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Dechelotte, P.; Darmaun, D.; Rongier, M.; Hecketsweiler, B.; Rigal, O.; Desjeux, J.F. Absorption and metabolic effects of enterally administered glutamine in humans. Am. J. Physiol. 1991, 260, G677–G682. [Google Scholar] [PubMed]
- Hankard, R.G.; Darmaun, D.; Sager, B.K.; D’Amore, D.; Parsons, W.R.; Haymond, M. Response of glutamine metabolism to exogenous glutamine in humans. Am. J. Physiol. 1995, 269, E663–E670. [Google Scholar] [PubMed]
- Evans, M.A.; Shronts, E.P. Intestinal fuels: Glutamine, short-chain fatty acids, and dietary fiber. J. Am. Diet. Assoc. 1992, 92, 1239–1246. [Google Scholar] [PubMed]
- Wang, B.; Wu, G.; Zhou, Z.; Dai, Z.; Sun, Y.; Ji, Y.; Li, W.; Wang, W.; Liu, C.; Han, F.; et al. Glutamine and intestinal barrier function. Amino Acids 2015, 47, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- McCauley, R.; Kong, S.E.; Hall, J. Glutamine and nucleotide metabolism within enterocytes. J. Parenter. Enter. Nutr. 1998, 22, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Glutamine as an immunonutrient. Yonsei Med. J. 2011, 52, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Grecu, I. Have we enough glutamine and how does it work? A clinician’s view. Ann. Nutr. Metab. 2012, 60, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Novak, F.; Heyland, D.K.; Avenell, A.; Drover, J.W.; Su, X. Glutamine supplementation in serious illness: A systematic review of the evidence. Crit. Care Med. 2002, 30, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T.; Griffiths, R.D.; McArdle, A. Exogenous glutamine: The clinical evidence. Crit. Care Med. 2007, 35, S545–S552. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.A.; Alison, M. The Biology of Epithelial Cell Populations; Oxford University Press: New York, NY, USA, 1984; Volume 1. [Google Scholar]
- Van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, M.; Cheng, H. Gastrointestinal stem cells. II. Intestinal stem cells. Am. J. Physiol. Gastrointest. Liv. Physiol. 2005, 289, G381–G387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.M.; Argenzio, R.A.; Chen, W.; Rippe, R.A.; Westwick, J.K.; Cox, A.D.; Berschneider, H.M.; Brenner, D.A. l-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am. J. Physiol. 1997, 272, G943–G953. [Google Scholar] [PubMed]
- Ko, T.C.; Beauchamp, R.D.; Townsend, C.M., Jr.; Thompson, J.C. Glutamine is essential for epidermal growth factor-stimulated intestinal cell proliferation. Surgery 1993, 114, 147–153. [Google Scholar] [PubMed]
- Ziegler, T.R.; Mantell, M.P.; Chow, J.C.; Rombeau, J.L.; Smith, R.J. Gut adaptation and the insulin-like growth factor system: Regulation by glutamine and IGF-I administration. Am. J. Physiol. 1996, 271, G866–G875. [Google Scholar] [PubMed]
- Blikslager, A.T.; Rhoads, J.M.; Bristol, D.G.; Roberts, M.C.; Argenzio, R.A. Glutamine and transforming growth factor-α stimulate extracellular regulated kinases and enhance recovery of villous surface area in porcine ischemic-injured intestine. Surgery 1999, 125, 186–194. [Google Scholar] [CrossRef]
- Gonzalez-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Mitic, L.L.; Anderson, J.M. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998, 60, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269, G467–G475. [Google Scholar] [PubMed]
- Harhaj, N.S.; Antonetti, D.A. Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 2004, 36, 1206–1237. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, J.; Kojima, T.; Ogasawara, N.; Kamekura, R.; Kurose, M.; Go, M.; Harimaya, A.; Murata, M.; Osanai, M.; Chiba, H.; et al. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol. Pharmacol. 2008, 74, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Basuroy, S.; Seth, A.; Elias, B.; Naren, A.P.; Rao, R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J. 2006, 393 Pt 1, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R.; Rill, B.K.; Carlson, S.L.; Carnes, D.; Kerner, R.; Mrsny, R.J.; Madara, J.L. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol.Cell Physiol. 1997, 273, C1378–C1385. [Google Scholar]
- Rigor, R.R.; Shen, Q.; Pivetti, C.D.; Wu, M.H.; Yuan, S.Y. Myosin light chain kinase signaling in endothelial barrier dysfunction. Med. Res. Rev. 2013, 33, 911–933. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lewis, P.; Samuelson, D.; Liboni, K.; Neu, J. Glutamine regulates Caco-2 cell tight junction proteins. Am. J. Physiol. Gastrointest. Liv. Physiol. 2004, 287, G726–G733. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, V.G.; Li, N.; Thomas, J.; West, C.M.; Neu, J. Glutamine and barrier function in cultured Caco-2 epithelial cell monolayers. J. Nutr. 2003, 133, 2176–2179. [Google Scholar] [PubMed]
- Beutheu, S.; Ghouzali, I.; Galas, L.; Dechelotte, P.; Coeffier, M. Glutamine and arginine improve permeability and tight junction protein expression in methotrexate-treated Caco-2 cells. Clin. Nutr. 2013, 32, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Basuroy, S.; Sheth, P.; Rao, R.K. l-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am. J. Physiol. Gastrointest. Liv. Physiol. 2004, 287, G510–G517. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Neu, J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J. Nutr. 2009, 139, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, A.; Furuse, M.; Saitou, M.; Ando-Akatsuka, Y.; Tsukita, S. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 1997, 137, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, R.; Dyukova, E.; Singh, N.K.; Ohba, M.; Mobley, J.A.; Rao, G.N. Vascular endothelial tight junctions and barrier function are disrupted by 15 S-hydroxyeicosatetraenoic acid partly via protein kinase Cϵ-mediated zona occludens-1 phosphorylation at threonine 770/772. J. Biol. Chem. 2014, 289, 3148–3163. [Google Scholar] [CrossRef] [PubMed]
- Dorfel, M.J.; Westphal, J.K.; Bellmann, C.; Krug, S.M.; Cording, J.; Mittag, S.; Tauber, R.; Fromm, M.; Blasig, I.E.; Huber, O. CK2-dependent phosphorylation of occludin regulates the interaction with ZO-proteins and tight junction integrity. Cell Commun. Signal. CCS 2013, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, Z.; Ji, Y.; Sun, K.; Dai, Z.; Wu, G. l-Glutamine Enhances Tight Junction Integrity by Activating CaMK Kinase 2–AMP-Activated Protein Kinase Signaling in Intestinal Porcine Epithelial Cells. J. Nutr. 2016, 146, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Slavov, D.; Wischmeyer, P.E. Glutamine-mediated dual regulation of heat shock transcription factor-1 activation and expression. J. Biol. Chem. 2012, 287, 40400–40413. [Google Scholar] [CrossRef] [PubMed]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.M.; Wu, G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 2009, 37, 111–122. [Google Scholar]
- Baltimore, D. NF-κB is 25. Nat. Immunol. 2011, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Chu, C.C.; Ko, T.L.; Yeh, C.L.; Yeh, S.L. Effects of alanyl-glutamine dipeptide on the expression of colon-inflammatory mediators during the recovery phase of colitis induced by dextran sulfate sodium. Eur. J. Nutr. 2013, 52, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Sufit, A.J.; Wischmeyer, P.E. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. J. Parenter. Enter. Nutr. 2011, 35, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Haynes, T.E.; Li, P.; Li, X.; Shimotori, K.; Sato, H.; Flynn, N.E.; Wang, J.; Knabe, D.A.; Wu, G. l-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 2009, 37, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, V.; Eaves-Pyles, T.; Odoms, K.; Quaid, G.; Shanley, T.P.; Wong, H.R. Heat shock inhibits activation of NF-κB in the absence of heat shock factor-1. Biochem. Biophys. Res. Commun. 2002, 291, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Liboni, K.C.; Li, N.; Scumpia, P.O.; Neu, J. Glutamine modulates LPS-induced IL-8 production through IkappaB/NF-κB in human fetal and adult intestinal epithelium. J. Nutr. 2005, 135, 245–251. [Google Scholar] [PubMed]
- Hubert-Buron, A.; Leblond, J.; Jacquot, A.; Ducrotte, P.; Dechelotte, P.; Coeffier, M. Glutamine pretreatment reduces IL-8 production in human intestinal epithelial cells by limiting IκBα ubiquitination. J. Nutr. 2006, 136, 1461–1465. [Google Scholar] [PubMed]
- Kretzmann, N.A.; Fillmann, H.; Mauriz, J.L.; Marroni, C.A.; Marroni, N.; Gonzalez-Gallego, J.; Tunon, M.J. Effects of glutamine on proinflammatory gene expression and activation of nuclear factor κB and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm. Bowel Dis. 2008, 14, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H. STAT signaling in inflammation. Jak-Stat 2013, 2, e24198. [Google Scholar] [CrossRef] [PubMed]
- Ihle, J.N. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 2001, 13, 211–217. [Google Scholar] [CrossRef]
- Liboni, K.; Li, N.; Neu, J. Mechanism of glutamine-mediated amelioration of lipopolysaccharide-induced IL-8 production in Caco-2 cells. Cytokine 2004, 26, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Kubes, P.; McCafferty, D.M. Nitric oxide and intestinal inflammation. J. Am. Med. 2000, 109, 150–158. [Google Scholar] [CrossRef]
- Hecker, M.; Sessa, W.C.; Harris, H.J.; Anggard, E.E.; Vane, J.R. The metabolism of l-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: Cultured endothelial cells recycle l-citrulline to l-arginine. Proc. Natl. Acad. Sci. USA 1990, 87, 8612–8616. [Google Scholar] [CrossRef] [PubMed]
- Swierkosz, T.A.; Mitchell, J.A.; Sessa, W.C.; Hecker, M.; Vane, J.R. l-Glutamine inhibits the release of endothelium-derived relaxing factor from the rabbit aorta. Biochem. Biophys. Res. Commun. 1990, 172, 143–148. [Google Scholar] [CrossRef]
- Houdijk, A.P.; Visser, J.J.; Rijnsburger, E.R.; Teerlink, T.; van Leeuwen, P.A. Dietary glutamine supplementation reduces plasma nitrate levels in rats. Clin. Nutr. 1998, 17, 11–14. [Google Scholar] [CrossRef]
- Suh, G.J.; Youn, Y.K.; Song, H.G.; Rhee, J.E.; Jung, S.E. The effect of glutamine on inducible nitric oxide synthase gene expression in intestinal ischemia-reperfusion injury. Nutr. Res. 2003, 23, 131–140. [Google Scholar]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Perez-Gomez, C.; Nunez de Castro, I.; Asenjo, M.; Marquez, J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int. J. Biochem. Cell Biol. 2002, 34, 439–458. [Google Scholar] [CrossRef]
- Watson, A.J.; Pritchard, D.M., VII. Apoptosis in intestinal epithelium: Lessons from transgenic and knockout mice. Am. J. Physiol. Gastrointest. Liv. Physiol. 2000, 278, G1–G5. [Google Scholar]
- Que, F.G.; Gores, G.J. Cell death by apoptosis: Basic concepts and disease relevance for the gastroenterologist. Gastroenterology 1996, 110, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Demehri, F.R.; Barrett, M.; Ralls, M.W.; Miyasaka, E.A.; Feng, Y.; Teitelbaum, D.H. Intestinal epithelial cell apoptosis and loss of barrier function in the setting of altered microbiota with enteral nutrient deprivation. Front. Cell. Infect. Microbiol. 2013, 3, 105. [Google Scholar] [CrossRef] [PubMed]
- Zatorski, H.; Marynowski, M.; Fichna, J. Is insulin-like growth factor 1 (IGF-1) system an attractive target inflammatory bowel diseases? Benefits and limitation of potential therapy. Pharmacol. Rep. 2016, 68, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Sanchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martinez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Koji, T.; Makiyama, K.; Kobayashi, N.; Nakane, P.K. Apoptosis of crypt epithelial cells in ulcerative colitis. J. Pathol. 1996, 180, 152–159. [Google Scholar] [CrossRef]
- Kim, J.M.; Eckmann, L.; Savidge, T.C.; Lowe, D.C.; Witthoft, T.; Kagnoff, M.F. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 1998, 102, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Schumann, M.; Siegmund, B.; Schulzke, J.D.; Fromm, M. Celiac Disease: Role of the Epithelial Barrier. Cellular Mol. Gastroenterol. Hepatol. 2017, 3, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Hyoh, Y.; Ishizaka, S.; Horii, T.; Fujiwara, A.; Tegoshi, T.; Yamada, M.; Arizono, N. Activation of caspases in intestinal villus epithelial cells of normal and nematode infected rats. Gut 2002, 50, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Madesh, M.; Balasubramanian, K.A. Apoptosis in the intestinal epithelium: Its relevance in normal and pathophysiological conditions. J. Gastroenterol. Hepatol. 2000, 15, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Papaconstantinou, H.T.; Hwang, K.O.; Rajaraman, S.; Hellmich, M.R.; Townsend, C.M., Jr.; Ko, T.C. Glutamine deprivation induces apoptosis in intestinal epithelial cells. Surgery 1998, 124, 152–159, discussion 159–160. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Fujii, J.; Brito, G.A.; Alcantara, C.; Oria, R.B.; Lima, A.A.; Obrig, T.; Guerrant, R.L. Caspase and bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro. Infect. Immun. 2006, 74, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Takayama, C.; Mukaizawa, F.; Fujita, T.; Ogawara, K.; Higaki, K.; Kimura, T. Amino acids suppress apoptosis induced by sodium laurate, an absorption enhancer. J. Pharm. Sci. 2009, 98, 4629–4638. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.; Oehler, R.; Manhart, N.; Exner, R.; Wessner, B.; Strasser, E.; Spittler, A. Regulative potential of glutamine—Relation to glutathione metabolism. Nutrition 2002, 18, 217–221. [Google Scholar] [CrossRef]
- Hall, A. The role of glutathione in the regulation of apoptosis. Eur. J. Clin. Investig. 1999, 29, 238–245. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.D.; Li, J.; Chung, D.H.; Evers, B.M. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am. J. Physiol. Gastrointest. Liv. Physiol. 2007, 293, G1262–G1271. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E. Glutamine and heat shock protein expression. Nutrition 2002, 18, 225–228. [Google Scholar] [CrossRef]
- Garrido, C.; Gurbuxani, S.; Ravagnan, L.; Kroemer, G. Heat shock proteins: Endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 2001, 286, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Singleton, K.D.; Serkova, N.; Beckey, V.E.; Wischmeyer, P.E. Glutamine attenuates lung injury and improves survival after sepsis: Role of enhanced heat shock protein expression. Crit. Care Med. 2005, 33, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Ropeleski, M.J.; Riehm, J.; Baer, K.A.; Musch, M.W.; Chang, E.B. Anti-apoptotic effects of l-glutamine-mediated transcriptional modulation of the heat shock protein 72 during heat shock. Gastroenterology 2005, 129, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Lee, A.-H.; Franke, A.; Glickman, J.N.; Zeissig, S.; Tilg, H.; Nieuwenhuis, E.E.; Higgins, D.E.; Schreiber, S.; Glimcher, L.H. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; San-Miguel, B.; Prause, C.; Marroni, N.; Cuevas, M.J.; Gonzalez-Gallego, J.; Tunon, M.J. Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis. PLoS ONE 2012, 7, e50407. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Hampe, J.; Franke, A.; Rosenstiel, P.; Till, A.; Teuber, M.; Huse, K.; Albrecht, M.; Mayr, G.; de La Vega, F.M.; Briggs, J.; et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007, 39, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Rioux, J.D.; Xavier, R.J.; Taylor, K.D.; Silverberg, M.S.; Goyette, P.; Huett, A.; Green, T.; Kuballa, P.; Barmada, M.M.; Datta, L.W.; et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 2007, 39, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, Y.; Nishiumi, S.; Masuda, A.; Inoue, J.; Nguyen, N.M.; Irino, Y.; Komatsu, M.; Tanaka, K.; Kutsumi, H.; Azuma, T.; et al. Autophagy in the intestinal epithelium reduces endotoxin-induced inflammatory responses by inhibiting NF-kappaB activation. Arch. Biochem. Biophys. 2011, 506, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K.; Patel, K.K.; Komatsu, M.; Virgin, H.W.T.; Stappenbeck, T.S. A common role for Atg16L1, Atg5 and Atg7 in small intestinal Paneth cells and Crohn disease. Autophagy 2009, 5, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Stappenbeck, T.S. Autophagy and intestinal homeostasis. Annu. Rev. Physiol. 2013, 75, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama, T.; Musch, M.W.; Ropeleski, M.J.; Tsubouchi, H.; Chang, E.B. Glutamine increases autophagy under Basal and stressed conditions in intestinal epithelial cells. Gastroenterology 2009, 136, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Sabatini, D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005, 17, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Van der Vos, K.E.; Eliasson, P.; Proikas-Cezanne, T.; Vervoort, S.J.; van Boxtel, R.; Putker, M.; van Zutphen, I.J.; Mauthe, M.; Zellmer, S.; Pals, C.; et al. Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat. Cell Biol. 2012, 14, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Sido, B.; Seel, C.; Hochlehnert, A.; Breitkreutz, R.; Droge, W. Low intestinal glutamine level and low glutaminase activity in Crohn’s disease: A rational for glutamine supplementation? Dig. Dis. Sci. 2006, 51, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, Y.C.; Liu, J.J.; Hou, Y.C.; Yeh, C.L.; Yeh, S.L. Effects of dietary glutamine on the homeostasis of CD4+ T cells in mice with dextran sulfate sodium-induced acute colitis. PLoS ONE 2014, 9, e84410. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.-H.; Liu, J.-J.; Yeh, S.-L.; Chen, W.-J.; Yeh, C.-L. Glutamine modulates acute dextran sulphate sodium-induced changes in small-intestinal intraepithelial γδ-T-lymphocyte expression in mice. Br. J. Nutr. 2014, 111, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Ameho, C.K.; Adjei, A.A.; Harrison, E.K.; Takeshita, K.; Morioka, T.; Arakaki, Y.; Ito, E.; Suzuki, I.; Kulkarni, A.D.; Kawajiri, A.; et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor α production in trinitrobenzene sulphonic acid induced colitis. Gut 1997, 41, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Souba, W.W.; Klimberg, V.S.; Hautamaki, R.D.; Mendenhall, W.H.; Bova, F.C.; Howard, R.J.; Bland, K.I.; Copeland, E.M. Oral glutamine reduces bacterial translocation following abdominal radiation. J. Surg. Res. 1990, 48, 1–5. [Google Scholar] [CrossRef]
- Lee, W.Y.; Hu, Y.M.; Ko, T.L.; Yeh, S.L.; Yeh, C.L. Glutamine modulates sepsis-induced changes to intestinal intraepithelial gammadeltaT lymphocyte expression in mice. Shock 2012, 38, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.N.; Lee, W.Y.; Pai, M.H.; Chen, W.J.; Yeh, C.L.; Yeh, S.L. Glutamine modulates CD8αα(+) TCRαβ(+) intestinal intraepithelial lymphocyte expression in mice with polymicrobial sepsis. Nutrition 2013, 29, 911–917. [Google Scholar] [CrossRef] [PubMed]
- García-de-Lorenzo, A.; Zarazaga, A.; García-Luna, P.P.; Gonzalez-Huix, F.; López-Martínez, J.; Miján, A.; Quecedo, L.; Casimiro, C.; Usán, L.; del Llano, J. Clinical evidence for enteral nutritional support with glutamine: A systematic review. Nutrition 2003, 19, 805–811. [Google Scholar] [CrossRef]
- Benjamin, J.; Makharia, G.; Ahuja, V.; Rajan, K.A.; Kalaivani, M.; Gupta, S.D.; Joshi, Y.K. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: A randomized controlled trial. Dig. Dis. Sci. 2012, 57, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.J.; Avenell, A.; Noble, D.W.; Campbell, M.K.; Croal, B.L.; Simpson, W.G.; Vale, L.D.; Battison, C.G.; Jenkinson, D.J.; Cook, J.A. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011, 342, d1542. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G.; Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N. Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K.; Miller, V.; Stanton, J.; Elbadri, A.M.; Thomas, A.G. Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn’s disease. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Hiele, M.; Peeters, M.; Ghoos, Y.; Rutgeerts, P. Effect of long-term oral glutamine supplements on small intestinal permeability in patients with Crohn’s disease. J. Parenter. Enter. Nutr. 1999, 23, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Scolapio, J.S.; Camilleri, M.; Fleming, C.R.; Oenning, L.; Burton, D.; Sebo, T.J.; Batts, K.; Kelly, D. Effect of growth hormone, glutamine, and diet on adaptation in short-bowel syndrome: A randomized, controlled study. Gastroenterology 1997, 113, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Carbonnel, F.; Hecketsweiler, B.; Dechelotte, P.; Gender, J.-P.; Cosnes, J. Effects of an isotonic oral rehydration solution, enriched with glutamine, on fluid and sodium absorption in patients with short-bowel syndrome. Aliment. Pharmacol. Ther. 1997, 11, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Scolapio, J.S. Effect of growth hormone, glutamine, and diet on body composition in short bowel syndrome: A randomized, controlled study. J. Parenter. Enter. Nutr. 1999, 23, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, J.; Jeppesen, P.; Mortensen, P. Effect of high dose growth hormone with glutamine and no change in diet on intestinal absorption in short bowel patients: A randomised, double blind, crossover, placebo controlled study. Gut 2000, 47, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.; Szkudlarek, J.; Høy, C.-E.; Mortensen, P. Effect of high-dose growth hormone and glutamine on body composition, urine creatinine excretion, fatty acid absorption, and essential fatty acids status in short bowel patients. A randomized, double-blind, crossover, placebo-controlled study. Scand. J. Gastroenterol. 2001, 36, 48–54. [Google Scholar] [PubMed]
- Scolapio, J.; McGreevy, K.; Tennyson, G.; Burnett, O. Effect of glutamine in short-bowel syndrome. Clin. Nutr. 2001, 20, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Alpers, D.H. Glutamine: Do the data support the cause for glutamine supplementation in humans? Gastroenterology 2006, 130, S106–S116. [Google Scholar] [CrossRef] [PubMed]
- Gore, D.C.; Wolfe, R.R. Metabolic response of muscle to alanine, glutamine, and valine supplementation during severe illness. J. Parenter. Enter. Nutr. 2003, 27, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Bosman, R.J.; Treskes, M.; van der Spoel, H.J.; Zandstra, D.F. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensiv. Care Med. 2001, 27, 84–90. [Google Scholar] [CrossRef]
- MacFie, J. Enteral versus parenteral nutrition. Br. J. Surg. 2000, 87, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Boelens, P.G.; Melis, G.C.; van Leeuwen, P.A.; ten Have, G.A.; Deutz, N.E. Route of administration (enteral or parenteral) affects the contribution of l-glutamine to de novo L-arginine synthesis in mice: A stable-isotope study. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E683–E690. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Huix, F.; Fernandez-Banares, F.; Esteve-Comas, M.; Abad-Lacruz, A.; Cabre, E.; Acero, D.; Figa, M.; Guilera, M.; Humbert, P.; de León, R. Enteral versus parenteral nutrition as adjunct therapy in acute ulcerative colitis. Am. J. Gastroenterol. 1993, 88, 2. [Google Scholar]
- Buchman, A.L.; Moukarzel, A.A.; Bhuta, S.; Belle, M.; Ament, M.E.; Eckhert, C.D.; Hollander, D.; Gornbeln, J.; Kopple, J.D.; Vijayaroghavan, S.R. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. J. Parenter. Enter. Nutr. 1995, 19, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Noyer, C.M.; Simon, D.; Borczuk, A.; Brandt, L.J.; Lee, M.J.; Nehra, V. A double-blind placebo-controlled pilot study of glutamine therapy for abnormal intestinal permeability in patients with AIDS. Am. J. Gastroenterol. 1998, 93, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Goeters, C.; Wenn, A.; Mertes, N.; Wempe, C.; Van Aken, H.; Stehle, P.; Bone, H.-G. Parenteral l-alanyl-l-glutamine improves 6-month outcome in critically ill patients. Crit. Care Med. 2002, 30, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.D.; Jones, C.; Palmer, T.A. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition 1997, 13, 295–302. [Google Scholar] [CrossRef]
- Griffiths, R.D.; Allen, K.D.; Andrews, F.J.; Jones, C. Infection, multiple organ failure, and survival in the intensive care unit: Influence of glutamine-supplemented parenteral nutrition on acquired infection. Nutrition 2002, 18, 546–552. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. https://doi.org/10.3390/ijms18051051
Kim M-H, Kim H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. International Journal of Molecular Sciences. 2017; 18(5):1051. https://doi.org/10.3390/ijms18051051
Chicago/Turabian StyleKim, Min-Hyun, and Hyeyoung Kim. 2017. "The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases" International Journal of Molecular Sciences 18, no. 5: 1051. https://doi.org/10.3390/ijms18051051
APA StyleKim, M.-H., & Kim, H. (2017). The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. International Journal of Molecular Sciences, 18(5), 1051. https://doi.org/10.3390/ijms18051051